Abstract
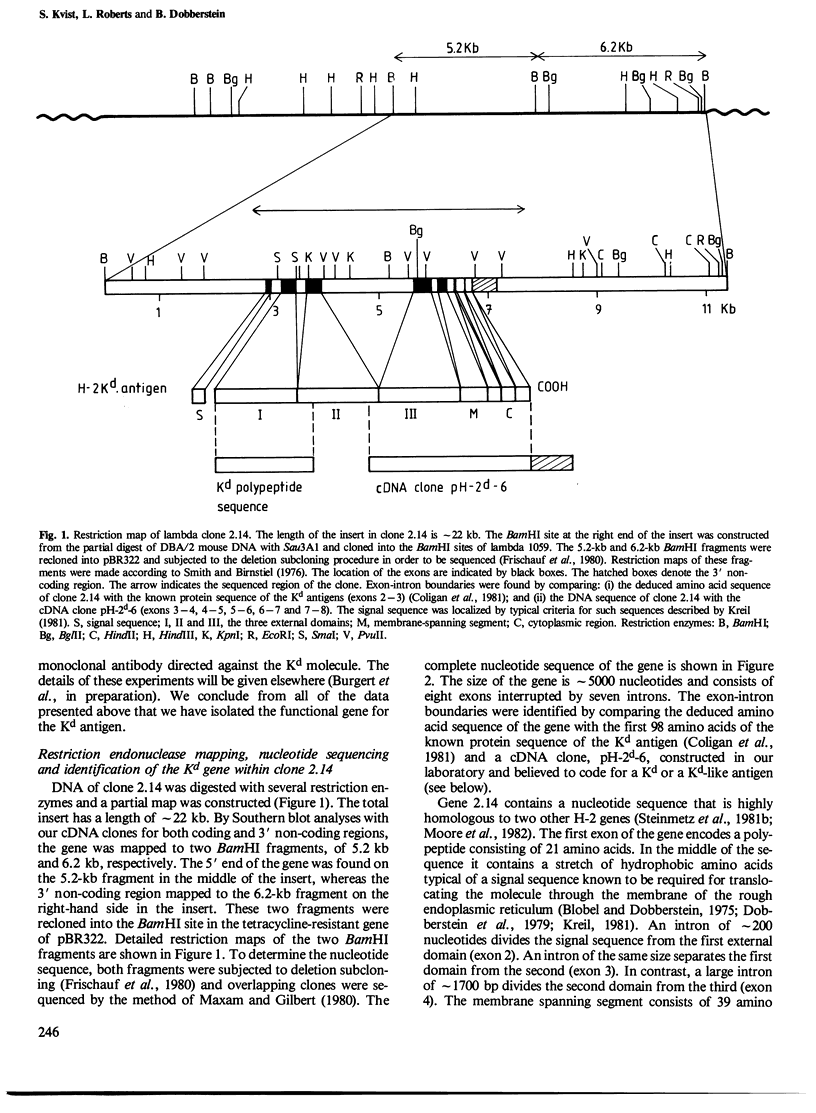
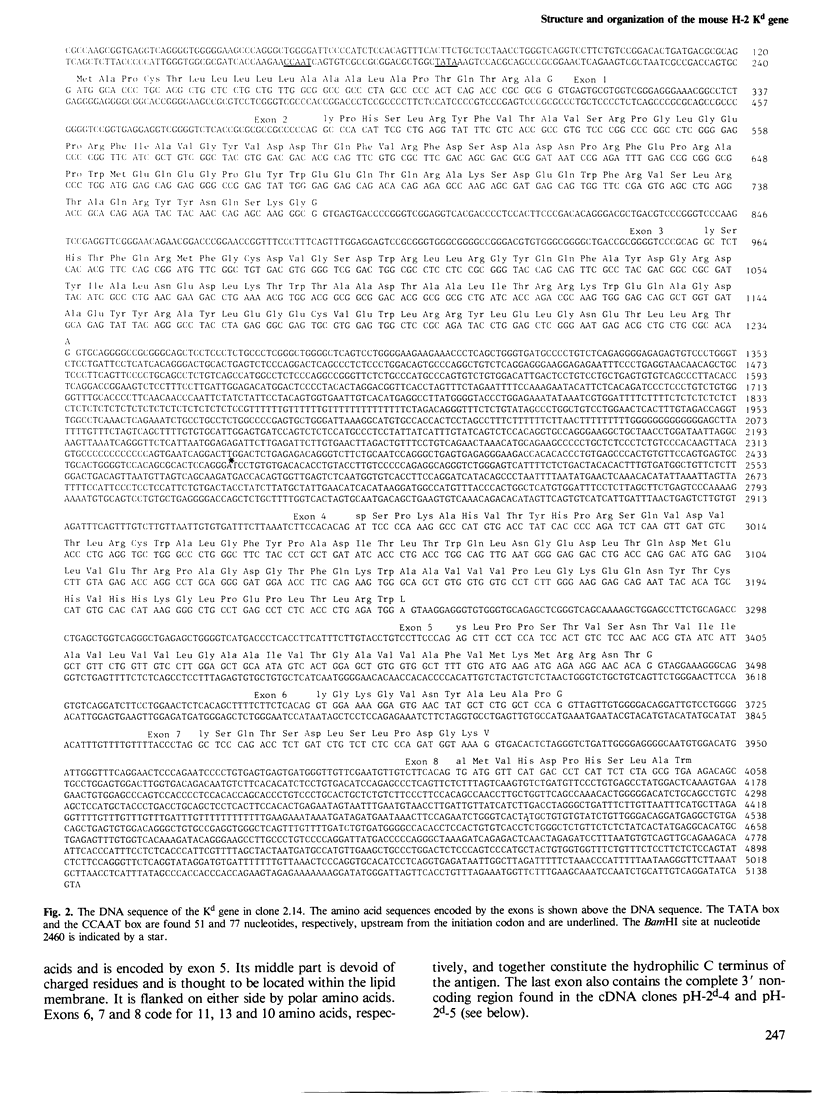
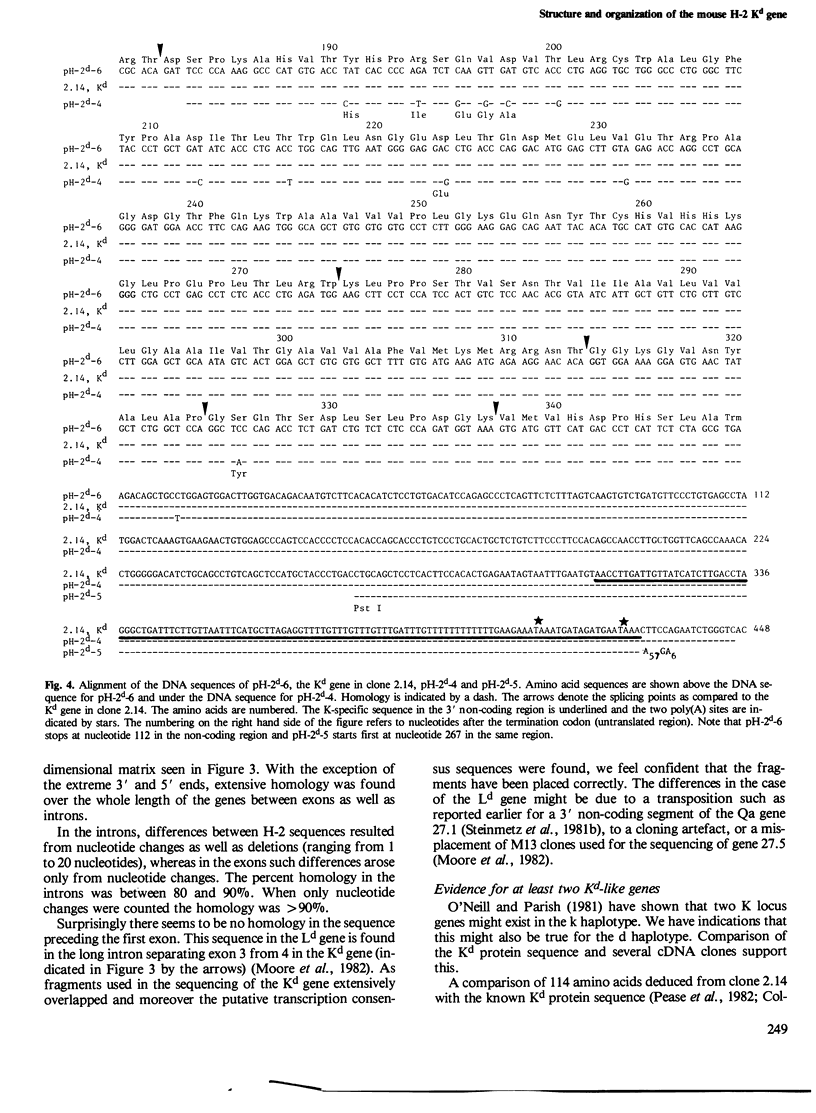
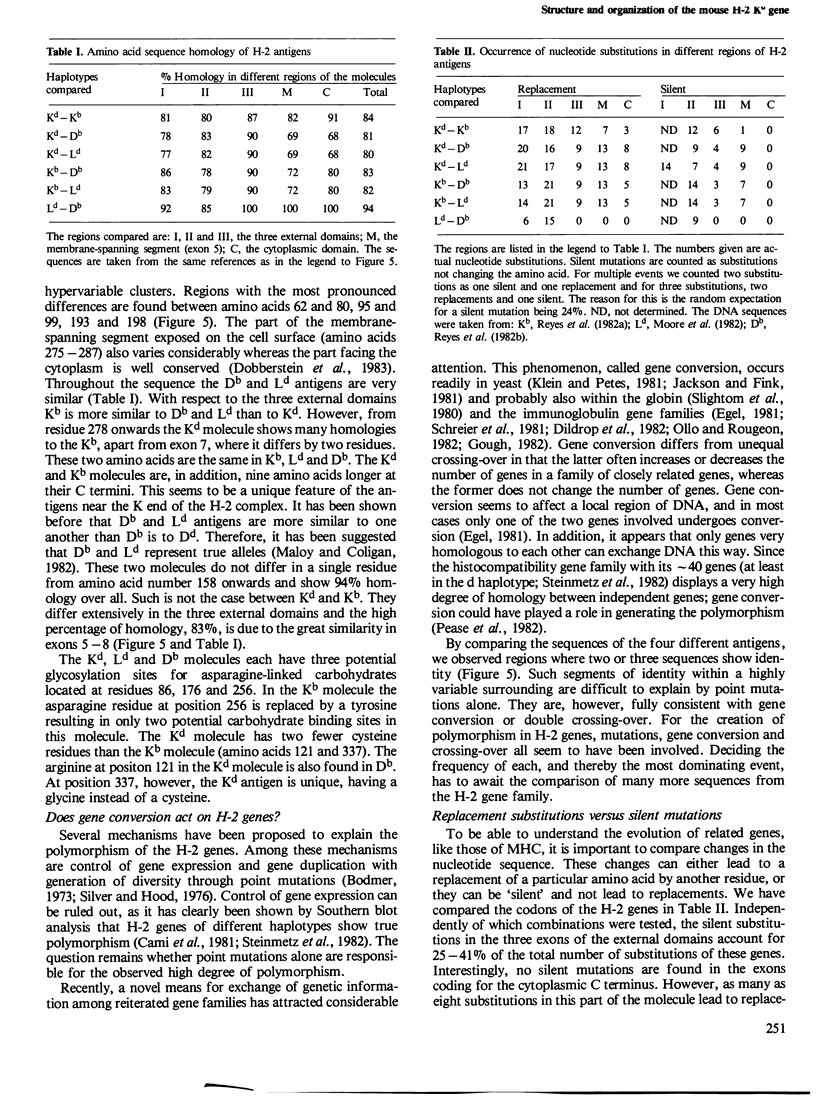
The gene coding for the mouse H-2Kd antigen has been isolated by using a K-locus specific cDNA probe. The complete nucleotide sequence of the gene reveals eight exons separated by seven introns. Transcriptionally important DNA sequences (CCAAT and TATA) precede the first exon. Comparison with other H-2 genes shows extensive homology in exons as well as in introns. Two cDNA clones encoding Kd antigens have been analysed and provide evidence for at least two expressed Kd genes in the DBA/2 mouse. Comparison of the Kd antigen sequence to three other H-2 antigens indicates that gene conversion mechanism(s) act on H-2 genes. Analyses of exon donor and acceptor sites of different H-2 genes and cDNAs show that alternative splicing sites are used by different genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary S., Mach B. Origin of transcription of a mouse immunoglobulin light chain gene. EMBO J. 1982;1(6):719–724. doi: 10.1002/j.1460-2075.1982.tb01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F. New genetic model for allelism at histocompatibility and other complex loci: polymorphism for control of gene expression. Transplant Proc. 1973 Dec;5(4):1471–1475. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cami B., Brégégère F., Abastado J. P., Kourilsky P. Multiple sequences related to classical histocompatibility antigens in the mouse genome. Nature. 1981 Jun 25;291(5817):673–675. doi: 10.1038/291673a0. [DOI] [PubMed] [Google Scholar]
- Coligan J. E., Kindt T. J., Uehara H., Martinko J., Nathenson S. G. Primary structure of a murine transplantation antigen. Nature. 1981 May 7;291(5810):35–39. doi: 10.1038/291035a0. [DOI] [PubMed] [Google Scholar]
- Dildrop R., Brüggemann M., Radbruch A., Rajewsky K., Beyreuther K. Immunoglobulin V region variants in hybridoma cells. II. Recombination between V genes. EMBO J. 1982;1(5):635–640. doi: 10.1002/j.1460-2075.1982.tb01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Egel R. Intergenic conversion and reiterated genes. Nature. 1981 Mar 19;290(5803):191–192. doi: 10.1038/290191a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. L., Petes T. D. Intrachromosomal gene conversion in yeast. Nature. 1981 Jan 15;289(5794):144–148. doi: 10.1038/289144a0. [DOI] [PubMed] [Google Scholar]
- Klein J. The major histocompatibility complex of the mouse. Science. 1979 Feb 9;203(4380):516–521. doi: 10.1126/science.104386. [DOI] [PubMed] [Google Scholar]
- Kreil G. Transfer of proteins across membranes. Annu Rev Biochem. 1981;50:317–348. doi: 10.1146/annurev.bi.50.070181.001533. [DOI] [PubMed] [Google Scholar]
- Kvist S., Bregegere F., Rask L., Cami B., Garoff H., Daniel F., Wiman K., Larhammar D., Abastado J. P., Gachelin G. cDNA clone coding for part of a mouse H-2d major histocompatibility antigen. Proc Natl Acad Sci U S A. 1981 May;78(5):2772–2776. doi: 10.1073/pnas.78.5.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Persson H., Philipson L., Peterson P. A. Molecular association between transplantation antigens and cell surface antigen in adenovirus-transformed cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5674–5678. doi: 10.1073/pnas.75.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne J. L., Bregegere F., Delarbre C., Abastado J. P., Gachelin G., Kourilsky P. Comparison of nucleotide sequences of mRNAs belonging to the mouse H-2 multigene family. Nucleic Acids Res. 1982 Feb 11;10(3):1039–1049. doi: 10.1093/nar/10.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy W. L., Coligan J. E. Primary structure of the H-2Db alloantigen. II. Additional amino acid sequence information, localization of a third site of glycosylation and evidence for K and D region specific sequences. Immunogenetics. 1982;16(1):11–22. doi: 10.1007/BF00364438. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michaelson J., Flaherty L., Vitetta E., Poulik M. D. Molecular similarities between the Qa-2 alloantigen and other gene products of the 17th chromosome of the mouse. J Exp Med. 1977 Apr 1;145(4):1066–1070. doi: 10.1084/jem.145.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- O'Neill H. C., Parish C. R. Monoclonal antibody detection of two classes of H-2Kk molecules. Mol Immunol. 1981 Aug;18(8):713–722. doi: 10.1016/0161-5890(81)90063-8. [DOI] [PubMed] [Google Scholar]
- Ollo R., Rougeon F. Mouse immunoglobulin allotypes: post-duplication divergence of gamma 2a and gamma 2b chain genes. Nature. 1982 Apr 22;296(5859):761–763. doi: 10.1038/296761a0. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Itakura K., Wallace R. B. Isolation of a cDNA clone for the murine transplantation antigen H-2Kb. Proc Natl Acad Sci U S A. 1982 May;79(10):3270–3274. doi: 10.1073/pnas.79.10.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Wallace R. B. The complete amino acid sequence of the murine transplantation antigen H-2Db as deduced by molecular cloning. Immunogenetics. 1982;16(1):1–9. doi: 10.1007/BF00364437. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Bothwell A. L., Mueller-Hill B., Baltimore D. Multiple differences between the nucleic acid sequences of the IgG2aa and IgG2ab alleles of the mouse. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4495–4499. doi: 10.1073/pnas.78.7.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Signäs C., Katze M. G., Persson H., Philipson L. An adenovirus glycoprotein binds heavy chains of class I transplantation antigens from man and mouse. Nature. 1982 Sep 9;299(5879):175–178. doi: 10.1038/299175a0. [DOI] [PubMed] [Google Scholar]
- Silver J., Hood L. Structure and evolution of transplantation antigens: partial amino-acid sequences of H-2K and H-2D alloantigens. Proc Natl Acad Sci U S A. 1976 Feb;73(2):599–603. doi: 10.1073/pnas.73.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Frelinger J. G., Fisher D., Hunkapiller T., Pereira D., Weissman S. M., Uehara H., Nathenson S., Hood L. Three cDNA clones encoding mouse transplantation antigens: homology to immunoglobulin genes. Cell. 1981 Apr;24(1):125–134. doi: 10.1016/0092-8674(81)90508-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Xin J. H., Kvist S., Dobberstein B. Identification of an H-2Kd gene using a specific cDNA probe. EMBO J. 1982;1(4):467–471. doi: 10.1002/j.1460-2075.1982.tb01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]



