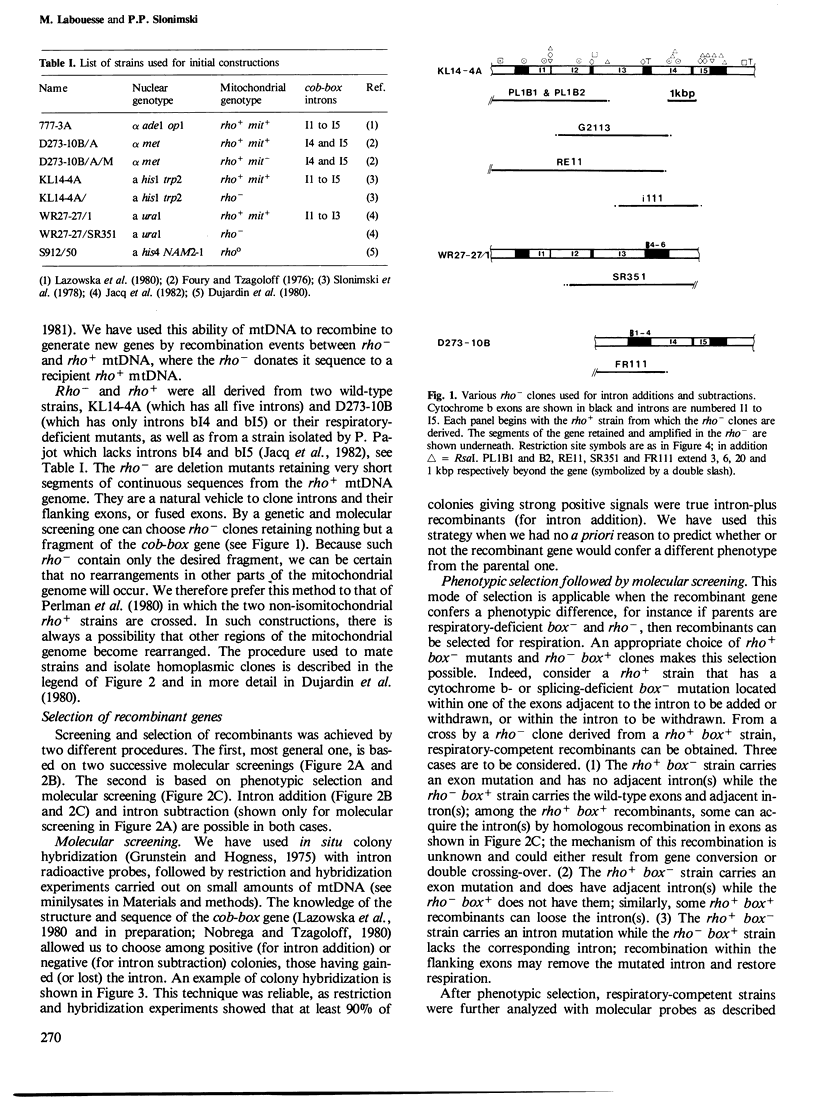
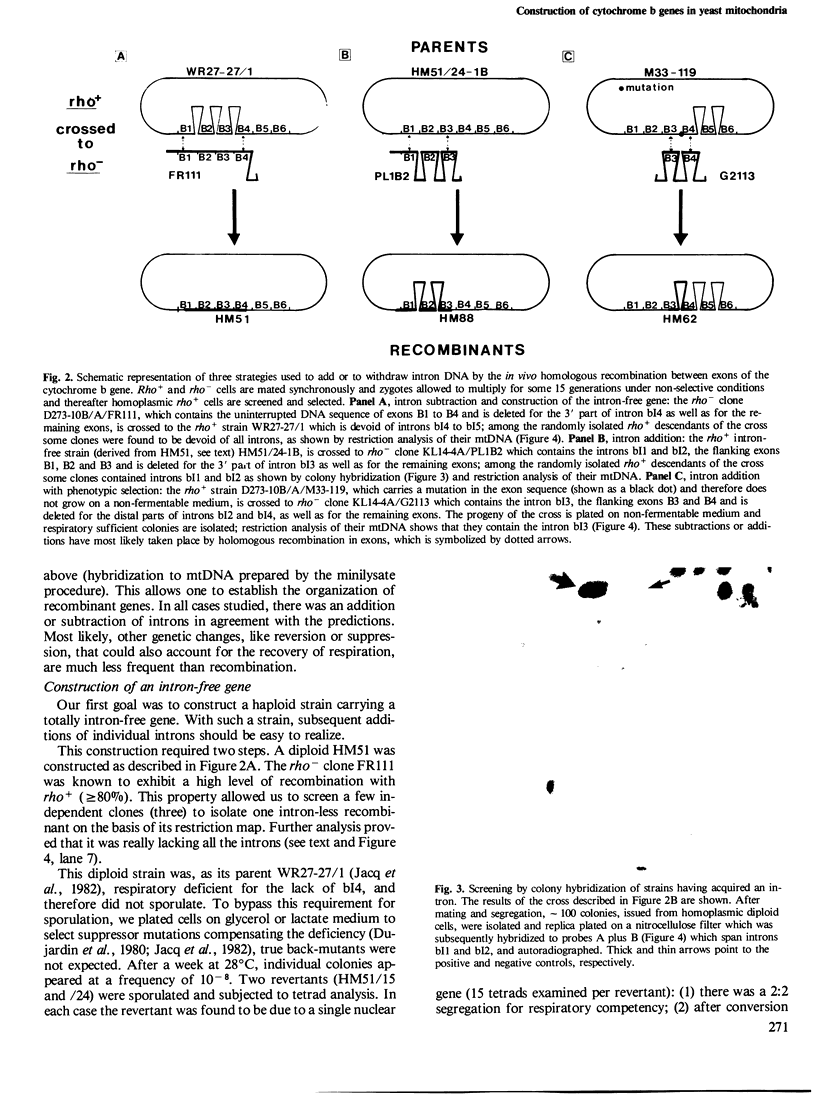
Abstract
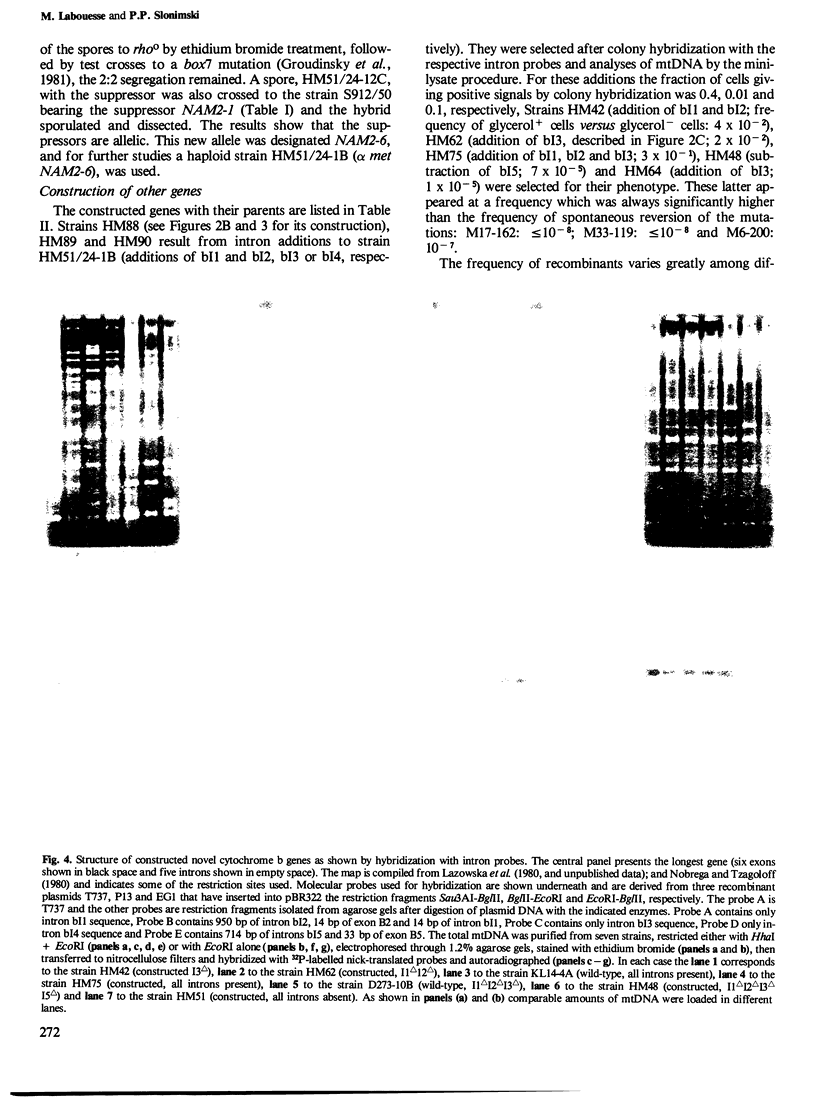
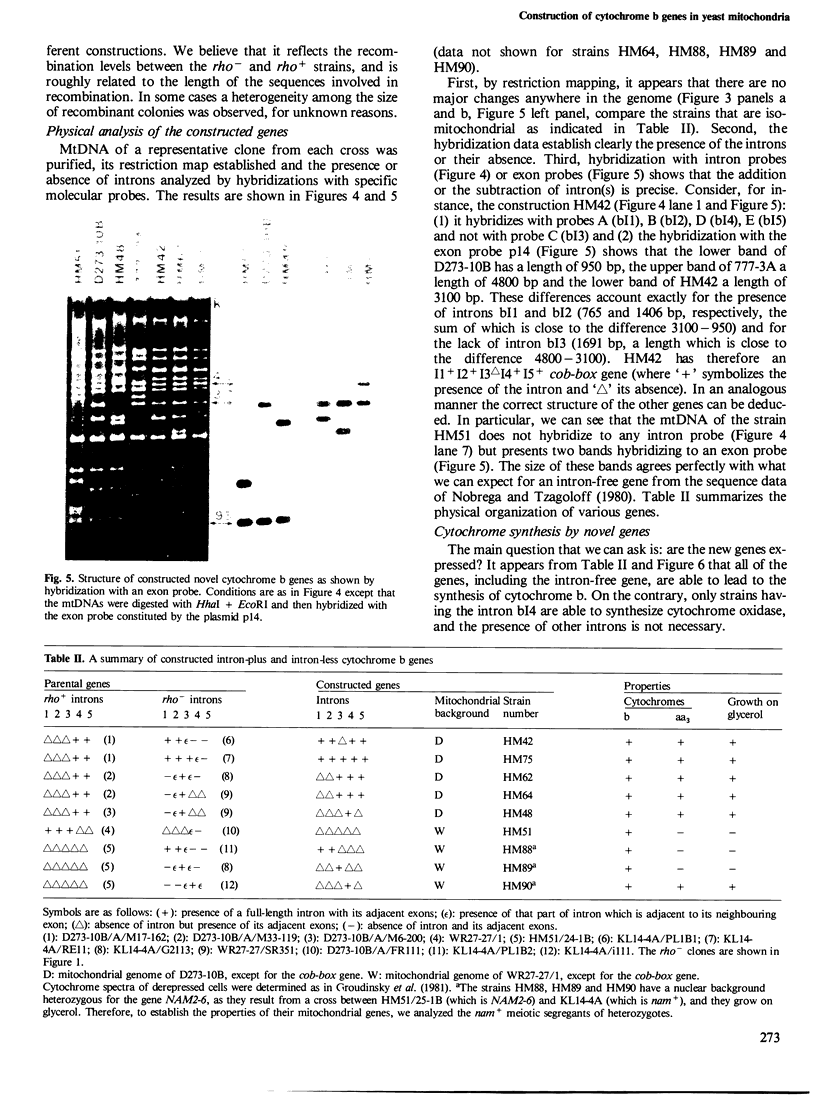
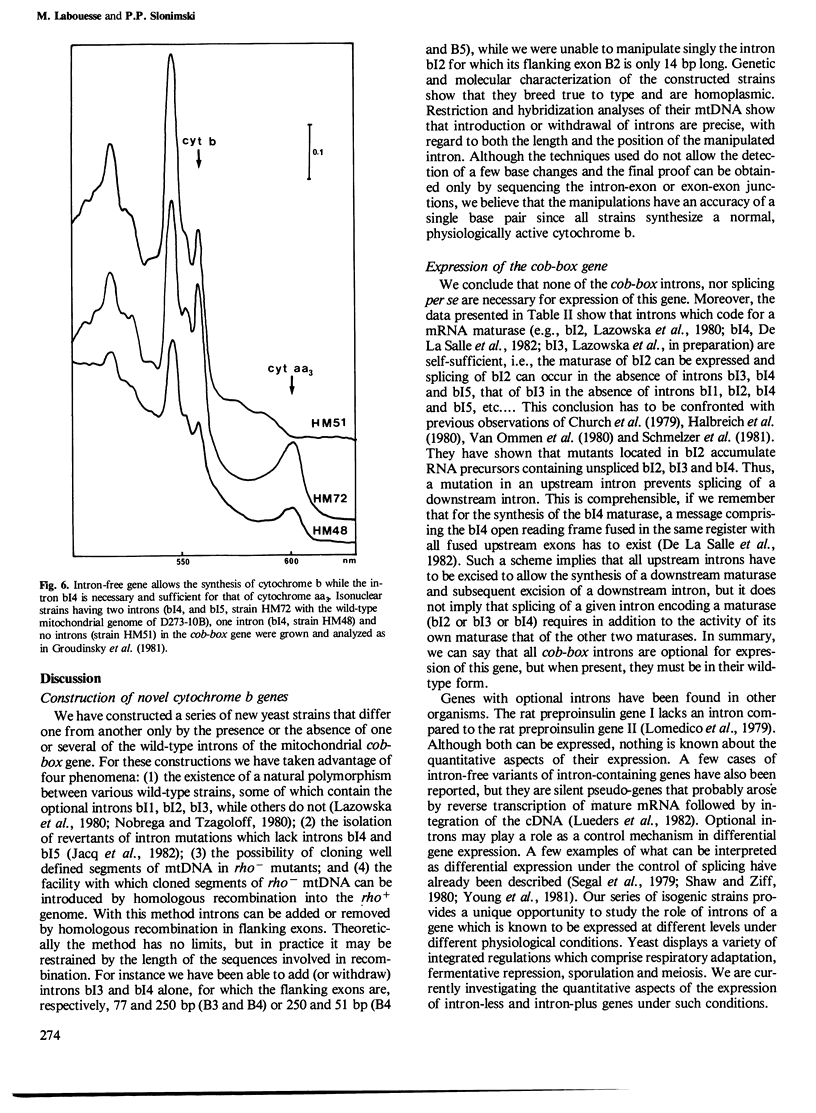
The mitochondrial cob-box gene coding for apocytochrome b in yeast has five introns and six exons or two introns and three exons depending on the wild-type strain considered. Some intron mutations in this gene affect not only its expression but also that of another mitochondrial gene: oxi3. To understand better the function of introns in gene expression, we have constructed a series of new strains that differ only by the presence or absence of one of the five wild-type introns in the cytochrome b gene, the rest of the mitochondrial and nuclear genome remaining unchanged. All constructions result from in vivo recombination events between rho- donor and rho+ recipient mtDNA. The following genes have been constructed: [see text]. Interestingly, all the genes lead to the synthesis of cytochrome b, while only the genes having the intron bI4 allow the expression of oxi3. A nuclear gene, when mutated, can compensate for the absence of the intron bI4.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc H., Dujon B., Guerineau M., Slonimski P. P. Detection of specific DNA sequences in yeast by colony hybridization. Mol Gen Genet. 1978 May 31;161(3):311–315. doi: 10.1007/BF00331006. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bonitz S. G., Coruzzi G., Thalenfeld B. E., Tzagoloff A., Macino G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit 1 of yeast cytochrme oxidase. J Biol Chem. 1980 Dec 25;255(24):11927–11941. [PubMed] [Google Scholar]
- Cami B., Kourilsky P. Screening of cloned recombinant DNA in bacteria by in situ colony hybridization. Nucleic Acids Res. 1978 Jul;5(7):2381–2390. doi: 10.1093/nar/5.7.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Slonimski P. P., Gilbert W. Pleiotropic mutations within two yeast mitochondrial cytochrome genes block mRNA processing. Cell. 1979 Dec;18(4):1209–1215. doi: 10.1016/0092-8674(79)90233-2. [DOI] [PubMed] [Google Scholar]
- De La Salle H., Jacq C., Slonimski P. P. Critical sequences within mitochondrial introns: pleiotropic mRNA maturase and cis-dominant signals of the box intron controlling reductase and oxidase. Cell. 1982 Apr;28(4):721–732. doi: 10.1016/0092-8674(82)90051-4. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dhawale S., Hanson D. K., Alexander N. J., Perlman P. S., Mahler H. R. Regulatory interactions between mitochondrial genes: interactions between two mosaic genes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1778–1782. doi: 10.1073/pnas.78.3.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Dujardin G., Jacq C., Slonimski P. P. Single base substitution in an intron of oxidase gene compensates splicing defects of the cytochrome b gene. Nature. 1982 Aug 12;298(5875):628–632. doi: 10.1038/298628a0. [DOI] [PubMed] [Google Scholar]
- Dujardin G., Pajot P., Groudinsky O., Slonimski P. P. Long range control circuits within mitochondria and between nucleus and mitochondria. I. Methodology and phenomenology of suppressors. Mol Gen Genet. 1980;179(3):469–482. doi: 10.1007/BF00271736. [DOI] [PubMed] [Google Scholar]
- Foury F., Tzagloff A. Assembly of the mitochondrial membrane system. XIX. Genetic characterization of mit- mutants with deficiencies in cytochrome oxidase and coenzyme qh2-cytochrome c reductase. Mol Gen Genet. 1976 Nov 24;149(1):43–50. doi: 10.1007/BF00275959. [DOI] [PubMed] [Google Scholar]
- Groudinsky O., Dujardin G., Slonimski P. P. Long range control circuits within mitochondria and between nucleus and mitochondria. II. Genetic and biochemical analyses of suppressors which selectively alleviate the mitochondrial intron mutations. Mol Gen Genet. 1981;184(3):493–503. doi: 10.1007/BF00352529. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Efstratiadis A., Karathanasis S., König M., Khoury G. Synthesis of stable unspliced mRNA from an intronless simian virus 40--rat preproinsulin gene recombinant. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6091–6095. doi: 10.1073/pnas.78.10.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Khoury G. Rescue of a splicing defective mutant by insertion of an heterologous intron. Nature. 1980 Aug 7;286(5773):634–637. doi: 10.1038/286634a0. [DOI] [PubMed] [Google Scholar]
- Haid A., Schweyen R. J., Bechmann H., Kaudewitz F., Solioz M., Schatz G. The mitochondrial COB region in yeast codes for apocytochrome b and is mosaic. Eur J Biochem. 1979 Mar;94(2):451–464. doi: 10.1111/j.1432-1033.1979.tb12913.x. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Pajot P., Foucher M., Grandchamp C., Slonimski P. A pathway of cytochrome b mRNA processing in yeast mitochondria: specific splicing steps and an intron-derived circular DNA. Cell. 1980 Feb;19(2):321–329. doi: 10.1016/0092-8674(80)90506-1. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Hanson D. K., Miller D. H., Mahler H. R., Alexander N. J., Perlman P. S. Regulatory interaction between mitochondrial genes. II. Detailed characterization of novel mutants mapping within one cluster in the cob2 region. J Biol Chem. 1979 Apr 10;254(7):2480–2490. [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Lomedico P., Rosenthal N., Efstratidadis A., Gilbert W., Kolodner R., Tizard R. The structure and evolution of the two nonallelic rat preproinsulin genes. Cell. 1979 Oct;18(2):545–558. doi: 10.1016/0092-8674(79)90071-0. [DOI] [PubMed] [Google Scholar]
- Lueders K., Leder A., Leder P., Kuff E. Association between a transposed alpha-globin pseudogene and retrovirus-like elements in the BALB/c mouse genome. Nature. 1982 Feb 4;295(5848):426–428. doi: 10.1038/295426a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Schmelzer C., Haid A., Grosch G., Schweyen R. J., Kaudewitz F. Pathways of transcript splicing in yeast mitochondria. Mutations in intervening sequences of the split gene COB reveal a requirement for intervening sequence-encoded products. J Biol Chem. 1981 Jul 25;256(14):7610–7619. [PubMed] [Google Scholar]
- Segal S., Levine A. J., Khoury G. Evidence for non-spliced SV40 RNA in undifferentiated murine teratocarcinoma stem cells. Nature. 1979 Jul 26;280(5720):335–338. doi: 10.1038/280335a0. [DOI] [PubMed] [Google Scholar]
- Shaw A. R., Ziff E. B. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3' coterminal mRNAs and five late families. Cell. 1980 Dec;22(3):905–916. doi: 10.1016/0092-8674(80)90568-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Ommen G. J., Boer P. H., Groot G. S., De Haan M., Roosendaal E., Grivell L. A., Haid A., Schweyen R. J. Mutations affecting RNA splicing and the interaction of gene expression of the yeast mitochondrial loci cob and oxi-3. Cell. 1980 May;20(1):173–183. doi: 10.1016/0092-8674(80)90245-7. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]