Abstract
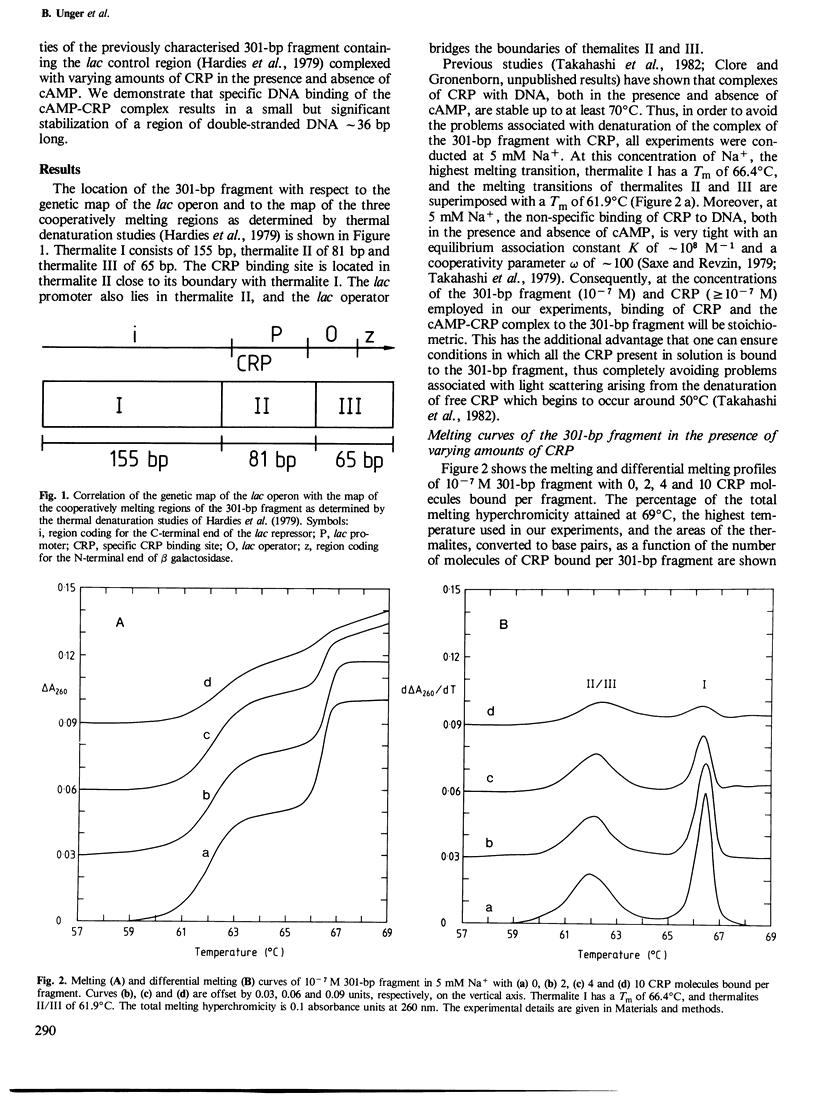
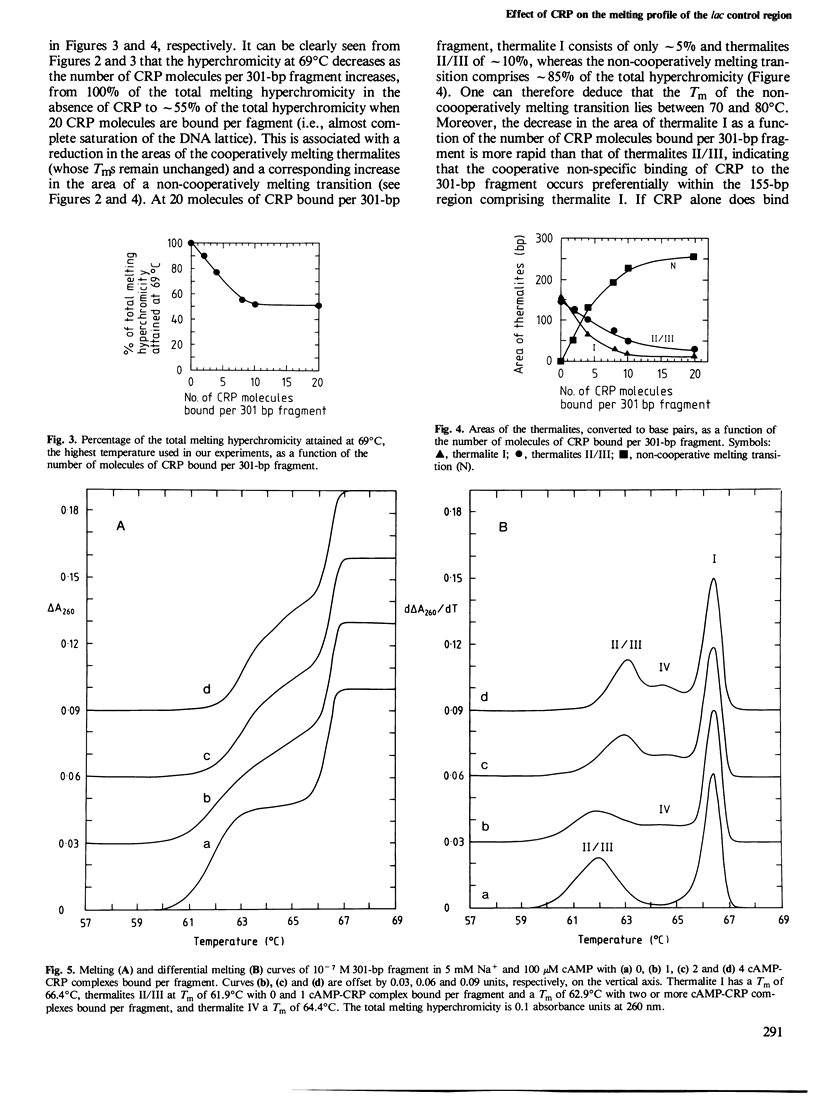
The effects of varying amounts of cAMP receptor protein (CRP) in the presence and absence of cAMP on the melting and differential melting curves of a 301-bp fragment containing the lac control region in 5 mM Na+ have been investigated. The native 301-bp fragment consists of three cooperatively melting thermalites. At 5 mM Na+, thermalite I (155 bp) has a Tm of 66.4 degrees C and the melting transitions of thermalites II (81 bp) and III (65 bp) are superimposed with a Tm of 61.9 degrees C. The specific DNA target site for CRP and the lac promotor are located within thermalite II. CRP alone exerts no specific effects on the melting of the 301-bp fragment, non-specific DNA binding of CRP resulting in a progressive stabilization of the double-stranded DNA by increasing the number of base pairs melting at a higher Tm in a non-cooperative transition. The cAMP-CRP complex, however, exerts a specific effect with a region of approximately 36 bp, comprising the specific CRP binding site and a neighbouring region of DNA, being stabilized. The appearance of this new cooperatively melting region, known as thermalite IV, is associated with a corresponding decrease in the area of thermalites II/III. The Tm of thermalite IV is 64.4 degrees C, 2.5 degrees C higher than that of thermalites II/III. With two or more cAMP-CRP complexes bound per 301-bp fragment, the stabilization also affects the remaining 110 bp now making up thermalites II/III whose Tm is increased by 1 degrees C to 62.9 degrees C. The implications of these findings for various models of the mode of action of the cAMP-CRP complex are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang J. J., Dubochet J., Baudras A., Blazy B., Takahashi M. Electron microscope observation of a fibre structure formed by non-specific binding of cAMP receptor protein to DNA. J Mol Biol. 1981 Aug 15;150(3):435–439. doi: 10.1016/0022-2836(81)90558-1. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Ebright R. H., Wong J. R. Mechanism for transcriptional action of cyclic AMP in Escherichia coli: entry into DNA to disrupt DNA secondary structure. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4011–4015. doi: 10.1073/pnas.78.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. Interaction of catabolite activator protein of Escherichia coli with single-stranded deoxyribonucleic acid. Biochemistry. 1981 Jan 20;20(2):306–312. doi: 10.1021/bi00505a012. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Hillen W., Goodman T. C., Wells R. D. High resolution thermal denaturation analyses of small sequenced DNA restriction fragments containing Escherichia coli lactose genetic control loci. J Biol Chem. 1979 Oct 25;254(20):10128–10134. [PubMed] [Google Scholar]
- Hillen W., Goodman T. C., Benight A. S., Wartell R. M., Wells R. D. High resolution experimental and theoretical thermal denaturation studies on small overlapping restriction fragments containing the Escherichia coli lactose genetic control region. J Biol Chem. 1981 Mar 25;256(6):2761–2766. [PubMed] [Google Scholar]
- Hillen W., Goodman T. C., Wells R. D. Salt dependence and thermodynamic interpretation of the thermal denaturation of small DNA restriction fragments. Nucleic Acids Res. 1981 Jan 24;9(2):415–436. doi: 10.1093/nar/9.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillen W., Klein R. D., Wells R. D. Preparation of milligram amounts of 21 deoxyribonucleic acid restriction fragments. Biochemistry. 1981 Jun 23;20(13):3748–3756. doi: 10.1021/bi00516a013. [DOI] [PubMed] [Google Scholar]
- Hillen W., Unger B. Correlation of thermodynamic and genetic properties in the Tn10 encoded TET gene control region. Nucleic Acids Res. 1982 Apr 24;10(8):2685–2700. doi: 10.1093/nar/10.8.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A., Buc H. Is DNA unwound by the cyclic AMP receptor protein? Nucleic Acids Res. 1982 Jan 22;10(2):473–485. doi: 10.1093/nar/10.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. L., Gielow W. O., Wallace R. G. Mechanism of araC autoregulation and the domains of two overlapping promoters, Pc and PBAD, in the L-arabinose regulatory region of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Feb;78(2):752–756. doi: 10.1073/pnas.78.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. Specific binding of CAP factor to lac promoter DNA. Nature. 1975 Aug 21;256(5519):672–674. doi: 10.1038/256672a0. [DOI] [PubMed] [Google Scholar]
- McKay D. B., Steitz T. A. Structure of catabolite gene activator protein at 2.9 A resolution suggests binding to left-handed B-DNA. Nature. 1981 Apr 30;290(5809):744–749. doi: 10.1038/290744a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Activation of transcription at specific promoters by glycerol. J Biol Chem. 1974 Jul 10;249(13):4050–4056. [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Selective effects of MgCl2 and temperature on the initiation of transcription at lac, gal, and lambda promoters. J Biol Chem. 1975 Oct 25;250(20):8202–8208. [PubMed] [Google Scholar]
- Ogden S., Haggerty D., Stoner C. M., Kolodrubetz D., Schleif R. The Escherichia coli L-arabinose operon: binding sites of the regulatory proteins and a mechanism of positive and negative regulation. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3346–3350. doi: 10.1073/pnas.77.6.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemme F. R. A model for catabolite activator protein binding to supercoiled DNA. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5263–5267. doi: 10.1073/pnas.79.17.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe S. A., Revzin A. Cooperative binding to DNA of catabolite activator protein of Escherichia coli. Biochemistry. 1979 Jan 23;18(2):255–263. doi: 10.1021/bi00569a003. [DOI] [PubMed] [Google Scholar]
- Schmitz A. Cyclic AMP receptor proteins interacts with lactose operator DNA. Nucleic Acids Res. 1981 Jan 24;9(2):277–292. doi: 10.1093/nar/9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. B. Interaction of the cAMP receptor protein with the lac promoter. Nucleic Acids Res. 1980 Feb 25;8(4):759–766. [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Blazy B., Baudras A. An equilibrium study of the cooperative binding of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate to the adenosine cyclic 3',5'-monophosphate receptor protein from Escherichia coli. Biochemistry. 1980 Oct 28;19(22):5124–5130. doi: 10.1021/bi00563a029. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Blazy B., Baudras A. Non-specific interactions of CRP from E. coli with native and denatured DNAs: control of binding by cAMP and cGMP and by cation concentration. Nucleic Acids Res. 1979 Nov 24;7(6):1699–1712. doi: 10.1093/nar/7.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P. Tandem CRP binding sites in the deo operon of Escherichia coli K-12. EMBO J. 1982;1(9):1049–1054. doi: 10.1002/j.1460-2075.1982.tb01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Goodman T. C., Hillen W., Horn G. T., Klein R. D., Larson J. E., Müller U. R., Neuendorf S. K., Panayotatos N., Stirdivant S. M. DNA structure and gene regulation. Prog Nucleic Acid Res Mol Biol. 1980;24:167–267. doi: 10.1016/s0079-6603(08)60674-1. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]



