Abstract
Tyrosine kinase enzymes are an attractive target for anticancer therapies. Tyrosine kinase inhibitors (TKI) are well tolerated; somehow severe systemic side effects are rarely seen during treatment. Toxicities of skin and appendages may lead to poor compliance, psychosocial inconvenience, and drug interruption. Changes of the hair can arise following cures with TKI. Nilotinib, a second-generation TKI, has been responsible for various cutaneous side effects including different clinical presentations of alopecia (scarring and nonscarring forms). This paper reports the case of a 45-year-old male diagnosed with chronic myelogenous leukemia (CML) treated with nilotinib, who presented with a keratosis pilaris (KP)-like eruption, autoresolutive alopecia areata plaque of the wrist and diffuse eyebrow thinning. To date, eight cases of nilotinib-induced KP were reported. However, none of them was associated with alopecia areata. Hence, physicians need to be aware of this new cutaneous side effect and investigating the reason of this phenomenon requires additional studies.
Key words: Alopecia areata, chronic myelogenous leukemia, keratosis pilaris, nilotinib
INTRODUCTION
New treatments for chronic myelogenous leukemia (CML) have recently emerged, including multitargeted tyrosine kinase inhibitors (TKI). These agents may have very distressing adverse reactions due to the long duration of treatment. Therefore, dermatologists and their patients are frequently confronted with the cutaneous reactions related to the use of such drugs. The second-generation Bcr-Abl TKI nilotinib has been implicated in multiple skin eruptions, especially pruritic erythematous and maculopapular exanthemas (the majority of which being transient and autoresolutive) as well as Lichenoid eruptions.[1] Herein, we report a unique case of nilotinib-induced keratosis pilaris (KP) with diffuse eyebrow thinning and autoresolutive alopecia areata plaque of the wrist.
CASE REPORT
A 45-year-old male presents to our clinic for an asymptomatic skin eruption of the upper and lower limbs that has been evolving for 2 months followed by bilateral eyebrow thinning. He was diagnosed with chronic myeloid leukemia with a Bcr-Abl kinase mutation 6 months ago then started, for the first time, on nilotinib 300 mg twice daily. The patient had been taking the same dose of nilotinib for 4 months before the onset of the eruption. A relevant episode of sudden hair loss occurred in a circular area of 4 cm on his right wrist; it appeared 2 months after he had started on nilotinib and spontaneously resolved 2 months later. Furthermore, 3 months after this event, there has been a rapidly progressing hair loss of his eyebrows and thinning of his chest hair. He was not treated with any other medication during this period and did not have any personal or family history of any similar rash nor alopecia.
On examination, the patient has extensive keratotic, red-brown, follicular papules over upper and lower limbs [Figures 1 and 2]. The rash was predominant over the extensor surfaces of the upper limbs, but the face and scalp remain intact. In addition, the patient presents diffuse thinning of his eyebrows without perifollicular erythema, hyperkeratosis, or any other evidence of scarring [Figure 3]. The disease does not affect scalp hair, eyelashes, or nails. The normal hair density on his right wrist is noted again. The remainder of the exam is normal and the patient declines any skin biopsy.
Figure 1.
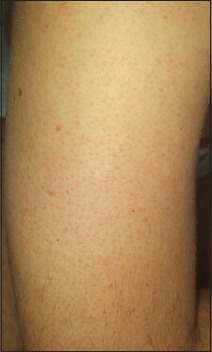
Follicular, keratotic, red-brown papules over the extensor surfaces of the right arm
Figure 2.
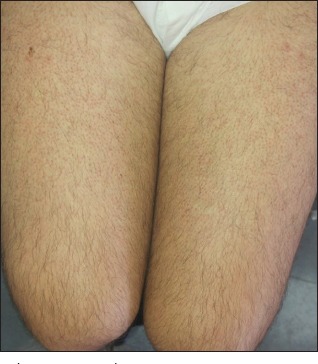
Follicular, keratotic, red-brown papules over the extensor surfaces of the thighs
Figure 3.
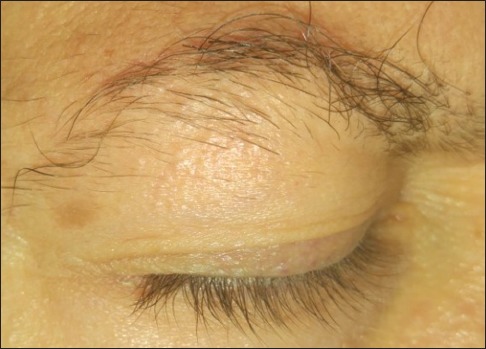
Diffuse eyebrow thinning
The follicular eruption is diagnosed as nilotinib-induced KP and the patient is given a topical 2% salicylic acid cream. For eyebrow thinning, clobetasol propionate 0.05% cream and minoxidil 5% spray are prescribed. Nilotinib cannot be discontinued due to its substantial role in the treatment of his chronic myeloid leukemia.
DISCUSSION
In this case, we report the unique association of nilotinib-induced KP eruption with diffuse eyebrow thinning and autoresolutive alopecia areata plaque of the wrist while the patient is still under treatment. The second-generation Bcr-Abl TKI nilotinib targets mainly the Bcr-Abl tyrosine kinase. Plus, it interacts with c-kit receptor, platelet-derived growth factor receptor (PDGFR), and discoidin domain receptors.[2,3] This targeted therapy has been approved for treatment of Philadelphia chromosome–positive CML and involved in numerous skin adverse reactions.[1] Although to the best of our knowledge, only eight cases of nilotinib-induced KP have been reported, none of which are associated with alopecia areata and eyebrows thinning.
The sudden appearance of red to brown follicular keratotic papules over the extensor surfaces of upper arms, thighs, and buttocks on a patient when treated with nilotinib without evidence of childhood and familial KP is highly suggestive of nilotinib-induced KP. The pathogenesis of these reactions incriminates nilotinib due to its action on tyrosine kinase receptor in the hair follicles, reproducing the same rash induced by vemurafenib through the same ERK/MAPK pathway.[3,4] Such reactions may be seen with another multikinase inhibitor, the sorafenib, due to its actions on PDGFR and c-Kit.[2] Treatment of this drug eruption consists of topical keratolytic agents, retinoids, urea, salicylic acid, and emollients with various results.[1,3]
Alopecia is well documented in the literature when associated with epidermal growth factor receptor-specific TKIs, which is not the case with multitargeted TKIs. The literature lacks clinical and histologic descriptions of nilotinib-induced alopecia. Here are few studies that are reported, such as Hansen et al.[1] who described one case of diffuse nilotinib-induced nonscarring alopecia and Patel et al.[2] reported two cases of scalp scarring lichen planopilaris-like eruption with nonscarring eyebrows alopecia and one case of nonscarring diffuse alopecia. Similarities in binding sites (PDGFR and c-Kit) of multitargeted TKIs nilotinib, sorafenib, and sunitinib are evocative of a potential single alopecia mechanism. Alteration of hair follicle cycle toward shedding was reported with PDGF inhibition, and spontaneous hair regrowth was only described during sorafenib therapy.[1]
In fact, it is challenging to determine whether the patient is experiencing an adverse effect from the drug. For evaluating the probability of this adverse drug reaction, the most widely accepted instrument is the Naranjo algorithm. In our patient, Naranjo score is of 5 revealing that alopecia areata is probably but not definitely induced by nilotinib. Some limitations exist in this case because the drug could not be discontinued nor an antagonist or a placebo could be given; moreover, drug detection in any body fluid was not performed. Nevertheless, the causality link is still probable. Hence, physicians need to be aware of this new cutaneous side effect and additional studies are required to investigate the reason of this phenomenon.
This new finding is revealed as a novel clinical presentation of nilotinib-induced alopecia. The benign course of such side effects does not contraindicate the pursuit of the treatment. Physicians should note and report any new symptoms or signs that appear during treatment with the new TKIs since their mechanism of action is still not fully elucidated. Yet, the distinction between an adverse drug reaction and an exacerbation of an existing disease or an emerging medical problem remains problematic because the clinical picture is so complex.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hansen T, Little AJ, Miller JJ, Ioffreda MD. A case of inflammatory nonscarring alopecia associated with the tyrosine kinase inhibitor nilotinib. JAMA Dermatol. 2013;149:330–2. doi: 10.1001/jamadermatol.2013.1375. [DOI] [PubMed] [Google Scholar]
- 2.Patel AB, Solomon AR, Mauro MJ, Ehst BD. Unique cutaneous reaction to second- and third-generation tyrosine kinase inhibitors for chronic myeloid leukemia. Dermatology. 2016;232:122–5. doi: 10.1159/000437383. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu A, Hattori M, Takeuchi Y, Ishikawa O. Generalized keratosis pilaris-like eruptions in a chronic myelogenous leukemia patient treated with Nilotinib. J Dermatol. 2016;43:1100–1. doi: 10.1111/1346-8138.13336. [DOI] [PubMed] [Google Scholar]
- 4.Leong WM, Aw CW. Nilotinib-induced keratosis pilaris. Case Rep Dermatol. 2016;8:91–6. doi: 10.1159/000445676. [DOI] [PMC free article] [PubMed] [Google Scholar]


