Abstract
Aim and Objective:
To evaluate the therapeutic efficacy of oral pentoxifylline in the treatment of oral submucous fibrosis (OSMF) patients by assessing the clinical symptoms such as burning sensation, mouth opening, and submucosal layer thickness and echogenicity using ultrasonography, both pre- and post-operatively.
Materials and Methods
Thirty study subjects were included in the study and divided into two groups in single-blind randomized manner, oral pentoxifylline and dexamethasone group. Burning sensation, mouth opening, ultrasonographic submucosal thickness, and echogenicity were recorded both pre- and post-operatively. Any adverse effects reported by the patients were also noted. The data collected were statistically analyzed, and response to pentoxifylline and intralesional dexamethasone with hyaluronidase was observed using ultrasonography.
Results
A highly significant reduction (P < 0.001) in burning sensation, improvement in mouth opening, and changes in submucosal thickness were noticed in both groups, and significant improvement (P < 0.05) in echogenicity in both the groups was noticed. However, pentoxifylline group showed marginally better improvement than dexamethasone group.
Conclusion:
Pentoxifylline can bring about significant improvement in OSMF, which can be used as better alternative where intralesional steroid was contraindicated or not well tolerated.
Keywords: Dexamethasone, hyaluronidase, oral submucous fibrosis, pentoxifylline, ultrasonography
Introduction
Schwartz in 1952 described a condition affecting the oral mucosa including the palate and faucial pillar, called “atrophia idiopathica mucosae oris” among five Indian women from Kenya. Later, the term “oral submucous fibrosis (OSMF)” was coined by S.G Joshi in 1953. The disease is predominantly seen in India among Asian countries, with a reported prevalence ranging up to 0.4% in Indian rural population. Based on a study conducted in 2002, more than 5 million people in India suffer from OSMF, 0.5% of Indian population.[1] The habit of betel quid chewing is widespread throughout India and Southeast Asia. Moreover, it is widely prevalent in teenagers and young adult.[2,3]
Buccal mucosa, faucial pillar, and soft palate are predominantly affected. Underlying muscles and the muscles of mastication can also be involved. Currently, pentoxifylline, a tri-substituted methylxanthine derivative, is reported to have satisfactory result in the management of OSMF due to their immune modulation, alteration of fibroblast physiology, rheologic modification, and anti-inflammatory property.[4] Very few studies have been done so far regarding effectiveness of pentoxifylline in OSMF, that too mainly subjective evaluation such as mouth opening, blanching of oral mucosa, and burning sensation.[5,6,7] Objective evaluation of the treatment outcome in OSMF patient can be carried out using ultrasonography (USG), which provides real-time imaging of soft tissue.
The purpose of the study is to evaluate objectively the effectiveness of oral pentoxifylline for the treatment of OSMF using high-frequency USG in comparison to conventional intralesional steroid therapy with dexamethasone and hyaluronidase.
Materials and Methods
The study was approved by the Institutional Ethical Committee. The study population included a total of thirty patients, who reported to the Department of Oral Medicine and radiology, either of the sex who were diagnosed as OSMF based on habitual history and clinical findings. Informed consent was obtained from every patient, and they were subjected to routine blood investigation and habit cessation counseling in our institution before and during the study.
A clinical diagnosis of OSMF was made, and patients were numbered serially as they entered the study and patients were graded clinically according to Khanna and Andrade (1995) classification. Only Grade II and Grade III were included in the study. Thirty patients were included conveniently in the study in single-blind randomized manner to the following groups alternatively irrespective of age, sex, and stage of OSMF.
Group A: Pentoxifylline group
Group B: Dexamethasone and hyaluronidase group.
Clinical parameters included in the study to evaluate the effectiveness of the drugs were burning sensation and mouth opening. USG parameters included were submucosal thickness and echogenicity. The intensity of burning sensation was determined using a visual analog scale of 0–10 with 10 mm division, where 0 was no burning sensation and 10 was worst possible burning sensation. The interincisal mouth opening was measured using a Vernier caliper from the mesioincisal angle of upper central incisor to the mesioincisal angle of lower central incisor and recorded in centimeters.
USG measurements of submucosal thickness and echogenicity were performed for 30 patients comprising 15 in each group. A pilot study was first done in ten healthy volunteers regarding accuracy and reproducibility of USG measurements. After the approval from the Ethical Committee, the study was conducted in OSMF patients with repeated measurements and averaging the three consecutive measurements, which was finally taken for statistical analysis.
Scanning was performed using multifrequency linear transducer with a frequency ranging from 3 to 12 MHz (Siemens) which was connected to the scanner; real-time imaging of buccal mucosae was performed. Buccal mucosa was selected for USG evaluation as it is commonly involved next to faucial pillar and it is easily approachable for proper evaluation. Patients were prior instructed to indicate the mucosa by placing the forefinger inside the mouth against the mucosa to delineate the lining mucosa and empty space of the oral cavity as stated by Wilson et al.[8] Patients were also told not to apply finger pressure against the mucosa but just a finger touch movement of the mucosa. The real-time imaging of the right and left buccal mucosa was carried out, and the measurements were taken. Mucosal lining was seen as a hyperechoic linear line, submucosa as a band of hypoechoic zone supported by a muscle planes in controls. This band of hypoechogenicity in between hyperechoic mucosa and muscle layer was measured as submucosa. All measurements were taken in centimeters.
With increased severity of OSMF, submucosa is thought to become more hyperechoic as compared to hypoechoic in normal patients. Hyperechogenicity is marked as 1 and hypoechogenicity is marked as 2. Since differences in reliability of ultrasonic assessments of mucosal thickness in different parts of oral cavity may depend on the difficulties of repeatedly measuring at the same location, on varying thickness of tissue. These problems might be resolved by averaging multiple measurements. After preoperative USG evaluation, patients in Group A were administered oral pentoxifylline 400 mg thrice daily after meals for 3 months, and patients in Group B were administered intralesional injection of 0.5 ml of local anesthesia with 2 ml of dexamethasone and 1500 I.U of hyaluronidase biweekly for 6 weeks. After the course of the treatment scheduled, all the patients of both the groups were subjected to postoperative USG evaluation of submucosal thickness and echogenicity similar to preoperative evaluation and the values are recorded. Figures 1–3 show pre- and post-operative evaluation of submucosa using USG. All the values were statistically analyzed and the results were drawn. Both groups were followed for 6 months; no significant recurrence or any compliance was noticed. However, further extended follow-up with increased sample is required for long-term outcome of the drug.
Figure 1.
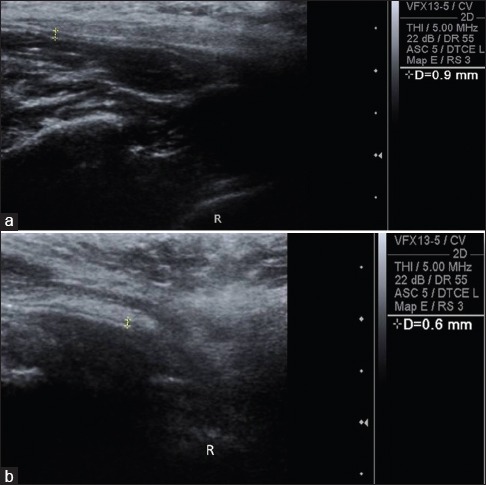
Preoperative (a) and postoperative (b) ultrasonographic evaluation of buccal mucosa of Case 1 showed submucosal thickness reduced from 0.9 to 0.6 mm and echogenicity also changes from hyper to hypo
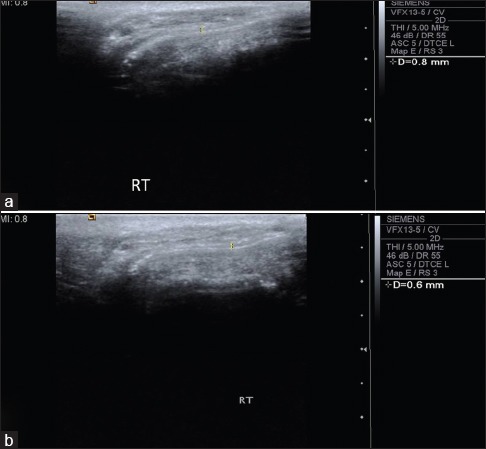
Figure 3.
Preoperative (a) and postoperative (b) ultrasonographic evaluation of buccal mucosa of Case 3 showed submucosal thickness reduced from 0.6 to 0.4 mm and echogenicity also changes from hyper to hypo
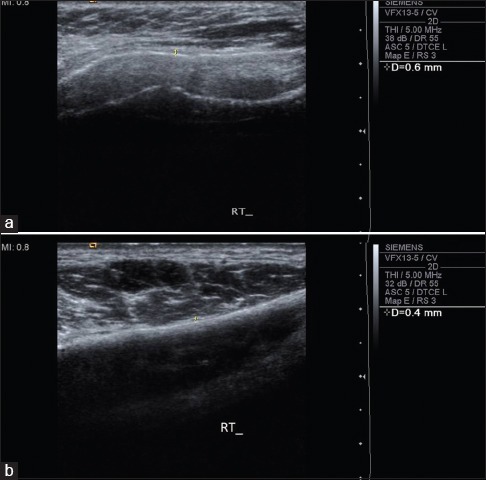
Figure 2.
Preoperative (a) and postoperative (b) ultrasonographic evaluation of buccal mucosa of Case 2 showed submucosal thickness reduced from 0.8 to 0.6 mm and echogenicity also changes from hyper to hypo
Results
A total of thirty patients with age ranging from 23 to 54 years, with the mean age being 35.4 years in Group A and 32.8 years in Group B, were included in the study. A male predilection was observed. In the present study, Stage II is more prevalent than Stage III was observed.
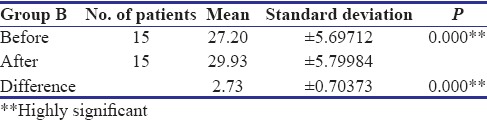
In the present study, all the patients in Group A showed complete reduction in burning sensation and two patients in Group B showed mild persistence of burning sensation after treatment. However, both the groups showed highly significant reduction (P < 0.001) in burning sensation [Table 1]. A study conducted by Rajendran et al. reported statistically significant improvement in burning sensation in 14 patients treated with pentoxifylline for 7 months. Both groups showed highly significant increase in mouth opening (P < 0.001), but Group A showed marginally higher degree of improvement (mean - 4.53 mm) as compared to Group B (mean - 2.73 mm) [Tables 2 and 3]. The maximum increase in mouth opening was 7 mm and the minimum was 3 mm in Group A whereas was 4 mm and 2 mm, respectively, in Group B.
Table 1.
Comparision of burning sensation between groups
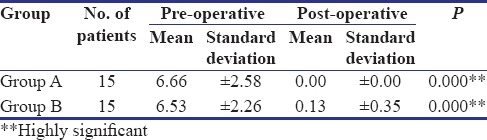
Table 2.
Evaluation of mouth opening in Group a OSMF
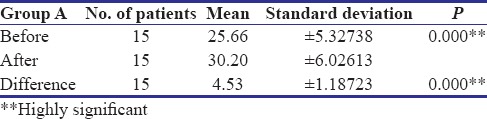
Table 3.
Evaluation of mouth opening in Group b OSMF
In the present study, the overall mean preoperative submucosal thickness of buccal mucosa is about 0.722 mm, which ranges from 0.43 to 1.12 mm. The mean preoperative submucosal thickness of buccal mucosa in Group A is about 0.715 ± 0.166 mm, which ranges from 0.46 to 1.12 mm; in case of Group B, mean submucosal thickness is about 0.729 ± 0.188 mm, which ranges from 0.43 to 1.08 mm. The overall mean postoperative submucosal thickness of buccal mucosa in Group A is about 0.494 ± 0.146 mm, which ranges from 0.26 to 0.84 mm, and in Group B, improvement is about 0.598 ± 0.181 mm, which ranges from 0.43 to 1.08 mm, and both are found to be highly significant [Tables 4 and 5]. McNemar's test is used to evaluate the echogenicity because of binomial distribution of values. Pentoxifylline group showed significant results in echogenicity on both right and left sides (P < 0.005) individually and combined showed highly significant result (P < 0.001).
Table 4.
Comparision of submucosal thickness - Group A
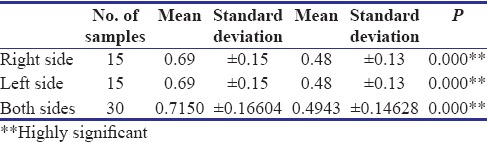
Table 5.
Comparision of submucosal thickness - Group B
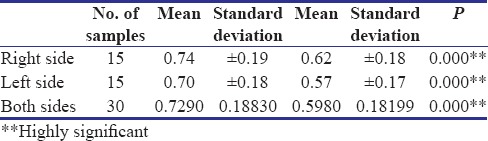
The evaluation of overall improvement in mouth opening, burning sensation, USG submucosal thickness, and echogenicity showed that Group A is found to have marginally better improvement than Group B [Chart 1]. is the first study which evaluates submucosa objectively, on OSMF patients to analyze the outcome of different medical mode of treatment.
Chart 1.
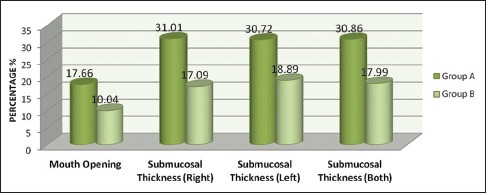
Overall percentage of improvement in parameters between groups
Discussion
Oral submucous fibrosis is a common premalignant condition affecting the oral mucosa with more prevalence among Indian population. Various treatment modalities have been elucidated to alleviate the symptoms associated with OSMF.[9] Currently, oral pentoxifylline has been proved to have beneficial result in treating OSMF because of its anti-inflammatory, fibrinolytic, immunomodulatory, and rheologic modifying property. Pentoxifylline improves red blood cell membrane deformability by increasing the amount of membrane adenosine triphosphate. It also alters red blood cell membrane protein phosphorylation patterns, increases protein kinase activity, and decreases Ca 2+-dependent K + efflux. The results of experimental studies have shown that fibroblasts cultured in the presence of pentoxifylline produced twice as much collagenase activity and decreased amounts of collagen, glycosaminoglycans, and fibronectin. Interleukin-1-induced fibroblast proliferation is also inhibited by pentoxifylline. All these actions of pentoxifylline have made to think in terms of management for oral submucous fibrosis.[10] The present study was done to evaluate the effectiveness of oral pentoxifylline in the management of OSMF; evaluation was done using USG as it provides both qualitative and quantitative assessment of oral mucosa.
Several categories of drugs have been used in the treatment of OSMF, but their effectiveness leaves much to be desired and no treatment regimen has afforded definitive cure.[11] While oral administration limits the concentration of drugs in lesional tissue and increases the potential for side effects, the intralesional injections are associated with significant mechanical injury and noncompliance on the patient's part because of the accompanying discomfort and pain.[12]
In this study, both pentoxifylline and dexamethasone groups showed a significant improvement in mouth opening and reduction in burning sensation. Pentoxifylline appears to be well tolerated. Only two patients experienced side effects, but neither had reason to discontinue the therapy. Most side effects caused by pentoxifylline involve the gastrointestinal tract and central nervous system. The most frequent gastrointestinal complaints include dyspepsia, nausea, vomiting, and bloating, in case of central nervous system side effects include dizziness and headache, tremor, anxiety, and confusion in a small percentage of patients. Both central nervous system and gastrointestinal side effects are dose related and are therefore minimized by dose reduction. Hence, pentoxifylline can be a good alternative in the management of OSMF, for whom intralesional steroids or hyaluronidase are contraindicated, for those who cannot make frequent visit and to avoid pain due to injection. Moreover, most importantly, pentoxifylline is cost-effective and more compliant to the patient.
Furthermore, qualitative and quantitative assessment of pentoxifylline effectiveness in treating OSMF was assessed by USG, which showed marked changes in submucosal thickness and echogenicity in lieu with the clinical improvement.[13,14,15] Hence, USG can be considered as a valuable tool in assessing the severity, extension, disease progression, and treatment outcome objectively and efficiently. However, further descriptive study is required to substantiate the sensitivity of USG in OSMF evaluation in all respects.
However, the results of our study need to be confirmed in a larger population of OSMF patients with a longer period of follow-up. In addition, further study is required to assess the effectiveness of pentoxifylline on the basis of extent of efficacy in different age groups, at various stages of OSMF, and duration of habit associated with OSMF.
Conclusion
Pentoxifylline can be used as promising alternative treatment modality to intralesional steroid for treatment of OSMF, where the later is contraindicated or not well tolerated by the patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge Bernard Institute of Radiology, Rajiv Gandhi Government General Hospital, Chennai.
References
- 1.Pindborg JJ, Mehta FS, Gupta PC, Daftary DK. Prevalence of oral submucous fibrosis among 50,915 Indian villagers. Br J Cancer. 1968;22:646–54. doi: 10.1038/bjc.1968.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Pindborg JJ, Mehta FS. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. J Oral Pathol Med. 1995;24:145–52. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 3.Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–8. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Zhang M, Xu YJ, Mengi SA, Arneja AS, Dhalla NS. Therapeutic potentials of pentoxifylline for treatment of cardiovascular diseases. Exp Clin Cardiol. 2004;9:103–11. [PMC free article] [PubMed] [Google Scholar]
- 5.Rajendran R, Rani V, Shaikh S. Pentoxifylline therapy: A new adjunct in the treatment of oral submucous fibrosis. Indian J Dent Res. 2006;17:190–8. doi: 10.4103/0970-9290.29865. [DOI] [PubMed] [Google Scholar]
- 6.Mehrotra R, Singh HP, Gupta SC, Singh M, Jain S. Pentoxifylline therapy in the management of oral submucous fibrosis. Asian Pac J Cancer Prev. 2011;12:971–4. [PubMed] [Google Scholar]
- 7.Vedeswari CP, Jayachandran S, Ganesan S. In vivo autofluorescence characteristics of pre- and post-treated oral submucous fibrosis: A pilot study. Indian J Dent Res. 2009;20:261–7. doi: 10.4103/0970-9290.57354. [DOI] [PubMed] [Google Scholar]
- 8.Wilson IR, Crocker EF, McKellar G, Rengaswamy V. An evaluation of the clinical applications of diagnostic ultrasonography in oral surgery. Oral Surg Oral Med Oral Pathol. 1989;67:242–8. doi: 10.1016/0030-4220(89)90345-9. [DOI] [PubMed] [Google Scholar]
- 9.Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995;24:402–6. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 10.Aara A, Satishkumar GP, Vani C, Venkat Reddy M, Sreekanth K, Ibrahim MD. Comparative study of intralesional dexamethasone, hyaluronidase and oral pentoxifylline in patients with oral submucous fibrosis. Glob J Med Res. 2012;12:1–14. [Google Scholar]
- 11.Borle RM, Borle SR. Management of oral submucous fibrosis: A conservative approach. J Oral Maxillofac Surg. 1991;49:788–91. doi: 10.1016/0278-2391(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 12.Sinha SN, Jain PK. Intraoral injection of hydrocortisone & placental extract in oral submucous fibrosis. Indian J Otolaryngol Head Neck Surg. 1978;30:103. [Google Scholar]
- 13.Manjunath K, Rajaram PC, Saraswathi TR, Sivapathasundharam B, Sabarinath B, Koteeswaran D, et al. Evaluation of oral submucous fibrosis using ultrasonographic technique: A new diagnostic tool. Indian J Dent Res. 2011;22:530–6. doi: 10.4103/0970-9290.90287. [DOI] [PubMed] [Google Scholar]
- 14.Devathambi JR, Aswath N. Ultrasonographic evaluation of oral submucous fibrosis and masseteric hypertrophy. J Clin Imaging Sci. 2013;3(Suppl 1):12. doi: 10.4103/2156-7514.124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangaiah P, Annigeri RG, Lingappa A. Transcutaneous ultrasonographic assessment of oral submucous fibrosis: A preliminary study. Int J Oral Med Sci. 2010;9:137–47. [Google Scholar]


