Abstract
Background:
For the first time in India, allografts from human extracted teeth were prepared. A randomized, prospective, clinicoradiographical, histological study was conducted to evaluate their efficacy in comparison with freeze-dried bone allograft (FDBA) in alveolar ridge preservation.
Materials and Methods:
Graft preparation: with written consent, teeth were collected from three donors (full mouth extraction cases). Once donors’ serums were tested negative for HIV, HBV, HCV, and Venereal disease research laboratory (VDRL), mineralized whole tooth allograft (WTA) and dentin allograft (DA) were prepared using the standard protocol of Tissue Bank at Tata Memorial Hospital, Mumbai, India.
Study Design:
In this randomized controlled trial, 15 patients undergoing extraction of at least four teeth were selected. In each patient after atraumatic extractions, one socket was grafted with WTA, second with DA, third with FDBA, and fourth was left ungrafted (control site). All the sites were covered with chorion membrane. To estimate three-dimensional alveolar crest changes, cone beam computed tomography scans were taken immediately after grafting and 4 months postoperatively. Bone biopsies using 3 mm trephine bur were obtained from four patients at the time of implant placement and evaluated histologically.
Results:
Clinically uneventful healing was observed at all sites. Compared to other sites, WTA and DA consistently showed superior results demonstrating least reduction in alveolar crest height and width which was statistically significant (P < 0.05). Between WTA and DA sites, there was no statistically significant difference. Histological analysis also confirmed more new bone formation at WTA and DA sites.
Conclusions:
Rather than disposing extracted human teeth as a biomedical waste (common practice), they can be collected from suitable systemically healthy donors. With the help of tissue bank, they can be processed into an allograft, serving as an excellent alternative to conventional allografts.
Keywords: Allografts, bone graft(s), bone regeneration, imaging, ridge preservation
Introduction
Bone grafts have been used for decades in reconstruction and regeneration of alveolar bone defects formed by traumatic extractions, periodontal or endodontic origin. However, complete preservation and reconstructions of alveolar ridge to its original dimensions still seem to be a distant goal. Autogenous bone graft being osteoinductive, osteoconductive, and osteoproliferative is considered a gold standard.[1] However, most of time its quantity is inadequate due to limited availability of donor site. Thus to suffice the required quantity of bone graft material, numerous alternatives are investigated for bone regeneration over the years.
Dentin and bone have similarities in terms of structural and biochemical properties. This led an idea of using dentin as a bone graft material..[2] Demineralized dentin matrix (DDM) has been shown to possess osteogenic capacity.[3] Use of autogenous dentin graft was developed and clinically applied from 2003 onward.[4] It has shown promising clinical and histological results as an alternative to autogenous bone graft. However, it still has its own sets of limitations such as:
Patient may not accept his/her own tooth as it is diseased and has to be extracted
Quantity is dependent on the number of teeth indicated for extraction, and quality of graft depends on the nature of extracted teeth
Chairside graft preparation is tedious as well as time consuming.
Thus, to overcome these shortcomings, we thought of preparing allografts from human extracted teeth. Allograft is a graft between genetically dissimilar members of the same species.
The primary objective of the present study is to clinically and radiographically evaluate the efficacy of mineralized human teeth allografts to preserve alveolar ridge dimensions in comparison to conventional allograft, i.e., freeze-dried bone allograft (FDBA). The secondary objective is to histologically determine bone formation potential of teeth allografts.
Materials and Methods
The present study was approved by the Institutional Ethical Committee (MGVKBHDC/15-16/1634). The current trial is registered with the Clinical Trials Registry–India with registration number-CTRI/2016/11/007441. All clinical procedures were performed in accordance with the Declaration of Helsinki.
Graft preparation
Allografts from human extracted teeth were prepared in collaboration with Tissue bank at Tata Memorial Hospital (TMH), Mumbai, India. Human extracted teeth were collected in the Department of Oral Surgery at MGV's KBH Dental College and Hospital, Nashik. From August 2015 to September 2015, full-mouth extraction cases were screened as potential donors for extracted teeth. Five patients met the inclusion criteria to be donors, all the donors were:
Systemically healthy
No history of, or current adverse habits such as smoking, gutka, or tobacco chewing
No caries and/or peri-apical infection.
All donors were females with a mean age of 41 ± 0.4 years. For each donor, detailed medical and dental histories were recorded by one single dental operator in the donor screening form provided by TMH which were duly signed by the donors. The purpose of teeth donation was explained to each donor and written consent was obtained. As a standard protocol for graft preparation using human tissues, a donor has to undergo testing for HIV, HBV, HCV, and syphilis antigens. Thus with consent of all the potential donors, 5 ml blood was collected, centrifuged, serum was separated, and it was transferred to vacutainers. These vacutainers were labeled with donors’ details and immediately refrigerated till further analysis. A total of 22 teeth were collected from five donors. All teeth were cleaned using an ultrasonic scaler.
For 11 teeth obtained from two donors, dentin was separated using carbide straight fissure bur by trimming superficial enamel and cemental portion. Rest of the 11 teeth from the remaining three donors were kept as whole teeth. Processing of all teeth was done immediately postextraction and was stored according to individual donors, in a separate sterile plastic container. Each container was labeled with donor's information and refrigerated till further processing. Once all the teeth were collected, all the containers and corresponding donors’ serums were packed and transferred in an organ donation box surrounded by frozen ice-pack gels to maintain the temperature during transportation. Box was sealed and transported to the Tissue Bank at TMH, Mumbai. Once the donors’ serums were tested non-reactive to HIV, HBV, HCV and VDRL antigens; using standard protocol of bone graft preparation by TMH, teeth were processed, sterilized, and packed as 1 cc graft of particle size 300–500 μm. Two different types of allografts, namely, whole tooth allograft (WTA) and dentin allograft (DA) were prepared. The purpose of preparing two different allografts was to evaluate the effect of only dentin as opposed to whole teeth independently. The grafts were then sent back to the Department of Periodontics at MGV's KBH Dental College, Nashik, to evaluate their clinical efficacy.
Study design
A randomized, controlled, prospective, clinical study was designed to investigate the efficacy of WTA and DA in alveolar ridge preservation as compared to conventional allograft, i.e., FDBA against ungrafted sites. Patients having at least four teeth indicated for extraction were selected. The four extraction sockets were allocated to one of the following groups using random number table:
Socket grafted with WTA
Socket grafted with DA
Socket grafted with FDBA
Socket left ungrafted (control).
Participants
Patients who reported to the Department of Oral Surgery between November and April 2016 were screened. A total of 15 patients (7 males and 8 females, aged 28–45 years; mean age: 35.6 ± 5.7 years) who met the following inclusion criteria were recruited [Figure 1]:
Figure 1.
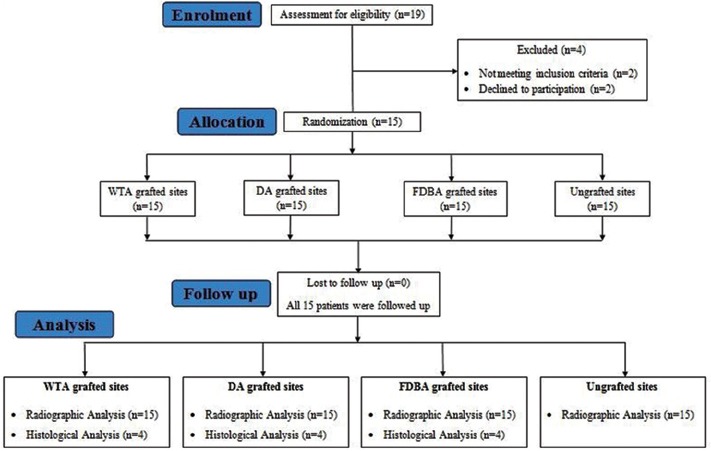
Study flow chart
Individuals having at least four teeth indicated for extraction
Systemically healthy patients
Alveolar sockets which were free of any preexisting periapical pathology based on intraoral periapical radiographs
Alveolar sockets with intact four-wall architecture and depth ≥5 mm based on assessment using UNC-15 periodontal probe (GDC, Hoshiarpur, Punjab, India).
Patients were excluded from the study based on the following criteria:
Pregnant or lactating
History of any surgical or nonsurgical therapy or antibiotic therapy in the last 6 months
Individuals with habits such as smoking, tobacco chewing, or alcohol
Individuals with a history of radiation therapy.
Surgical protocol
With written informed consent from all the study participants, flapless, atraumatic extraction of the teeth was performed using periotomes and forceps. To confirm the eligibility of surgical site, integrity of the alveolar wall postextraction was confirmed using UNC-15 periodontal probe (GDC, Hoshiarpur, Punjab, India). Three extracted sockets were filled with graft materials and one extraction socket was left ungrafted as a control site. All the grafted and ungrafted sites were covered with a chorion membrane (Tissue bank, TMH, Mumbai, India). A crisscross suture was given to stabilize the membrane. Cone beam computed tomography (CBCT) scan was taken immediately after grafting. Postoperative instructions were given, and amoxicillin (500 mg TDS) was prescribed for 5 days. Patients were recalled for suture removal after 7 days. Second CBCT scan was taken after 4 months.
Assessment
The total 4 months of study period was divided into three time intervals, i.e., day of extraction and bone grafting, 7th day postgrafting, and 4 months’ postgrafting. Clinical evaluation was done at all visits, and CBCT scans were obtained immediately after grafting and at the end of 4 months. Three-dimensional radiographic alveolar crestal bone changes in height and width after 4 months were selected as the primary outcome and histological new bone formation as the secondary outcome.
Cone beam computed tomography analysis
Frame/slice of each CBCT scan was adjusted in an axial view and center aligned over the area of interest. For the buccal and lingual cortical height, the measurements were done using linear measurement tool in a cross-sectional slice. For mesial and distal cortical height, measurements were done in a tangential slice. All the vertical measurements were done from the most coronal aspect of cortical bone to the fixed anatomical point apically. Buccolingual width was measured in a cross-sectional slice and mesiodistal in the tangential slice. The amount of crestal bone loss is the difference between independently measured bone height width immediately after grafting and 4 months’ postgrafting using the above-mentioned protocol.
Histological evaluation
Individuals in whom implant placement was performed 4 months after the alveolar ridge preservation, an additional informed written consent for bone biopsy was obtained. Bone tissues were harvested using a trephine bur (3 mm in diameter) at the time of osteotomy preparation. The collected specimens were sent to the Department of Oral Pathology for histological evaluation of new bone formation.
Statistical analyses
For all treatment groups, comparison of change in ridge mean width and height dimensions was done using Student's unpaired t-test. The Student's t-test was performed at the degree of freedom “9” and confidence interval of 95%. For all tests, P < 0.05 was considered statistically significant. The obtained results were described and plotted. For statistical analysis, “SPSS-9” (IBM India Pvt. LTD, Bengaluru, Karnataka, India) software was used.
Results
Patient-based analysis
In 15 patients, 45 sites out of total 60 extraction sites were grafted with ridge preservation technique (15 grafted with WTA, 15 grafted with DA, and 15 grafted with FDBA) and the remaining 15 were left ungrafted as control sites. Among 45 grafted sites, 26 were in the maxilla (9 - WTA, 7 - DA, and 10 - FDBA) and 19 were in mandible (6 - WTA, 8 - DA, and 5 - FDBA). Seven ungrafted sites were in maxilla and the remaining eight were in mandible.
Seven days’ postoperatively, uneventful healing was observed in all the patients at all the sites. No sign of infection or graft rejection was observed. At the end of the study period of 4 months, satisfactory clinical healing was observed in all the patients. Visually, WTA and DA sites showed minimum alveolar ridge width shrinkage compared to sites grafted with FDBA and ungrafted sites [Figure 2].
Figure 2.
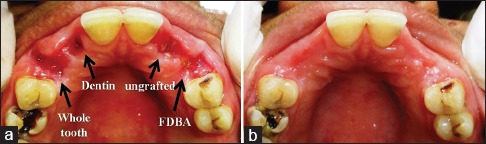
(a) 7-day follow-up. (b) 4-month follow-up
Dimensional changes
The dimensional changes were calculated by comparing the CBCT scans taken immediately after grafting procedure and 4 months’ postgrafting [Figure 3].
Figure 3.
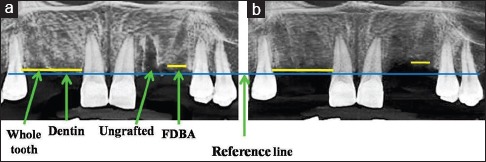
(a) 7-day follow-up radiograph. (b) 4-month follow-up radiograph
Vertical ridge height changes
Mean and standard deviation values based on a comparison of CBCT scans for changes in ridge height related to the baseline buccal, lingual, mesial, and distal socket walls are shown graphically in Figure 4. Statistically significant differences were obtained when the mean (buccal, lingual, mesial, and distal) vertical resorption for all the experimental groups was compared. WTA- and DA-grafted sites consistently showed statistically significant least reduction in ridge height, i.e., 0.36 ± 0.04 mm and 0.31 ± 0.07 mm, respectively, in comparison with FDBA-grafted sites with 0.87 ± 0.07 mm reduction and ungrafted sites with 1.96 ± 0.24 mm reduction (P < 0.05).
Figure 4.
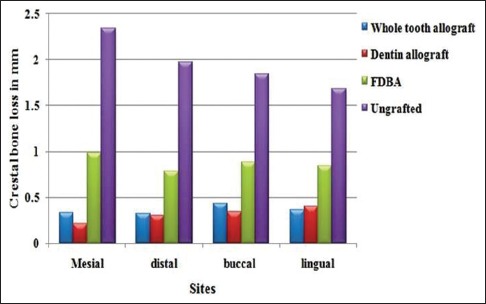
Mean height change in millimeter
Horizontal ridge width changes
Mean and standard deviation values based on a comparison of the CBCT scans for changes in ridge width related to the baseline buccolingual and mesiodistal width of extraction socket are shown graphically in Figure 5. The mean width of the socket was calculated as a mean of buccolingual and mesiodistal widths. The mean width change was highest for ungrafted sites (1.66 ± 0.97 mm) followed by FDBA-grafted sites (0.82 ± 0.50 mm) and least for WTA and DA sites (0.49 ± 0.19 mm and 0.50 ± 0.29 mm, respectively). A mean width change for WTA and DA sites was significantly lesser when compared to FDBA-grafted sites and FDBA-ungrafted sites (P < 0.05).
Figure 5.
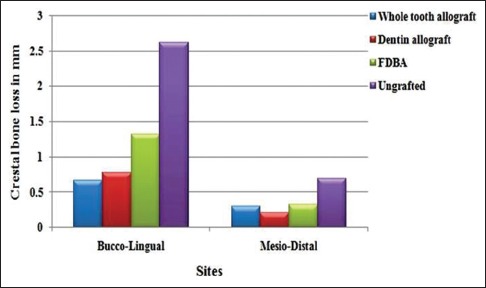
Mean width change in millimeter
Histological observations
In four patients who chose to opt for the implant placement after completion of the study period, bone biopsy was taken from the grafted sites using trephine bur. Out of the 12 grafted sites from these patients, seven sites were in maxilla and five were in mandible. Histology specimens from WTA- and DA-grafted sites showed better integration of graft particles and more newly formed bone. It was associated with connective tissue stroma rich in angiogenesis. No inflammatory cellular infiltration was observed [Figure 6].
Figure 6.
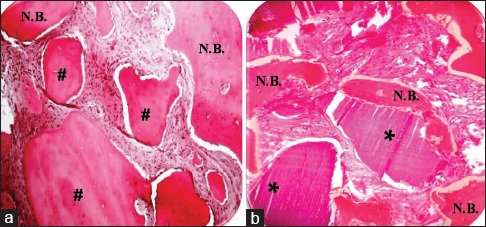
Hematoxylin and eosin staining of biopsy tissue taken from: (a) Freeze-dried bone allograft-grafted site after 4 months (×40). (b) Teeth grafted site after 4 months (×40). *Residual teeth particles; #Residual freeze-dried bone allograft particles. NB: New bone formation
In comparison, FDBA sites showed less new bone formation. FDBA graft particles were also well integrated with newly forming bone. The connective tissue stroma surrounding FDBA graft particles showed less evidence of angiogenesis [Figure 6].
Discussion
Tooth extraction invariably leads to loss of height and width of the alveolar bone. If the extraction socket is left to heal by its natural course, reduction of 2.6 mm to 4.6 mm in width and 0.4 mm to 3.9 mm in height of alveolar bone is observed. This ultimately results in overall shrinkage in dimensions of alveolar bone.[5,6] Later, at the time of prosthetic replacements, especially in case of dental implants, additional procedure of osseous reconstruction might be required to meet optimal prosthetic, functional, and esthetic demands. Thus, procedures such as “ridge preservation” using various bone graft materials immediately after extraction can be employed.
Literature has demonstrated the availability and efficacy of different types of bone graft materials in terms of osteoinductive and/or osteoconductive properties that could preserve the alveolar ridge postextraction and accelerate the bone repair process.[7] Among the various options in literature, autogenous bone graft is still considered an ideal regenerative material. Alternative material such as autogenous DDM has gained a lot of momentum these days, since it has demonstrated excellent biocompatibility and favorable osteogenic remodeling properties.[8] Research involving dentin as bone graft material began with a case report in 1967 that demonstrated animal-derived DDM-induced bone formation in the intramuscular pockets.[3] Until now, several dentin studies have reported the osteoinductive potency of DDM and presence of BMP molecule in dentin matrix.[9,10,11,12] In addition, it was noted that DDM derived from human teeth, induced bone and cartilage independently in subcutaneous tissues of nude mice at 4 weeks after implantation.[13] Having intrinsic chemotactic property, DDM has shown to attract osteoprogenitor cells and osteoblasts for the promotion of new bone formation.[14] Clinical effectiveness of the autogenous DDM inside the extracted socket was tested wherein the bone radiopacity with enhanced healing process was observed.[15]
However, chairside preparation of autogenous DDM is still a time-consuming process and may not be a feasible option all the time. Thus, to tackle this issue, we decided to prepare allografts from extracted human teeth. Subsequently, in this randomized, clinicoradiographic pilot study, we evaluated their efficacy in comparison to conventional allograft. This is a prospective study having 4-month follow-up period. Radiographically, change in mean width and height of alveolar bone was assessed as primary outcome variables. Histological healing pattern was considered as the secondary outcome variable.
Human teeth have three distinct calcified layers. All these layers are thought to impart different properties in terms of bone graft material. Since it is difficult to collect enamel and cementum by separating them from underlying dentin, we decided to prepare two separate grafts, i.e., dentin only and whole teeth. The process of removal of enamel and cementum from dentin was standardized and evaluated histologically before conducting a trial. In the present study, the WTA- and DA-grafted sites demonstrated statistically significant least amount of alveolar ridge width as well as height reduction in comparison with FDBA- and non-grafted sites (P < 0.05). However, among WTA- and DA-grafted sites, there was no statistically significant difference when mean reduction in height and width of alveolar ridge was analyzed (P > 0.05). This simply implies that there is no need of putting extra efforts to separate dentin from tooth when the entire tooth can be utilized as bone graft material with similar results.
Results of the current clinical trial evaluating the use of human teeth as allograft are in accordance with various animal studies and case reports published in the 1980s. These studies have validated the osteoinductive potential of allogenic, demineralized, lyophilized dentin.[16,17,18,19] From a histological standpoint, WTA and DA particles were surrounded by vital bone with few areas of resorption. This indicates induction of new bone formation around these graft particles. This is consistent with a study done by Kim et al.[20] Similarly, FDBA sites also showed better graft integration and new bone formation, but the amount of new bone formation was generally moderate.
Murata et al. in the first clinical case of sinus augmentation using auto-dentin as a bone graft material stated that composition of dentin and bone is comparable. They consist of body fluid (10%), collagen (18%), noncollagenous proteins (2%), and hydroxyapatite (70%) in weight volume.[2] According to Urist in 1965, DDM and demineralized bone matrix contain mainly Type-I collagen with growth factors such as bone morphogenetic protein 2 and fibroblast growth factors. These bioactive molecules are thought to contribute to osteoinductive and osteoconductive properties of human tooth as a graft material.[3] Various in vivo and animal studies have demonstrated its biocompatibility, osteoinductive, and osteoconductive potential.[21,22,23,24,25]
Limitations
This is a pilot study with small sample size. Additional studies are needed to validate these findings using larger sample size. Longer follow-up period will give more predictable results and would confirm its stability.
Conclusions
Alveolar ridge preservation procedure yielded satisfactory clinical and radiographical outcome with no reported complications. The study validated the need of alveolar ridge preservation technique in maintaining height and width of residual alveolar ridge immediately after extraction. Human teeth allografts were prepared for the first time in India. They have shown more promising results as compared to FDBA in achieving minimum volumetric alveolar bone loss in alveolar ridge preservation procedure. Histological evidence of new bone formation by teeth allografts corroborated better clinical and radiographical results by them. Derived from an extracted human tooth, it is the most easily available and cost-effective material. Thus, rather than disposing extracted teeth as biomedical waste, they can be collected from healthy donors (tested negative for triple H along with syphilis antigens) and syphilis and pooled together. At tissue banks, they can be processed in large batches into an allograft. Being an economical, natural, biocompatible, versatile, and predictable grafting material and having demonstrated better results, it can serve as a better alternative to most of the conventional allografts. This opens up whole new avenue of deciduous as well as permanent exfoliated and extracted teeth banking. Further randomized clinical trials are needed to establish its regenerative potential in various periodontal defects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to gratefully acknowledge the valuable support and timely guidance given by Dr. (Ms) Astrid Lobo Gajiwala, Tissue Bank, Tata Memorial Hospital and Research Centre, Mumbai, India.
References
- 1.Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 2.Murata M, Akazawa T, Mitsugi M, Kabir MA, UM IW, Minamida Y, et al. Autograft of dentin materials for bone regeneration. In: Pignatello R, editor. Advances in Biomaterials Sciences and Biomedical Applications. 1st ed. Croatia: InTech; 2013. pp. 391–404. [Google Scholar]
- 3.Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12:999–1008. doi: 10.1016/0003-9969(67)90095-7. [DOI] [PubMed] [Google Scholar]
- 4.Murata M. Autogenous Demineralized Dentin Matrix for Maxillary Sinus Augmentation in Humans: The First Clinical Report. 81th. Gothenburg: International Association for Dental Research; 2003. [Google Scholar]
- 5.Van der Weijden F, Dell’Acqua F, Slot DE. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J Clin Periodontol. 2009;36:1048–58. doi: 10.1111/j.1600-051X.2009.01482.x. [DOI] [PubMed] [Google Scholar]
- 6.Ten Heggeler JM, Slot DE, Van der Weijden GA. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: A systematic review. Clin Oral Implants Res. 2011;22:779–88. doi: 10.1111/j.1600-0501.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 7.Scheyer ET, Schupbach P, McGuire MK. A histologic and clinical evaluation of ridge preservation following grafting with demineralized bone matrix, cancellous bone chips, and resorbable extracellular matrix membrane. Int J Periodontics Restorative Dent. 2012;32:543–52. [PubMed] [Google Scholar]
- 8.Lee JY, Lee JH, Kim YK. Comparative analysis of guided bone regeneration using autogenous tooth bone graft material with and without resorbable membrane. J Dent Sci. 2013;8:281–6. [Google Scholar]
- 9.Bang G, Urist MR. Bone induction in excavation chambers in matrix of decalcified dentin. Arch Surg. 1967;94:781–9. doi: 10.1001/archsurg.1967.01330120035008. [DOI] [PubMed] [Google Scholar]
- 10.Huggins CB, Urist MR. Dentin matrix transformation: Rapid induction of alkaline phosphatase and cartilage. Science. 1970;167:896–8. doi: 10.1126/science.167.3919.896. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Deporter DA, Melcher AH. Induction of chondrogenesis in muscle, skin, bone marrow, and periodontal ligament by demineralized dentin and bone matrix in vivo and in vitro. J Dent Res. 1986;65:12–22. doi: 10.1177/00220345860650010101. [DOI] [PubMed] [Google Scholar]
- 12.Bessho K, Tanaka N, Matsumoto J, Tagawa T, Murata M. Human dentin-matrix-derived bone morphogenetic protein. J Dent Res. 1991;70:171–5. doi: 10.1177/00220345910700030301. [DOI] [PubMed] [Google Scholar]
- 13.Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, et al. Human acid-insoluble dentin with BMP2 accelerates bone induction in subcutaneous and intramuscular tissues. J Ceram Soc Jpn. 2010;118:438–41. [Google Scholar]
- 14.Gomes MF, Banzi EC, Destro MF, Lavinicki V, Goulart MD. Homogenous demineralized dentin matrix for application in cranioplasty of rabbits with alloxan-induced diabetes: Histomorphometric analysis. Int J Oral Maxillofac Implants. 2007;22:939–47. [PubMed] [Google Scholar]
- 15.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Bang G. Induction of heterotopic bone formation by demineralized dentin in guinea pigs: Antigenicity of the dentin matrix. J Oral Pathol. 1972;1:172–85. doi: 10.1111/j.1600-0714.1972.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 17.Bang G. Induction of heterotopic bone formation by demineralized dentin in rats and guinea pigs. Scand J Dent Res. 1973;81:230–9. doi: 10.1111/j.1600-0722.1973.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 18.Fugazzotto PA, De Paoli S, Benfenati SP. The use of allogenic freeze-dried dentin in the repair of periodontal osseous defects in humans. Quintessence Int. 1986;17:461–77. [PubMed] [Google Scholar]
- 19.Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation by osteoinduction in rats. Scand J Dent Res. 1990;98:434–41. doi: 10.1111/j.1600-0722.1990.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011;11:7442–5. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 21.Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010;81:1264–72. doi: 10.1902/jop.2010.100016. [DOI] [PubMed] [Google Scholar]
- 22.Kim YK, Yun PY, Um IW, Lee HJ, Yi YJ, Bae JH, et al. Alveolar ridge preservation of an extraction socket using autogenous tooth bone graft material for implant site development: Prospective case series. J Adv Prosthodont. 2014;6:521–7. doi: 10.4047/jap.2014.6.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong HR, Hwang JH, Lee JK. Effectiveness of autogenous tooth bone used as a graft material for regeneration of bone in miniature pig. J Korean Assoc Oral Maxillofac Surg. 2011;37:375–9. [Google Scholar]
- 24.Jeong KI, Lee J, Um IW, Kim YK. Alveolar cleft restoration using autogenous tooth bone graft material for implant placement: A case report. J Oral Implantol. 2015;41:487–90. doi: 10.1563/AAID-JOI-D-12-00265. [DOI] [PubMed] [Google Scholar]
- 25.Kim SK, Kim SW, Kim KW. Effect on bone formation of the autogenous tooth graft in the treatment of peri-implant vertical bone defects in the minipigs. Maxillofac Plast Reconstr Surg. 2015;37:2. doi: 10.1186/s40902-015-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


