Abstract
Objectives:
Oral submucous fibrosis (OSMF) caused by dense deposition of collagen fibers which is a protein. There is a plethora of research to evaluate degree of collagen deposition using various simple histochemical techniques, but its correlation with total serum protein (TSP) level has not been explored so far.
Materials and Methods:
This case–control study comprised total forty samples with thirty cases of OSMF and ten cases were selected as control group, divided into four groups as per Lai et al. classification. Histological grading was also done according to the Rooban et al.'s classification. Blood sample was collected to evaluate TSP estimation. Findings were tabulated, and comparisons were made between clinical, histological, and TSP estimation. Discrete statistical data were analyzed by Chi-square test, ANOVA, and t-test with a statistical analysis package (SPSS version software 6.0).
Results:
No significant correlation was obtained between clinical staging and histopathological grading. Definite correlation was obtained in TSP and globulin levels of OSMF patients and their grades of fibrosis histopathologically.
Conclusion:
In the present study, it was observed that biochemical investigations involving assessment of TSP can be used as a diagnostic tool in OSMF, along with histopathological examination.
Keywords: Albumin, correlation, functional grading, globulin, histopathological grading, Masson's Trichrome staining, oral submucous fibrosis, total protein estimation
Introduction
Cancer is by no means a modern disease. It was known to the ancient Egyptians as early as 1500 B.C.[1]
Several lines of evidence including clinical, experimental, and morphological data support the concept that oral squamous cell carcinoma arises from noninvasive lesions which encompass a histological continuum between the normal mucosa at one end and high-grade dysplasia/carcinoma in situ, at the other, establishing a model of neoplastic progression and are termed as premalignant or precancerous lesions or conditions.[2] Oral submucous fibrosis (OSMF) is one of the examples of such lesions which cannot be cured permanently.
Various health organizations recommended that the research efforts should be focused on developing novel diagnostic techniques.[3]
The role of the constituents of areca nut in the pathogenesis of OSMF has been studied in detail over few decades, and it has been proved that it leads to excessive deposition of collagen which is a protein and ultimately leads to hyalinization of subepithelial tissues causing restricted mouth opening.[4,5,6] In addition, protein levels in the serum of OSMF patients are also found to be altered compared to normal individuals,[5] which could be an indicator of excessive collagen deposition in OSMF.
There are numerous studies on clinical grading and histopathological grading of OSMF,[6] but metabolic aspects in head and neck carcinogenesis have been studied less extensively which are frequently associated with carcinoma.
OSMF is also a “collagen metabolic disorder,” and various researchers have done biochemical investigations to outline the changes in the blood, serum, or tissues of these patients and have given insights on the possible pathogenesis of OSMF.[2]
Thus, the present study was undertaken with an aim to determine if the diseased individuals reveal any changes in the total serum protein (TSP) level too along with its correlation with functional grading and histopathological grade of fibrosis using special stain, i.e. Masson's Trichrome stain which has not been explored so far in the literature.
Aims and Objectives
The following aims were considered for the present study:
Correlation of functional grading according to Lai et al.[6] criteria with histopathological grade according to Rooban et al.[7]
Correlation between TSP, globulin, albumin, and albumin and globulin (A/G) ratio with histopathological grades of fibrosis
Correlation between all three, i.e., clinical, biochemical, and histopathological grades.
Materials and Methods
This case–control study comprised total forty samples from D.Y. Patil School of Dentistry, Nerul, Navi Mumbai, from the Department of Oral Pathology and Microbiology and patients were categorized into the following subdivision:
Thirty cases of OSMF as study group, randomly selected from outpatient department (OPD) [Figure 1]
Ten cases were selected as control group.
Figure 1.
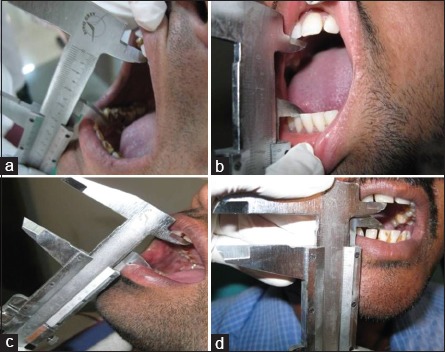
Group a, b, c, and d in the study group
Methods and criteria used for sample selection.
Inclusion criteria
The control patients were selected according to criteria such as clinically healthy persons without the history of any oral habits such as tobacco, pan, and alcohol consumption, smoking, and normal appearing oral mucosa.
Exclusion criteria
Antibiotics in the course of the previous month, bleeding risk (anticoagulant therapy) chemotherapy and/or immunosuppressant therapy, endocarditis risk, patients radiated in the head and neck region, and infection risk (e.g., HIV, hepatitis B virus, hepatitis C virus, tuberculosis).
All the study participants from the OPD of Dr. D.Y. Patil Dental College and Hospital, Nerul, Navi Mumbai, Maharashtra, were given clear explanations about the objective of the study and a written informed consent was taken. Ethical clearance was not considered as patients’ identity was not revealed.
For functional grading of OSMF, criteria of Lai et al.[6] were taken into consideration. Mouth opening of each patient was measured using a Vernier caliper.
Biopsy was taken, and sections were then subjected to H and E and Masson's Trichrome staining. The H- and E-stained slides were interpreted for the confirmation of the diagnosis and Masson's Trichrome staining was used for the histological grading according to the Rooban et al.'s[7] criterion under ×4, ×10, and ×40 magnifications by two experienced oral pathologists who were blindfolded.
Blood sample collection
Five milliliters of blood was collected in plain vacutainers by venipuncture using 24-gauge needles for routine hematological and biochemical investigations. Then, blood serum was separated by centrifugation at 3000 rpm to estimate TSP.
Total serum protein estimation
For estimation of TSP, Johnson and Johnson (J and J) Vitros 250 machines is fully automated analyzers from Ortho Clinical Diagnostics was used, works on bromocresol green (BCG) principle.
The method is based on the protein error of indicators. Binding of a protein to an indicator changes its color. Among serum proteins, only albumin binds to BCG. This binding produces a change in the color of BCG which is measured calorimetrically. The pH is maintained during the reaction by a buffer.
As automated machine Vitros 250 was used, commercially available reagents were used in this study [Figure 2].
Figure 2.

(a and b) Vitrose tips are loaded to Vitros sample tray and are positioned in Vitros 250 machine. (c) Sample programming in Vitros 250 machine
The blood samples collected in plain vacutainer were stored at 6°C for 24 h. Then, samples were transported to department of biochemistry, in a sterile plastic air tight bag.
The samples were then labeled as per patients identification (ID) (medical records department number) and serial number
Processing number was given to each sample to facilitate easy record track
All the patient data were then fed into “sample collection data system” to generate a record and report
Simultaneously, all the data from “sample collection data system” are transferred to Vitros 250 machine (J and J) to avoid any confusion
The samples were loaded into the centrifuge machine (Remi-R-8C)
In centrifuge machine (Remi-R-8C), sample balancing was done
Then, the machine was run at 3000 rpm (revolution per minute) for 5 min
The vacutainers were removed from centrifuge machine
Serum (minimum 50 μl) was transferred into Vitros sample cup or tips with the help of dry and sterile plastic piped
Sample cups were then placed in the Vitros sample tray, and tray is positioned in Vitros 250 machine
Sample programming was done in Vitros 250 machine
The patient ID which was generated previously was selected for individual sample
Test selection was done for TSP, globulin, albumin, and A/G ratio
The test program was run in Vitros 250 machine
Readings on the Vitros 250 were recorded after the processing was complete
The reports were generated from the sample collection data system.
All the findings of functional and histological grading were recorded and compared with total protein estimation using discrete statistical data were analyzed by Chi-square test, ANOVA and t-test with a statistical analysis package (SPSS software version 6.0, Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp).
Observations and Results
All the observations were tabulated and statistically analyzed.
Correlation of functional grading with histopathological grade of fibrosis (Masson's Trichrome stain)
When functional grades according to Lai et al. criteria and histopathological grades of fibrosis according to Rooban et al. were compared, out of thirty cases, 12 (40%) cases which were in clinical Group A, 1 (3%) had histopathological grading of 2, 7 (23.3%) had histopathological Grade 3, 4 (13.2%) had Grade 4, and none of the cases was in histopathological Grade 1 [Figures 3 and 4].
Figure 3.
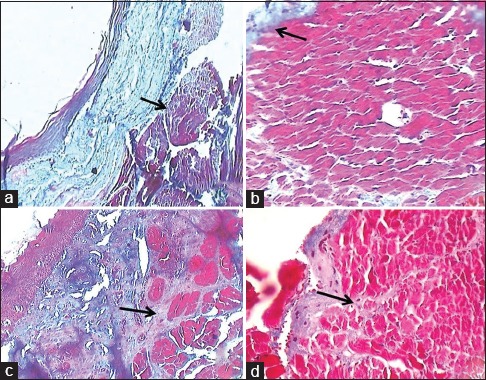
(a) Masson's Trichrome stained slide showing Grade 1 - fibrosis limiting to lamina propria (×10). (b) Masson's Trichrome stained slide showing Grade 1 - fibrosis limiting to lamina propria (×40). (c) Masson's Trichrome stained slide showing Grade 2 - fibrosis involving superficial region of muscle bundle (×10). (d) Masson's Trichrome stained slide showing Grade 2 - fibrosis involving superficial region of muscle bundle (×40)
Figure 4.
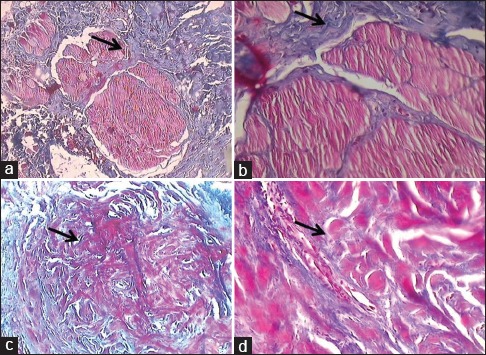
(a) Masson's Trichrome stained slide showing Grade 3 - fibrosis involving deeper regions of muscle bundle (×10). (b) Masson's Trichrome stained slide showing Grade 3 - fibrosis involving deeper regions of muscle bundle (×40).(c) Masson's Trichrome stained slide showing Grade 4 - muscle bundle replaced by fibrosis (×10). (d) Masson's Trichrome stained slide showing Grade 4 - muscle bundle replaced by fibrosis (×40)
In clinical Group B, 1 (3%) had a histopathological grading of 2, 4 (13.2%) had histopathological Grade 3, 2 (6.6%) had Grade 4, and none of the cases were in histopathological Grade 1.
In clinical Group C, 1 (3%) showed a histopathological Grade of 1, 1 (3%) were Grade 2, and none in Grades 3 and 4.
In clinical Group D, 2 (6.6%) had histopathological grading of 1, 3 (10%) had histopathological Grade 2, 2 (6.6%) were Grade 3, and 2 (6.6%) were Grade 4, this correlation had P = 0.181 which is higher than the level of significance, i.e., 0.005, therefore, did not show any significant correlation [Table 1].
Table 1.
Correlation of histopathological grade by Masson’s Trichrome and clinical groups
Total serum protein correlation with histopathological grades of fibrosis
When Chi-square test was applied to correlate TSP and histopathological, P value of Chi-square was 0.000 which indicates that histopathological grades and TSP groups were related to each other [Table 2]. Correlation coefficient was 0 suggestive of a strong positive relationship between histopathological grade and TSP.
Table 2.
Correlation between total serum protein, globulin, and histopathological grades
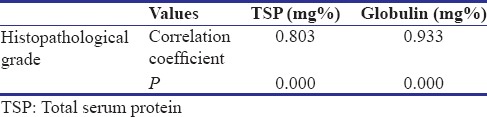
Correlation between globulin with histopathological grade of fibrosis
Globulin of all the participants of the study group was subjected to ANOVA test to find out the correlation between globulin and histopathological grading and since P = 0.000 which is less than that of level of significance, i.e. 0.0005 [Table 3]. There was definite correlation between globulin and histopathological grades.
Correlation coefficient between histopathological grade and globulin was 0.933, which showed that there is a strong positive correlation between these two [Table 2].
Table 3.
ANOVA test for globulin and histopathological grades

Correlation of mean total serum protein, globulin, and albumin with Oral submucous fibrosis
The significance between the mean values of TSP [Table 4], mean values of globulin [Table 5], and mean values of albumin [Table 6] of both study and control groups was tested using independent t-test, which showed that there was a significant increase in the mean TSP and mean globulin and mean albumin values of study group when compared to control group as P value for TSP, globulin, and albumin is <0.0005. The mean TSP value for study group is greater than that of mean TSP value of control group and is as well true for globulin and albumin.
Table 4.
Total serum protein mean value

Table 5.
Globulin mean value

Table 6.
Albumin mean value

Mean A: G ratio comparison with histopathological grade shows a decrease in A: G as the grades of fibrosis increases [Table 7].
Table 7.
Albumin and globulin ratio mean value

Discussion
There are many studies which correlate clinical features, histopathological grading, and functional grading.[8,9,10,11] There are also studies only on protein estimation.[12,13,14,15,16,17] However, there are not any studies which correlate functional, clinical, and histopathological grading with total protein estimation.
Fibrous collagen is biochemically protein in nature (procollagen) similar to protein of other cells as suggested by Van Wyk et al.[18] OSMF characterized by definite increase of collagen deposition which is a protein, in the areas affected by this condition locally. According to a study by Goel et al., there is a definite alteration in plasma amino acid level in OSMF patients as compared to control.[19]
Kamath et al. in his extensive review article stated that biochemical investigations can be considered as biomarkers in OSMF and amino acid levels are increased in OSMF.[20]
Therefore, the aim of this study was to determine if the affected individuals reveal any changes in the TSP level too and to determine its correlation with histopathological grading using Masson's Trichrome stain. This correlation has not been studied so far which makes this study unique.
As per functional grading, 12 (40%) were in Group A: mouth opening >35 mm, 7 (23%) were Group B: mouth opening between 30 and 35 mm, 2 (7%) were under Group C: mouth opening between 20 and 30 mm, and 9 (30%) were in Group D: mouth opening <20 mm. Majority of study group falls in clinical Group A. Because most of the patients had fibrosis in the posterior area which usually does not affect the mouth opening.
Histopathological grading revealed that three (10%) of the study group had Grade 1 OSMF which is fibrosis limiting to lamina propria alone, 6 (20%) had Grade 2 OSMF which is fibrosis involving superficial region of muscle bundle, 13 (43%) had Grade 3 OSMF which means fibrosis involving deeper regions of muscle bundle, and 8 (26.7%) had Grade 4 OSMF means muscle bundle replaced by fibrosis. A maximum number of study group had Grade 3 OSMF. This was similar to Pindborg et al.,[21] according to whom a pathological alteration in OSMF begins in the lamina propria and the epithelium responds only secondarily to it. In the advanced grades of OSMF, there was degeneration and replacement of muscle bundles by collagen fibers. Same findings were also noted by Gupta et al.[22]
When both histopathological grade and functional grading were correlated using Chi-square test, there was no significant correlation found between clinical grading and histopathological grading. Similar to Kiran Kumar et al.[7] and Gajendra et al.,[23] this variation could be due to the site of biopsy, which was mostly from less fibrous mid-buccal mucosa, but the fibrosis in most of the cases was present in the retromolar area.
In addition, even though patient showed extensive fibrosis and if it is present in the posterior region as compared to anterior region, it does not restrict the mouth opening. Therefore, even advanced histological grades in few patients showed lesser functional grading.
Studies have shown that the TSP level in the serum of OSMF patients was found to be significantly higher than that of normal individuals.[14,15,16,17,18,20]
On the contrary, Rajendran et al. found a low level of serum proteins in OSMF patients with advanced grade.[19] In the present study, participants with a mean value for TSP in 3 (10%) of histopathological Grade 1 was 6.3433 mg%, 6 (20%) of histopathological Grade 2 was 6.8533 mg%, 13 (43.3%) of histopathological Grade 3 was 7.4854 mg%, and 8 (26.6%) of histopathological Grade 4 was 8.4112 mg%, which shows a constant increase in the values of mean TSP as the grades of fibrosis increases. Similarly, the mean value of globulin in 3 (10%) of histopathological Grade 1 was 3.1100 mg%, 6 (20%) of histopathological Grade 2 was 3.3033 mg%, 13 (43.3%) of histopathological Grade 3 was 4.1023 mg%, and 8 (26.6%) of histopathological Grade 4 was 5.2538 mg%. Thus, as the histopathological grade of fibrosis increased, the protein level also increased. Saurabh et al. also found increased mean serum level of protein as compared to normal participants.[14]
The TSP of an individual is said to be dependent on many local, and systemic factors such as acute infection, chronic illness, and many authors believe that level of TSP in serum is also related to the nutritional deficiencies.[4]
There is an increasing amount of evidence that damage to the gut wall plays a role in autoimmune disease. A significantly high number of patients with autoimmune diseases have been shown to have histological indications of chronic gastrointestinal (GI) inflammation and damage.[24]
OSMF too has been demonstrated the presence of various autoantibodies and genetic predisposition with specific human leukocyte antigen suggesting an autoimmune etiology.[25,26]
This damaged gut wall is known as leaky gut syndrome which is nothing but minute perforations in intestinal mucosa caused by mechanical and chemical trauma due to which undigested food molecules which enters the blood stream and elicit an immune response in the connective tissue.[24] In OSMF also, GI tract is hit by persistent microtrauma caused by continuous chewing and swallowing of coarse fibers of areca nut, and it could be hypothesized that undigested nutrients can pass through the leaky gut could be responsible for increased serum protein levels.
Furthermore, the microtrauma caused due to the friction of coarse fibers of areca nut also leads to the diffusion of betel quid alkaloids and flavonoids into the subepithelial connective tissue, which results in juxta-epithelial inflammatory cell infiltration.
Yet, leaky gut syndrome as a diagnosis remains overlooked. The current standard of care paradigm is to treat the symptoms of disease, not the cause of disease, but reversing this paradigm and healing leaky gut syndrome would prevent, reverse, or delay disease such as OSMF. Extensive research needs to be done to study leaky gut and its cure in OSMF.
A leaky gut also damages the absorptive capacity of the epithelium and thus plays an important role in nutrient deficiency. Persistent GI inflammation eventually disrupts the integrity of the mucosal lining of the gut, and tiny perforations allow for molecules larger than usual to pass across this barrier including molecules from dietary protein and fats, bacteria, parasites, and fungi.
Both animal models and recent clinical evidence support this new paradigm of leaky gut syndrome and provide the rationale for innovative approaches to prevent and treat autoimmune diseases as per Fasano's study.[24]
Walker and Benditt studied the serum proteins in diseases of connective tissue disorders such as lupus erythematosus, scleroderma, dermatomyositis, rheumatic fever, and normal individuals, using electrophoresis. They found that in subacute and acute disseminated, i.e., in diffuse scleroderma, and in rheumatic fever, there is a decrease in serum albumin and an increase in serum gamma globulin.[27]
This study showed an increase in the TSP and globulin and hence decreased albumin and A/G ratio which was also observed in findings of Phatak,[4] 1978, who performed a study on 34 OSMF patients has shown a significant increase in the globulin fraction as compared with the controls. Anuradha and Devi,[28] 1993, in a study on 36 patients with OSMF revealed that there was an increase in the globulin fraction of protein and hence a decreased A/G ratio in these patients. There was a significant increase in total protein levels possibly due to the increase in globulin fractions and other serum proteins.
A study by Anil et al.[29] studied thirty cases of OSMF, thirty patients of leukoplakia, and thirty cases of oral cancer and reported defined increase in β2-microglobulin in patients of OSMF and oral cancer. Opposing results were noted in a study by Rajendran et al.[16] where serum protein values were significantly lower in all the patients. Another study conducted by Kalpana A. et al.[30] reported that OSMF shows a significant decrease in TSP in all OSMF cases as compared to normal control group.
Conclusion
A maximum number of study group had histopathological Grade 3 OSMF with muscle degeneration seen only among histopathological Grade 4 OSMF.
No significant correlation was obtained between functional grading and histopathological grading in the patients of OSMF. The possibility of difference in the severity and extent of fibrosis in different regions of the oral mucosa histopathologically and clinically could be because of the difference in location of fibrous bands and site of biopsy.
Definite correlation was obtained in TSP and globulin levels of OSMF patients and their grades of fibrosis histopathologically. Constant increase in the values of mean TSP and globulin was noted as the grades of fibrosis increased. Hence, decreased albumin and A/G ratio in these patients was observed.
The grades of fibrosis were observed to be directly proportional to the level of TSP in patients with OSMF and increase in the levels of TSP could be considered as a possible etiological factor for increased fibrosis in OSMF patients.
Merits of biochemical investigations are that they are economic, easy to perform, less time consuming, and can be performed by trained technical staff.
In the present study, it was observed that biochemical investigations involving assessment of TSP can be used as a diagnostic tool in OSMF, along with histopathological examination using special stains such as Masson's Trichrome.
Yet, further research studies are essential to explore the possible correlation between TSP and fibrosis with equal and more number of patients in each clinical and histopathological groups.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Primrose A. Cancer. Can Med Assoc J. 1935;32:233–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Shafer , Levy and Hine. Textbook of oral pathology. 5th ed. India: ELSEVIER; 2006. Benign and malignant tumours of oral cavity. [Google Scholar]
- 3.The US Commitment to Global Health: Recommendations for the Public and Private Sectors. Washington (DC): National Academies Press (US); 2009. [Last accessed on 2015 Jul 15]. Institute of Medicine (US) Committee on the US Commitment to Global Health. Available from: https://www.ncbi.nlm.nih.gov/books/NBK23788/ [PubMed] [Google Scholar]
- 4.Phatak AG. Fibrin producing factor in oral submucous fibrosis. Indian J Otolaryngol. 1979;31:103–4. [Google Scholar]
- 5.Kiran Kumar K, Saraswathi TR, Ranganathan K, Uma Devi M, Elizabeth J. Oral submucous fibrosis: A clinico-histopathological study in Chennai. Indian J Dent Res. 2007;18:106–11. doi: 10.4103/0970-9290.33785. [DOI] [PubMed] [Google Scholar]
- 6.Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995;24:402–6. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 7.Rooban T, Saraswathi TR, Al Zainab FH, Devi U, Eligabeth J, Ranganathan K. A light microscopic study of fibrosis involving muscle in oral submucous fibrosis. Indian J Dent Res. 2005;16:131–4. doi: 10.4103/0970-9290.29909. [DOI] [PubMed] [Google Scholar]
- 8.Goel S, Ahmed J, Singh M, Nahar P. Oral submucous fibrosis: A clinico-histopathological comparative study in population of Southern Rajasthan. J Carcinog Mutagen. 2010;1:1–4. [Google Scholar]
- 9.Singh M, Chaudhary AK, Pandya S, Debnath S, Singh M, Singh PA, et al. Morphometric analysis in potentially malignant head and neck lesions: Oral submucous fibrosis. Asian Pac J Cancer Prev. 2010;11:257–60. [PubMed] [Google Scholar]
- 10.Pandya S, Chaudhary AK, Singh M, Singh M, Mehrotra R. Correlation of histopathological diagnosis with habits and clinical findings in oral submucous fibrosis. Head Neck Oncol. 2009;1:10. doi: 10.1186/1758-3284-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy V, Wanjari PV, Banda NR, Reddy P. Oral Submucous Fibrosis: Correlation of Clinical Grading to various habit factors. Int J Dental Clinics. 2011;3:21–4. [Google Scholar]
- 12.Aswath N, Balakrishnan C. Estimation of serum, salivary immunoglobulin G, immunoglobulin A levels and total protein, hemoglobin in smokeless tobacco chewers and oral submucous fibrosis patients. Contemp Clin Dent. 2015;6:157–62. doi: 10.4103/0976-237X.166820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadani M. Evaluation of plasma fibrinogen degradation products and total serum protein concentration in oral submucous fibrosis. Journal of Clinical and Diagnostic Research. 2014;8:ZC54–7. doi: 10.7860/JCDR/2014/9061.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saurabh S, Sunil M, Kumar R, Mishra A, Singh Birring O, Khan L. Estimation of serum iron and protein levels in oral submucous fibrosis: A clinical research. J Med Radiol Pathol Surg. 2015;1:3–6. [Google Scholar]
- 15.Taneja L, Arya V, Bagewadi A, Keluskar V. Estimation of major immunoglobulin levels in patients with oral submucous fibrosis. J Cranio Maxillary Dis. 2015;4:16. [Google Scholar]
- 16.Rajendran R, Vasudevan DM, Vijayakumar T. Serum levels of iron and proteins in oral submucous fibrosis (OSMF) Ann Dent. 1990;49:23–5, 45. [PubMed] [Google Scholar]
- 17.Phatak AG. Molecules immunologically similar to fibrinogen (MISFI) in oral submucous fibrosis. Indian J Otolaryngol. 1984;36:45–7. [Google Scholar]
- 18.van Wyk CW, Seedat HA, Phillips VM. Collagen in submucous fibrosis: An electron-microscopic study. J Oral Pathol Med. 1990;19:182–7. doi: 10.1111/j.1600-0714.1990.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 19.Goel R, S G, Chandrasekhar T, Ramani P, Sherlin HJ, Natesan A, et al. Amino Acid profile in oral submucous fibrosis: A high performance liquid chromatography (HPLC) study. J Clin Diagn Res. 2014;8:ZC44–8. doi: 10.7860/JCDR/2014/10201.5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamath V, Krishnamurthy S, Rajkumar K, Satelur K. Transforming growth factor β2 (TGF-β2) in pathogenesis of oral submucous fibrosis: An immunohistochemical study. J Orofacial Sci. 2014;6:108. [Google Scholar]
- 21.Pindborg JJ, Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Mehta FS. Oral submucous fibrosis as a precancerous condition. Scand J Dent Res. 1984;92:224–9. doi: 10.1111/j.1600-0722.1984.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 22.Gupta SC, Khanna S, Singh M, Singh PA. Histological changes to palatal and paratubal muscles in oral submucous fibrosis. J Laryngol Otol. 2000;114:947–50. doi: 10.1258/0022215001904428. [DOI] [PubMed] [Google Scholar]
- 23.Gajendra D, Arora S, Ahmed Mujib BR. A Clinico-histopathological study of association between fibrosis and mouth opening in oral submucous fibrosis. J Oral Biosci. 2009;51:23–30. [Google Scholar]
- 24.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–8. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 25.Tilakaratne WM, Klinikowski MF, Saku T, Peters TJ, Warnakulasuriya S. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42:561–8. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Pindborg JJ, Mehta FS. Etiology of oral submucous fibrosis with special reference to the role of areca nut chewing. J Oral Pathol Med. 1995;24:145–52. doi: 10.1111/j.1600-0714.1995.tb01156.x. [DOI] [PubMed] [Google Scholar]
- 27.Walker SA, Benditt EP. The serum proteins in diseases of connective tissue; an electrophoretic study. J Invest Dermatol. 1950;14:113–20. doi: 10.1038/jid.1950.17. [DOI] [PubMed] [Google Scholar]
- 28.Anuradha CD, Devi CS. Serum protein, ascorbic acid & iron & tissue collagen in oral submucous fibrosis – A preliminary study. Indian J Med Res. 1993;98:147–51. [PubMed] [Google Scholar]
- 29.Anil S, Beena VT, Nair RG, Vijayakumar T. Evaluation of serum beta 2-microglobulin in premalignant and malignant lesions of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:750–2. doi: 10.1016/s1079-2104(05)80311-7. [DOI] [PubMed] [Google Scholar]
- 30.Patidar KA, Parwani RN, Wanjari SP. Correlation of salivary and serum IgG, IgA levels with total protein in oral submucous fibrosis. J Oral Sci. 2011;53:97–102. doi: 10.2334/josnusd.53.97. [DOI] [PubMed] [Google Scholar]


