Abstract
Purpose:
To evaluate autologous platelet-rich fibrin (PRF) and autogenous connective tissue graft (CTG) in gingival recession defects in conjunction with coronally advanced flap (CAF) using a microsurgical technique.
Materials and Methods:
Forty-five Class I and II recession defects were randomly equally (n = 15) divided into three groups: Group I sites treated with CAF with PRF, Group II sites treated with CAF with CTG, and Group III sites treated with CAF alone using microsurgical approach. Parameters recorded were vertical gingival recession (VGR) and horizontal gingival recession (HGR), % complete root coverage (CRC), patient comfort score (PCS), patient esthetic score (PES), and hypersensitivity score (HS) at 10 days, 3 months, and 6 months.
Results:
CAF surgery alone and in combination with PRF or CTG are effective procedures to cover denuded roots with mean VGR values of 1.26 ± 0.70 mm (74.4%), 1.26 ± 0.59 mm (58%), and 1.06 ± 0.79 mm (53.3%) for Groups I, II, and III, respectively. In terms of CRC achieved at 6 months, results showed that 100% CRC was obtained in 60% sites of Group I, 20% sites of Group II, and 27% sites of Group III. Patient response and acceptance for surgical treatment modality in terms of PCS and PES were highest for Group I (PRF and CAF) followed by Group III and Group II, and there was decrease in HS for Group I (PRF and CAF) while no significant changes in HS were observed for Group II and Group III. At the end of 6 months follow-up, there was a significant increase in gingival thickness measurements using transgingival probing in Group II, whereas nonsignificant changes were observed in Group I and Group III.
Conclusions:
A long-term multicenter randomized controlled clinical study may be necessary to evaluate the clinical outcome for autologous PRF in comparison to CTG and CAF alone.
Keywords: Connective tissue graft, coronally advanced flap, gingival recession, platelet-rich fibrin
Introduction
The goal of periodontal plastic surgery is to develop less invasive techniques that favor rapid healing, less postoperative discomfort, and greater patient satisfaction. The inception of the surgical operating microscope and microsurgical procedures is an important step to achieve this goal. These advances could lead to more precise and less traumatic tissue manipulation, enabling precise coaptation of wound edges and healing by the first intention.[1] Surgical trauma is minimized during microsurgery, thus less cell damage and necrosis occur, resulting in less inflammation and reduced pain.[2] Furthermore, in root surface coverage procedures, a microsurgical approach using ophthalmic blades and microsurgical suture (6-0) substantially improved the vascularization of the grafts as compared with applying a conventional macroscopic approach.[3]
The coronally advanced flap (CAF) procedure is a very common approach for root coverage. This procedure is based on the coronal shift of the soft tissues on the exposed root surface. It has been documented as an effective surgical technique and a predictable mucogingival surgical procedure used to achieve root coverage in the treatment of Millers Class I and II gingival recessions.[4]
A recent innovation in dentistry is the use of second-generation platelet concentrate which is an autologous platelet-rich fibrin (PRF) with growth factors and cicatricial properties for root coverage procedures. This material can be used as a reservoir for active biochemicals which are released slowly maintaining the grafted biomaterials in their respective positions. It not only reduces the bulk of graft material required but also makes a desirable connection between bone particles.[5]
The use of connective tissue grafts (CTGs) has made esthetic root coverage a predictable procedure. The connective tissue autograft technique was originally described by Edel in 1974 and is based on the fact that connective tissue carries the genetic message for the overlying epithelium to become keratinized.[6] Improvements in clinical outcomes have been reported by adding CTG to the CAF. This approach is associated with greater probability of obtaining complete root coverage (CRC) in the treatment of localized recession compared with other technique. When carefully closed, palatal donor sites can heal by primary intention without a painful period of open granulation after trap door (TD) CTG procurement technique. This greatly reduces postoperative morbidity.[7] Hence, the present study was conducted to evaluate autologous PRF and autogenous CTG in gingival recession defects in conjunction with CAF using the microsurgical technique.
Materials and Methods
This randomized controlled clinical study was conducted in the Department of Periodontology from November 2013 to December 2015. The study was conducted by the ethical principles described in the Declaration of Helsinki 1998 revised 2008 after approval from IRDC (SDC/IRDC/2013/MDS-P/26) and IHEC (SDC/IHEC/2013/MDS-P/26).
Nonalcoholic, nonsmoker (self-reported) patients of both genders of age more than 18 years, with no contributory medical history, were recruited among those visiting the outpatient Department of Periodontology based on the following inclusion criteria. Patients (1) having at least one tooth in maxillary anterior teeth region with Millers Class I or Class II labial gingival recession defect; (2) in good general health with no contraindications for periodontal surgery; (3) not using antibiotics, corticosteroids, chemotherapeutics, immunomodulators, or others that modify the results of periodontal therapy during the last 6 months; (4) selected teeth must be free of endodontic treatment, buccal or interproximal restoration; and (5) who have not undergone any periodontal treatment in the preceding 6 months of initial examination were included in the study. All the participants received verbal information regarding the study protocol, and written informed consent was obtained from each patient for participation in the study.
Sample size determination
For the present study to ascertain the sample size (number of sites to be treated in each group), pre hoc analysis with the following method was done:
n = 2×(Zα/2 + Zβ)2 SD2/d2 (where n: sample size per group; SD: pooled standard deviation being 0.5 in this; d: difference in the means (effect size); Zα/2: significance level, Zβ: power of the study).
Assuming 80% power, 5% significance level with 95% confidence interval as well as assuming standard 0.50, the required sample size per group is 11 subjects in each group. Assuming 20% loss to follow-up, the final sample size is 13 in each group.
Design of the study
For this randomized clinical study, randomization was conducted with the help of a random selection of sealed envelope having an equal chance of selection. These identical looking sealed envelopes consist of one of the three treatment modalities. Envelopes for each treatment modalities were equal in number to avoid heterogeneous sample size. The randomized controlled clinical trial comprised 36 patients (34 men and 2 women) with Millers Class I or II gingival recession present on the facial aspect of maxillary teeth with total sites of 45, which were divided into three groups as shown in flowchart [Figure 1].
Figure 1.
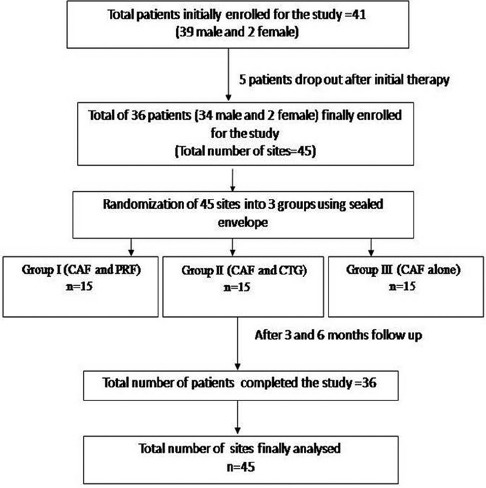
Flowchart showing design of the study
Platelet-rich fibrin
For the present study, PRF was prepared as per the following protocol given by Patel et al.[5] For the successful preparation of PRF, speedy blood collection and immediate centrifugation before the clotting cascade is initiated are essential. PRF clot in the middle was thereby carefully removed by sterilized scissors. The centrifuge machine was placed close to the operatory, and all the efforts were made to minimize the time between the preparation of PRF and its placement in the defect so as to retain the maximum regenerative potential.
Connective tissue graft
The connective tissue autograft for the present study was obtained from the palate using TD technique.[6] Excess connective tissue and fat was carefully removed with the help of Castroviejo scissors. Care was taken to ensure that the incision does not extend more apical than 10 mm from cementoenamel junction (CEJ) of the maxillary posterior teeth and the distal extent of the incision should end no further than the mesial border of maxillary first molar.[8]
Initial therapy
All the selected patients were informed about the cause of the recession and given detailed instruction for performing meticulous plaque control measures. All patients enrolled in the study underwent Phase I therapy. Reevaluation of Phase I therapy was done after 1 month. On the follow-up visit, an acrylic stent was fabricated as described by Lekovic et al.[9] using cold cure resin for record of the clinical parameters at baseline, and the stent after that was preserved on the cast which was again taken into consideration after 3 and 6 months for recording the clinical parameters. The apical margin of the stent served as the measurement point: it was linear and positioned in the coronal third of the tooth, leaving the interdental papillae visible. A reference point (slot) was carved on the stent at the midbuccal area of the experimental tooth, to allow a reproducible periodontal probe positioning. All the clinical parameters were recorded at baseline, 3 months, and 6 months with the use of the same acrylic stent.
Surgical procedure
Compliant patients with satisfactory oral hygiene maintenance were appointed for surgical therapy. Baseline clinical parameters were recorded on the day of the surgical appointment. Immediately before surgery, selected recession defects were randomly assigned to one of the three treatment modalities. All surgical procedures were performed under the microscopic vision using a surgical operating microscope (3D Medical system company, USA, with the magnification of 10×–20×). All the incisions were given with microsurgical (ophthalmic disposable) blade. All the surgical procedures (experimental and control) were performed by the same investigator.
Before surgery, patients were asked to rinse their mouth with 10 ml of 0.2% chlorhexidine gluconate solution as a presurgical rinse. The extraoral surfaces of the patient were swabbed with betadine (10% povidone iodine). The operative site was anesthetized with 2% lignocaine HCl with adrenaline (1:200,000) using block or infiltration technique. In case of experimental Group I (CAF with PRF), patient's blood sample was taken out for preparing PRF as explained above before giving incisions.
CAF performed in the study was according to de Sanctis and Zucchelli.[10] Two horizontal releasing incisions, mesial and distal to the recession defect located at a distance from the tip of the anatomical papillae equal to the depth of the recession plus 1 mm, without involving the gingival margin of adjacent teeth were given. An intrasulcular incision was made on the buccal aspect of the involved tooth. This was followed by two beveled oblique, slightly divergent, incisions starting at the end of the two horizontal incisions and extending to the alveolar mucosa. The resulting trapezoidal-shaped flap was elevated with a split–full–split approach in the coronal–apical direction: the surgical papillae comprised between the horizontal incisions and the probable sulcular area apical to the root exposure were elevated split thickness keeping the blade almost parallel to the root, and the soft tissue apical to the root exposure was elevated full thickness inserting a small periosteum elevator into the probable sulcus and proceeding in the apical direction up to exposing 3–4 mm of bone apical to the bone dehiscence. The releasing vertical incisions were elevated split thickness keeping the blade parallel to the bone plane, thus leaving the periosteum to protect the underlying bone in the lateral areas of the flap [Figure 2]. To permit the coronal advancement of the flap, all muscle insertions present in the thickness of the flap were eliminated. This was done keeping the blade parallel to the external mucosal surface. Coronal mobilization of the flap was considered “adequate” when the marginal portion of the flap was able to passively reach a level coronal to the CEJ of the tooth with the recession defect. Exposed root surfaces were gently scaled and planed with Gracey curettes, followed by thorough saline irrigation.
Figure 2.
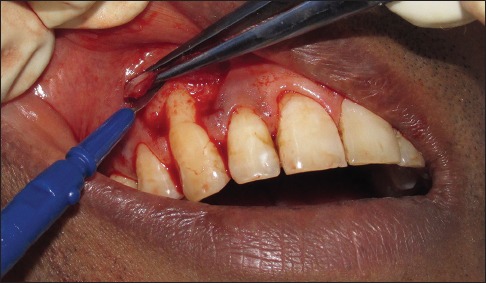
Recipient surgical site preparation using disposable ophthalmic blade
For experimental Group I (CAF with PRF), surgical site was flushed with saline. PRF was then positioned on the recession defect and squeezed to form a membrane that covers the defect. The fluid obtained during squeezing was thus confined to the treated site [Figure 3a].
Figure 3.
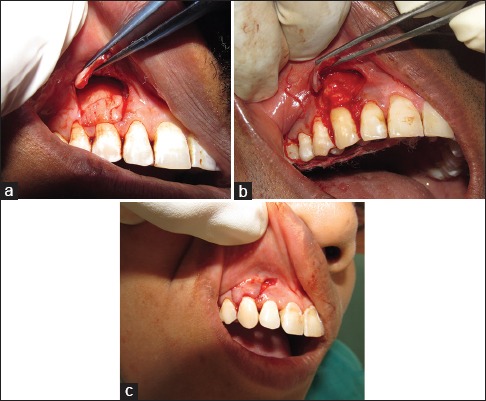
(a) Surgical site treated with coronally advanced flap and platelet-rich fibrin (Group I). (b) Surgical site treated with coronally advanced flap and connective tissue graft (Group II). (c) Surgical site treated with coronally advanced flap alone (Group III)
For experimental Group II (CAF with CTG), after the preparation of recipient site, CTG obtained from the palate as explained above was placed at the site [Figure 3b].
Both the experimental groups after placement of the PRF and CTG were placed at the recipient site; the flap was coronally positioned to fully cover the membrane and sutured using 6-0 black silk sutures (Mersilk). In the case of the control Group III (CAF alone), no membrane was used to cover the recession defects [Figure 3c]. The gingival flap was repositioned with its margin located on the enamel in the both experimental and control sites and was held at that position with horizontal sling suture. Interrupted sutures were placed along the vertical releasing incisions.
Light cure periodontal dressing (Barricaid, Dentsply) was applied at the surgical site [Figure 4]. Postsurgically, both test and control group patients were prescribed systemic antibiotics amoxicillin 500 mg TID for 5 days and the combination of brufen and paracetamol (ibugesic plus) bid 3 days. Postoperative written instructions were given to all the patients, and they were instructed to report to the department after 10 days. During this, plaque control was achieved with a 0.2% chlorhexidine solution rinse used twice a day.
Figure 4.
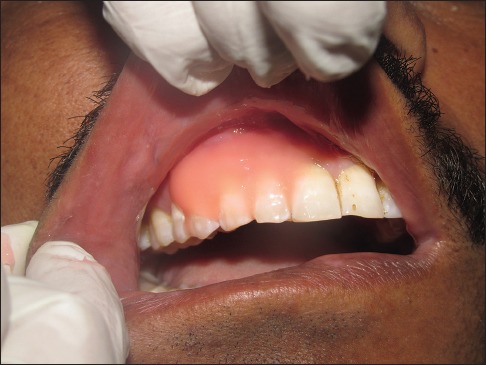
Surgical site covered with light cure dressing
Patient follow-up
After 10 days of the surgery, the dressings and sutures were carefully removed without hampering the healing of soft tissue, and the surgical site was irrigated with normal saline. Inquiry regarding postsurgical procedure was made, and patient satisfaction scores were recorded. Recall appointment of the patient was made after 3 and 6 months [Figure 5a–c]. At each visit, oral hygiene instructions were reinforced. Supragingival scaling was done if required. Postoperative clinical parameters were recorded on recall appointments.
Figure 5.
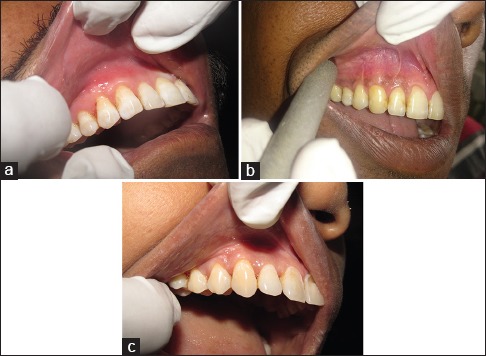
(a) Follow-up of surgical site treated with coronally advanced flap and platelet-rich fibrin after 6 months (Group I). (b) Follow-up of surgical site treated with coronally advanced flap and connective tissue graft after 6 months (Group II). Follow-up of surgical site treated with coronally advanced flap alone after 6 months (Group III)
Clinical parameters recorded were plaque index (PI)[11] and gingival index (GI)[12] at selected teeth; vertical gingival recession (VGR), horizontal gingival recession (HGR) at CEJ, probing pocket depth (PPD), clinical attachment level (CAL), measurement of gingival thickness (GT), width of attached gingiva (AG), and width of keratinized gingiva (KG), as detailed in Agarwal et al.[13]
The gingival thickness was recorded using transgingival probing (TGP) as mentioned by Vandana and Savitha.[14] The gingival thickness was assessed midbuccally in the attached gingival (GT-MB), half way between the mucogingival junction and free gingival groove and at the base of the interdental papilla. The gingival thickness was assessed by anesthetizing the facial gingiva with lignocaine spray (lignocaine 15.0 g [nummit spray]) and infiltration using 2% lignocaine HCl with 1:200,000 adrenaline injection; using a UNC-15 probe with a rubber stopper, the gingival thickness was assessed 20 min after injection. The readings on the probe were transferred to a digital Vernier caliper to measure the gingival thickness [Figure 6a and b].
Figure 6.
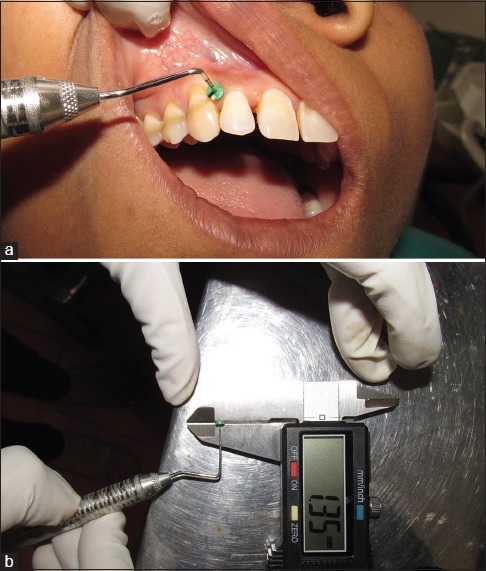
(a) Gingival thickness measurement using transgingival probing. (b) Measurement recorded using a Vernier caliper
Patient satisfaction analysis
Patient satisfaction regarding comfort, hypersensitivity, and esthetic appearance was analyzed subjectively based on visual analog scale (VAS),[15] to record patient comfort score (PCS), patient esthetic score (PES), and hypersensitivity score (HS) at baseline, 10 day, 3 months, and 6 months.[13]
Intraobserver reliability
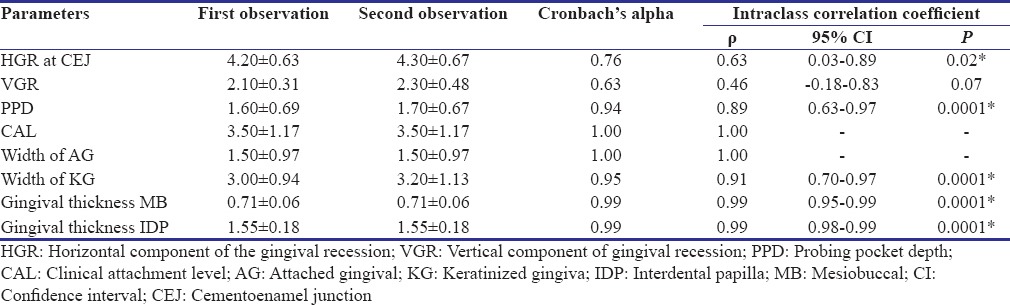
All the clinical parameters were recorded at baseline, 3 months, and 6 months. To maintain accuracy and reliability of clinical parameters, the clinical measurements were recorded by a single investigator throughout the study. Intraexaminer calibration was assessed by performing test-retest exercises in 10 patients before initiating the study. The reliability was expressed regarding Cronbach's alpha and intraclass correlation coefficient. There was no significant difference between the two observations in all the parameters showing better interobserver variations. Both Cronbach's alpha and intraclass correlation coefficient were found to be good for all the parameters showing good intraobserver variation as shown in Table 1.
Table 1.
Cronbach's alpha and intraclass correlation coefficient
Statistical analysis
The results are presented in mean ± standard deviation and percentages. The discrete variables were tested for normalcy using Kolmogorov test, and all the study variables were found to be nonnormal, except for age. Hence, the nonparametric statistical methods were employed for comparison purpose. The Kruskal–Wallis test was used to compare the study variables among the groups at baseline, 3 months, and 6 months. The Wilcoxon rank-sum test was used to compare the changes in the study variables from baseline to follow-ups within the groups. The Chi-square test was used to compare the categorical variables. P < 0.05 was considered statistically significant. The Cronbach's alpha and the intraclass correlation coefficient were calculated to find the intraobserver variations. All the analysis was carried out on SPSS 16.0 version (Chicago, Inc., USA).
Results
A total of 36 patients with 45 sites completed the present study uneventfully, for which equal number (n = 15) of sites were randomly distributed each for both experimental (Groups I and II) and control (Group III). The mean age of the patients of Group I (CAF and PRF), Group II (CAF and CTG), and Group III (CAF alone) was 30.27 ± 3.91, 33.93 ± 5.54, and 35.53 ± 6.52 years, respectively. There was no significant (P > 0.05) difference in the age among the Groups showing comparability of the groups regarding age. The majority of the patients in all the groups were males. There was no significant (P > 0.05) difference in the gender among the groups showing comparability of the groups regarding gender.
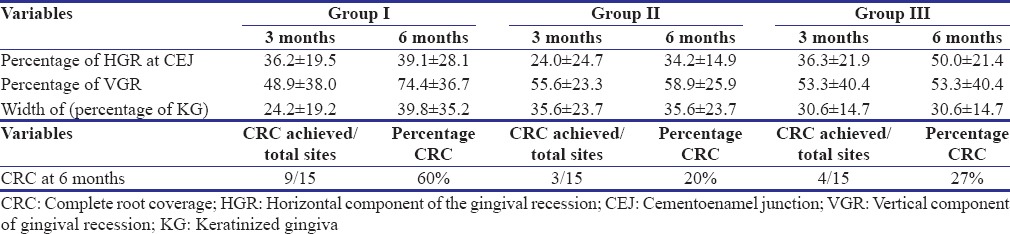
Table 2 shows mean values, change in mean values, as well as their comparison for various parameters (PI, GI, PPD, CAL, HGR, VGR, PCS, HS, and PES), recorded at baseline, 3 months, and 6 months intervals. Between-group comparisons at different time intervals are summarized in Table 3. Mean percentage root coverage of different groups at 3 and 6 months and mean percentage of CRC achieved in different groups achieved at 6 months have been summarized in Table 4.
Table 2.
Comparison of parameters among the groups at time periods
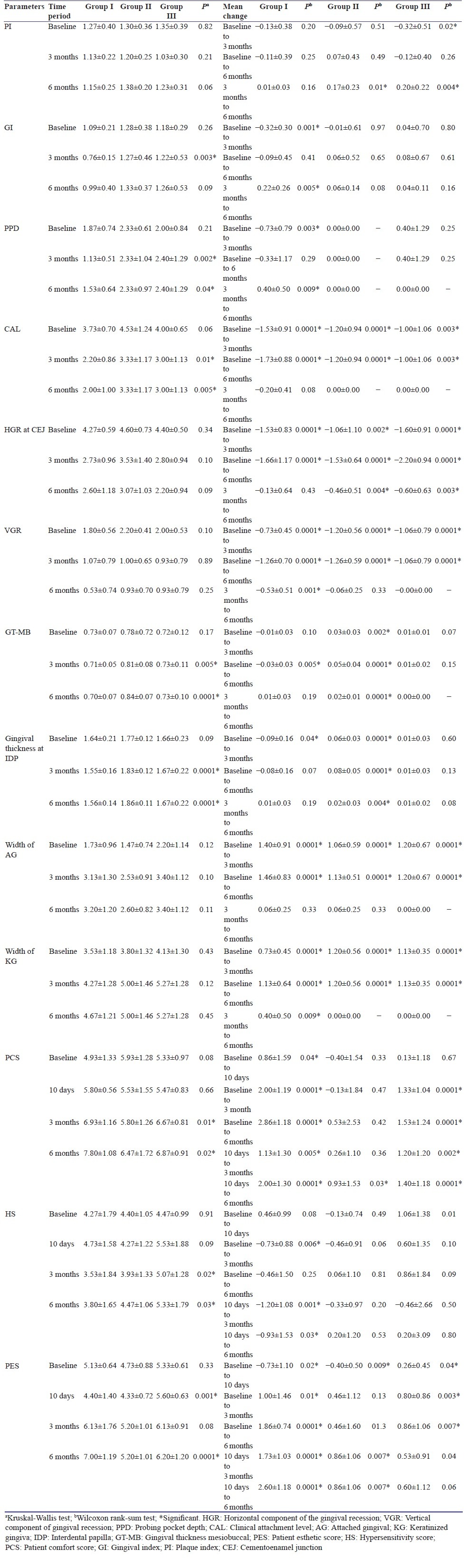
Table 3.
Comparison of various parameters between the groups at different time intervals
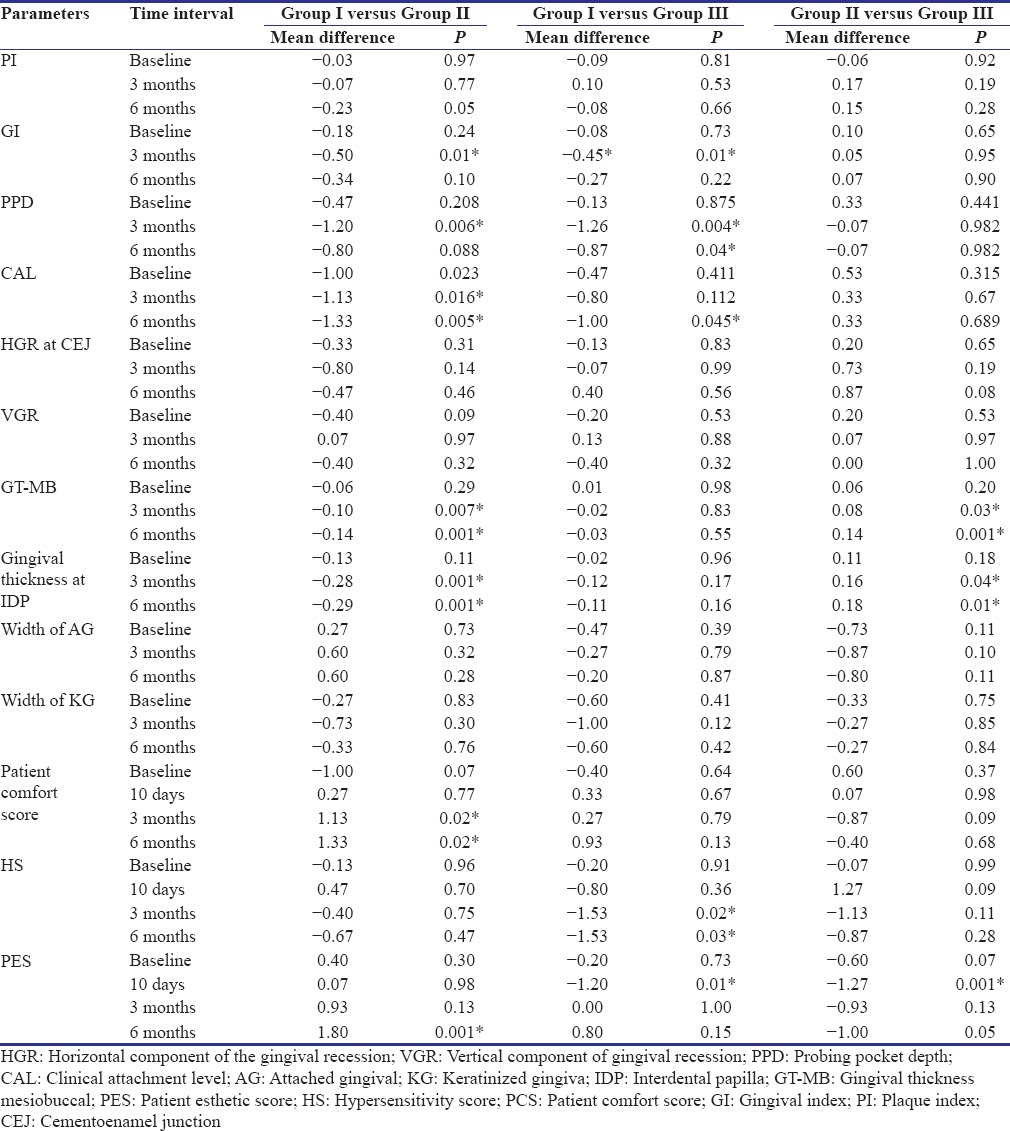
Table 4.
Comparison of percentage mean root coverage at 3 and 6 months among the groups
Discussions
The present study primarily compared the regenerative potential of autologous PRF and autogenous CTG in the management of Miller's Class I and Class II gingival recession defects in conjunction with CAF using the microsurgical technique. All 36 patients completed the study uneventfully and thus representing excellent healing properties of test materials (CTG and PRF) as well as the surgical techniques (CAF) employed.[16,17] There was a significant decrease in the PPD from baseline to 3 months and increased in PPD from 3 to 6 months in Group I (CAF and PRF). However, there was no change in Group II (CAF and CTG). For Group III (CAF alone), there was a nonsignificant increase in PPD values as compared to baseline values. Jankovic et al.,[16] however, reported nonsignificant increase PPD in both PRF- and CTG-treated sites at 6 months. Eren and Atilia[18] reported no change in PPD in CAF with PRF-treated sites whereas 1 mm increase in PPD in CAF with subepithelial CTG-treated sites. These were significantly more gain in CAL in Group I (CAF and PRF) at 3 and 6 months as compared to Group II (CAF and CTG) and at 6 months as compared to Group III (CAF alone). Jankovic et al.[16] reported a nonsignificant gain in CAL in both PRF and CTG controlled sites, whereas Aroca et al.[19] and da Silva et al.[20] reported statistically significant changes in control, as well as the PRF- and CTG-treated sites, respectively. Improvement in CAL was because of recession coverage that results from the coronal shift of attachment apparatus during CAF procedures.
There was a significant (P < 0.01) decrease in the horizontal component at CEJ (mm) from baseline to 3 and 6 months in all the groups. In contrast to the present study, where mean decrease in HGR values were 39%, 34%, and 50% for Group I, Group II, and Group III, Eren and Atilla[18] reported greater reduction in recession width in CTG-treated sites as compared to PRF-treated sites after 6 months; however, at the end of 1 year, they reported complete coverage of recession width in both test and control sites.
At baseline, there was no significant (P > 0.05) difference in the VGR between the groups. The decrease in mean value of VGR, i.e., mean root coverage from baseline to 6 months, was 1.26 ± 0.70 mm (74.4%), 1.26 ± 0.59 mm (58%), and 1.06 ± 0.79 mm (53.3%) for Group I, Group II, and Group III, respectively. Jankovic et al.[16] and Eren and Atilla[18] reported a greater reduction in gingival recession depth in CTG-treated sites as compared to PRF-treated sites after 6 months. Compared to the present study where mean root coverage of 74% was observed in PRF-treated group, Aroca et al.[19] reported 80.7% of mean root coverage at 6 months.
In terms of CRC achieved at 6 months, in the present study, results showed that 100% CRC was obtained in 60% sites of Group I (CAF and PRF), 20% sites of Group II (CAF and CTG), and 27% sites of Group III (CAF alone). In contrast to Aroca et al.,[19] who reported CRC in 19% patients of CAF and PRF group as compared to CAF alone, where 100% root coverage was obtained in 52.3% patients, our results reported superior root coverage in PRF-treated site as compared to control group. Previous studies have reported CRC in about 18%–93.5% of CTG-treated sites.[20,21,22] The variation can be attributed to differences in defect severity, surgical protocol, and other factors. When comparing results from different studies, definition and measurement of CRC may influence the results.
There was a significant increase in gingival thickness (GT-MB) in Group II (CAF and CTG) from baseline to 3 months and 3 months to 6 months. Regarding the increase in thickness, CTG-treated sites obtained better results as compared to PRF with CAF and CAF alone. Furthermore, studies have reported that increased gingival thickness is essential for the stable clinical outcome,[23] as well as creeping attachment that occur after 6–9 months. Although every care was taken while recording gingival thickness measurements using TGP technique, probe angulations, precision of manual probe markings, and reproducibility of the sites may be the source of error in TGP.[13]
There was a significant increase in AG and KG from baseline to 6 months in all the groups. In CAF sites alone or with PRF and CTG, increase in KG may be explained by the fact that mucogingival junction that demarcates the junction between the basal bone and the alveolar process has the tendency to reestablish itself to the original position, leading to gain in keratinized tissue height.[24]
There was no significant (P > 0.05) difference in the PCS that represent patient's comfort regarding postsurgical pain and inflammation among the groups at baseline and 10 days. Similar to Bittencourt et al.,[1] even in the present study, no patient considered any one surgery more painful than other and considered all procedures uncomfortable for the first 10 days. There was a significant mean increase in PCS at 6 months in PRF-treated group, followed by CAF alone and least for CTG treatment groups. In accordance to the present study, Agarwal et al.[13] also reported a mean increase in PCS of 2.43 in PRF-treated sites after 6 months postsurgery. The homogenous fibrin network in PRF is considered by the promoters to be healing biomaterials containing platelets, growth factors, and cytokines that enhance soft tissue wound healing.[2,5] Jankovic et al.[16] also reported improvements in early wound healing (first and second week postsurgery) in the group treated with PRF as compared to CTG-treated group. They advocated that patient discomfort data in PRF could be explained as a result of enhanced tissue healing and avoidance of a donor site surgical procedure. Cortellini et al.[21] reported more number of patients experienced discomfort (swelling and pain) after CAF with CTG as compared to CAF alone, as observed in the present study.
PES was found to be significantly (P < 0.05) increased from baseline to 6 months in Group I and Group III, whereas the increase in PES was nonsignificant for Group II (CAF and CTG). Bitterncout et al.[1] reported 100% patient satisfaction with esthetic results after recession coverage using microsurgical approach. Agarwal et al.[13] reported an increase in PES values in PRF treated-groups after 6 months postsurgery. The absence of scars and controlled increase in KG could justify patients’ satisfaction. However, the frequency of CRC could explain the difference in PES scores between various groups.[1] Further, a bulky look was obtained after CTG may be responsible for less PES in Group II (CAF and CTG).[25]
There was statistically significant decrease in HS for Group I (CAF and PRF) but nonsignificant increase in Group III (CAF alone) followed by Group II (CAF and CTG) after 6 months postsurgery. Aroca et al.[19] and Agarwal et al.[13] reported a decrease in HS in PRF-treated groups after 6 months postsurgery. Variation in HS observed in the study could be related to root coverage obtained.[21]
The procurement of CTG is technique sensitive procedure, and the optimal thickness of graft is an important determining factor. In cases of CTG-treated sites, the placement of CTG graft, the thickness of CTG, and size of the graft may somehow preclude its complete coverage by CAF. In contrast, PRF clot can be pressed into a thin membrane and easily covered by CAF. Therefore, in surgical technique, where vertical releasing incisions were performed for better visualization at the recipient site and reduction in surgical time,[26] in contrast to envelope flap or tunneling approach, vascularization of graft not covered by flap due to bulky size may result in the inferior clinical outcome as compared to CAF with PRF. Further, increased tension during suturing may cause impaired esthetics, disturb the initial wound healing, and result in less root coverage.[26] Therefore, in the present study, CRC in cases of CTG-treated sites was less as compared to PRF. However, if envelope flap or pouch and tunnel techniques are used, chances of complete coverage would have been more, even if some amount of graft is not covered by flap.[25]
Although periodontal microsurgery plays a pivotal role in periodontal procedures, it is a skill that requires practice to achieve proficiency and presents special challenges in dexterity and perception. Its execution is technique sensitive and is thereby more demanding than conventional periodontal procedures.[27] Microsurgical technique results in better postoperative outcomes; however, training and extra space in clinical setup for surgical operating microscope may preclude its use in routine practice.
The drawbacks of the study were low sample size, short-term follow-up with fair oral hygiene instead of meticulous plaque control among subjects, and lack of histological evaluation.
Conclusion
The study inferred that although CTG has been considered as a gold standard regenerative material for the treatment of gingival recession defects, PRF may not be considered less than an alternative material for the management of recession defects regarding the percentage of root coverage as well as patient acceptance. PRF is autologous, simple to procure due to avoidance of donor site surgical procedure as seen in CTG, cost-effective, nonimmunogenic biomaterial with excellent handling properties. Concerning gingival thickness, CAF with CTG has a definitive edge over PRF with CAF and CAF alone and thus may have more predictable and stable long-term results in terms of creeping attachment.[23] However, technique-sensitive CTG procurement requires precision and expertise and additional training.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bittencourt S, Del Peloso Ribeiro E, Sallum EA, Nociti FH, Jr, Casati MZ. Surgical microscope may enhance root coverage with subepithelial connective tissue graft: A randomized-controlled clinical trial. J Periodontol. 2012;83:721–30. doi: 10.1902/jop.2011.110202. [DOI] [PubMed] [Google Scholar]
- 2.Jain R, Kudva P, Kumar R. Periodontal microsurgery-magnifying facts, maximizing results. J Adv Med Dent Scie Res. 2014;2:24–34. [Google Scholar]
- 3.Burkhardt R, Lang NP. Coverage of localized gingival recessions: Comparison of micro- and macrosurgical techniques. J Clin Periodontol. 2005;32:287–93. doi: 10.1111/j.1600-051X.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 4.Pini Prato G, Pagliaro U, Baldi C, Nieri M, Saletta D, Cairo F, et al. Coronally advanced flap procedure for root coverage. Flap with tension versus flap without tension: A randomized controlled clinical study. J Periodontol. 2000;71:188–201. doi: 10.1902/jop.2000.71.2.188. [DOI] [PubMed] [Google Scholar]
- 5.Patel JS, Patel SG, Kadam C. Choukroun's platelet rich fibrin in regenerative dentistry. Univ Res J Dent. 2013;3:22–5. [Google Scholar]
- 6.Edel A. Clinical evaluation of free connective tissue grafts used to increase the width of keratinised gingiva. J Clin Periodontol. 1974;1:185–96. doi: 10.1111/j.1600-051x.1974.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 7.Pini-Prato GP, Cairo F, Nieri M, Franceschi D, Rotundo R, Cortellini P. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: A split-mouth study with a 5-year follow-up. J Clin Periodontol. 2010;37:644–50. doi: 10.1111/j.1600-051X.2010.01559.x. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui ZR, Jhingran R, Bains VK, Srivastava R, Madan R, Rizvi I. Comparative evaluation of platelet-rich fibrin versus beta-tri-calcium phosphate in the treatment of Grade II mandibular furcation defects using cone-beam computed tomography. Eur J Dent. 2016;10:496–506. doi: 10.4103/1305-7456.195160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lekovic V, Camargo PM, Weinlaender M, Vasilic N, Kenney EB. Comparison of platelet-rich plasma, bovine porous bone mineral, and guided tissue regeneration versus platelet-rich plasma and bovine porous bone mineral in the treatment of intrabony defects: A reentry study. J Periodontol. 2002;73:198–205. doi: 10.1902/jop.2002.73.2.198. [DOI] [PubMed] [Google Scholar]
- 10.de Sanctis M, Zucchelli G. Coronally advanced flap: A modified surgical approach for isolated recession-type defects: Three-year results. J Clin Periodontol. 2007;34:262–8. doi: 10.1111/j.1600-051X.2006.01039.x. [DOI] [PubMed] [Google Scholar]
- 11.Silness J, Löe H. Periodontal disease in pregnancy 3.Response to local treatment. Acta Odontol Scand. 1966;24:747–59. doi: 10.3109/00016356609028739. [DOI] [PubMed] [Google Scholar]
- 12.Loe H, Silness J. Periodontal disease in pregnancy. I Prevalence and severity Periodontal disease in pregnancy I Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal SK, Jhingran R, Bains VK, Srivastava R, Madan R, Rizvi I. Patient-centered evaluation of microsurgical management of gingival recession using coronally advanced flap with platelet-rich fibrin or amnion membrane: A comparative analysis. Eur J Dent. 2016;10:121–33. doi: 10.4103/1305-7456.175686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandana KL, Savitha B. Thickness of gingiva in association with age, gender and dental arch location. J Clin Periodontol. 2005;32:828–30. doi: 10.1111/j.1600-051X.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 15.Gould D, Kelly D, Goldstone L, Gammon J. Examining the validity of pressure ulcer risk assessment scales: Developing and using illustrated patient simulations to collect the data. J Clin Nurs. 2001;10:697–706. doi: 10.1046/j.1365-2702.2001.00525.x. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic S, Aleksic Z, Klokkevold P, Lekovic V, Dimitrijevic B, Kenney EB, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int J Periodontics Restorative Dent. 2012;32:e41–50. [PubMed] [Google Scholar]
- 17.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Eren G, Atilla G. Platelet-rich fibrin in the treatment of bilateral gingival recessions. Clin Adv Periodontics. 2012;2:154–60. [Google Scholar]
- 19.Aroca S, Keglevich T, Barbieri B, Gera I, Etienne D. Clinical evaluation of a modified coronally advanced flap alone or in combination with a platelet-rich fibrin membrane for the treatment of adjacent multiple gingival recessions: A 6-month study. J Periodontol. 2009;80:244–52. doi: 10.1902/jop.2009.080253. [DOI] [PubMed] [Google Scholar]
- 20.da Silva RC, Joly JC, de Lima AF, Tatakis DN. Root coverage using the coronally positioned flap with or without a subepithelial connective tissue graft. J Periodontol. 2004;75:413–9. doi: 10.1902/jop.2004.75.3.413. [DOI] [PubMed] [Google Scholar]
- 21.Cortellini P, Tonetti M, Baldi C, Francetti L, Rasperini G, Rotundo R, et al. Does placement of a connective tissue graft improve the outcomes of coronally advanced flap for coverage of single gingival recessions in upper anterior teeth? A multi-centre, randomized, double-blind, clinical trial. J Clin Periodontol. 2009;36:68–79. doi: 10.1111/j.1600-051X.2008.01346.x. [DOI] [PubMed] [Google Scholar]
- 22.Zucchelli G, Clauser C, De Sanctis M, Calandriello M. Mucogingival versus guided tissue regeneration procedures in the treatment of deep recession type defects. J Periodontol. 1998;69:138–45. doi: 10.1902/jop.1998.69.2.138. [DOI] [PubMed] [Google Scholar]
- 23.Harris RJ. The connective tissue with partial thickness double pedicle graft: The results of 100 consecutively-treated defects. J Periodontol. 1994;65:448–61. doi: 10.1902/jop.1994.65.5.448. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Banthia R, Singh P, Banthia P, Raje S, Aggarwal N. Clinical evaluation and comparison of the efficacy of coronally advanced flap alone and in combination with platelet rich fibrin membrane in the treatment of Miller Class I and II gingival recessions. Contemp Clin Dent. 2015;6:153–60. doi: 10.4103/0976-237X.156034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jahnke PV, Sandifer JB, Gher ME, Gray JL, Richardson AC. Thick free gingival and connective tissue autografts for root coverage. J Periodontol. 1993;64:315–22. doi: 10.1902/jop.1993.64.4.315. [DOI] [PubMed] [Google Scholar]
- 26.Tözüm TF, Keçeli HG, Güncü GN, Hatipoglu H, Sengün D. Treatment of gingival recession: Comparison of two techniques of subepithelial connective tissue graft. J Periodontol. 2005;76:1842–8. doi: 10.1902/jop.2005.76.11.1842. [DOI] [PubMed] [Google Scholar]
- 27.Shah C, Shah S, Modi D. Microsurgery in periodontics – Re-visited. J Res Adv Dent. 2012;1:107–23. [Google Scholar]


