Abstract
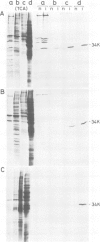
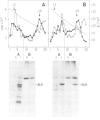
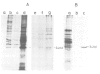
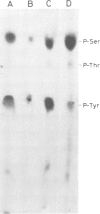
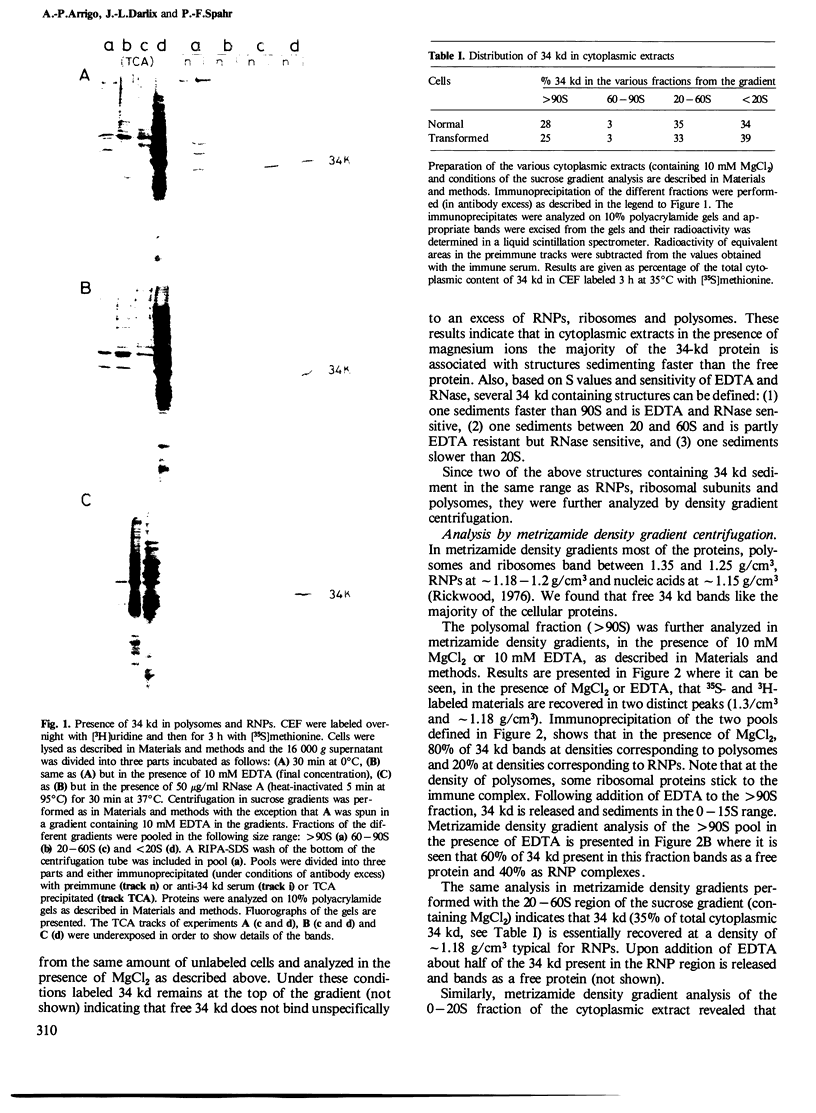
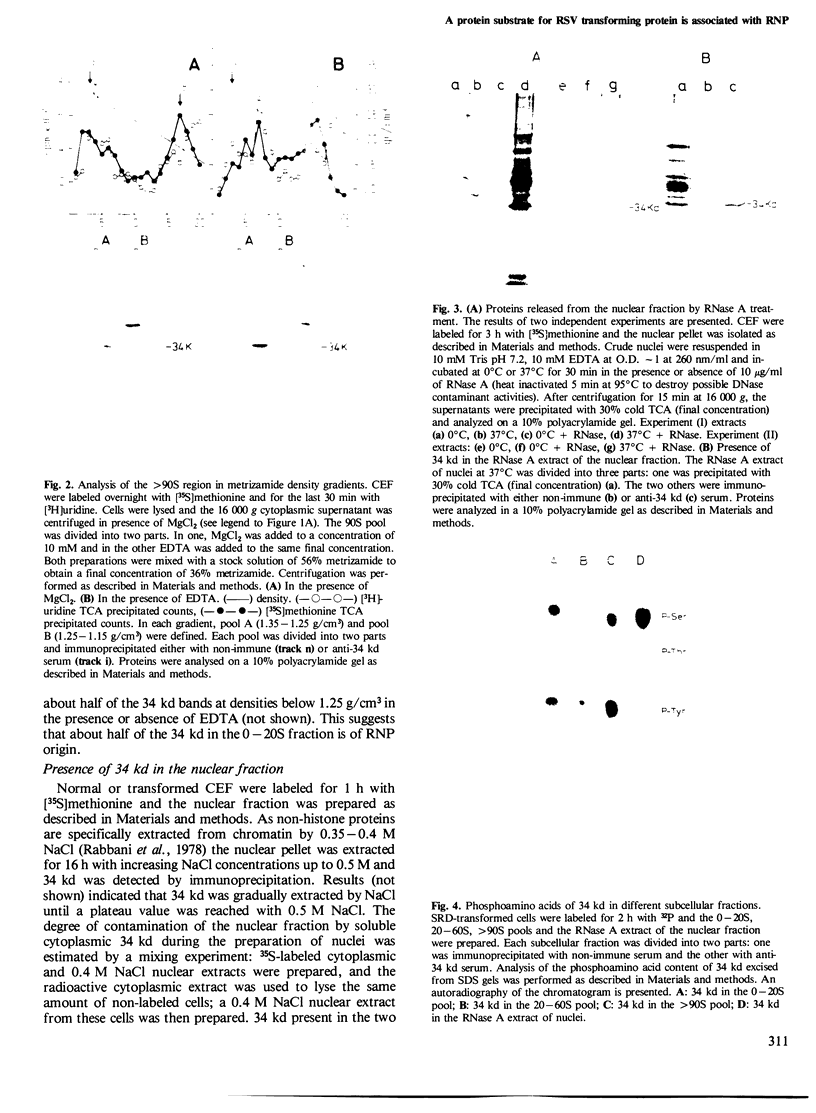
In chick embryo fibroblasts transformed by Rous sarcoma virus (RSV) the tyrosine phosphorylation of a cellular protein of 34,000 daltons mol. wt. (34 kd) is greatly enhanced; this was shown to be catalyzed by the phosphotransferase activity of RSV transforming protein pp60src. We report here that in cytoplasmic extracts of both normal and transformed cells, in the presence of magnesium ions, the majority of the 34-kd protein is associated with large structures and that a fraction of 34 kd appears to be associated with ribonucleoprotein particles (RNPs). In addition, upon u.v. light cross-linking of RNA to protein in normal or transformed cells, an anti-34 kd serum immunoprecipitates RNA fragments of apparent low sequence complexity as detected by T1 fingerprint analysis. Our results indicate that the 34-kd protein may play a role in the cell at the level of RNPs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Fakan S., Tissières A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev Biol. 1980 Jul;78(1):86–103. doi: 10.1016/0012-1606(80)90320-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Discrete primary locations of a tyrosine-protein kinase and of three proteins that contain phosphotyrosine in virally transformed chick fibroblasts. J Cell Biol. 1982 Aug;94(2):287–296. doi: 10.1083/jcb.94.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Levray M., Bromley P. A., Spahr P. F. Characterization of the genomic RNA from a Rous sarcoma virus mutant temperature sensitive for cell transformation. Nucleic Acids Res. 1979 Feb;6(2):471–485. doi: 10.1093/nar/6.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Brugge J. S., Erikson R. L. Phosphorylated and nonphosphorylated forms of avian sarcoma virus polypeptide p19. Virology. 1977 Jul 1;80(1):177–185. doi: 10.1016/0042-6822(77)90390-7. [DOI] [PubMed] [Google Scholar]
- Erikson E., Cook R., Miller G. J., Erikson R. L. The same normal cell protein is phosphorylated after transformation by avian sarcoma viruses with unrelated transforming genes. Mol Cell Biol. 1981 Jan;1(1):43–50. doi: 10.1128/mcb.1.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. I., Collett M. S., Erikson E., Purchio A. F., Brugge J. S. Protein phosphorylation mediated by partially purified avian sarcoma virus transforming-gene product. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):907–917. doi: 10.1101/sqb.1980.044.01.098. [DOI] [PubMed] [Google Scholar]
- Hancock R. Interphase chromosomal deoxyribonucleoprotein isolated as a discrete structure from cultured cells. J Mol Biol. 1974 Jul 5;86(3):649–663. doi: 10.1016/0022-2836(74)90187-9. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Möller K., Brimacombe R. Specific cross-linking of proteins S7 and L4 to ribosomal RNA, by UV irradiation of Escherichia coli ribosomal subunits. Mol Gen Genet. 1975 Dec 9;141(4):343–355. doi: 10.1007/BF00331455. [DOI] [PubMed] [Google Scholar]
- Nakamura K. D., Weber M. J. Phosphorylation of a 36,000 Mr cellular protein in cells infected with partial transformation mutants of rous sarcoma virus. Mol Cell Biol. 1982 Feb;2(2):147–153. doi: 10.1128/mcb.2.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani A., Goodwin G. H., Johns E. W. High mobility group non-histone chromosomal proteins from chicken erythrocytes. Biochem Biophys Res Commun. 1978 Mar 30;81(2):351–358. doi: 10.1016/0006-291x(78)91540-1. [DOI] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickwood D., Hell A., Birnie G. D. Isopycnic centrifugation of sheared chromatin in metrizamide gradients. FEBS Lett. 1973 Jul 1;33(2):221–224. doi: 10.1016/0014-5793(73)80197-8. [DOI] [PubMed] [Google Scholar]
- Rickwood D., Hell A., Malcolm S., Birnie G. D., MacGillivray A. J., Paul J. Fractionation of unfixed chromatin by buoyant-density centrifugation. Biochim Biophys Acta. 1974 Jul 11;353(3):353–361. doi: 10.1016/0005-2787(74)90029-x. [DOI] [PubMed] [Google Scholar]
- Sherton C. C., Di Camelli R. F., Wool I. G. Separation of large quantities of eukaryotic ribosomal subunits by zonal ultracentrifugation. Methods Enzymol. 1974;30:354–367. doi: 10.1016/0076-6879(74)30038-9. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Reinders R. J., van Venrooij W. J. Cross-linking of mRNA to proteins by irradiation of intact cells with ultraviolet light. Eur J Biochem. 1980 Nov;112(2):323–330. doi: 10.1111/j.1432-1033.1980.tb07207.x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]