Abstract
Background:
The fructus of Kochia scoparia Schrader (Chenopodiaceae) is a traditional herbal medicine that has been used for treating gonorrhea and dermatitis.
Objective:
We investigated the anti-inflammatory activities of three marker compounds, including 20-hydroxyecdysone, momordin Ic, and oleanolic acid, from the fructus of K. scoparia.
Materials and Methods:
The simultaneous analysis of three components was performed using high-performance liquid chromatography and high-performance thin-layer chromatography. We evaluated the anti-inflammatory effects of the nine marker compounds by determining their anti-inflammatory activities in the murine macrophage cell line RAW 264.7.
Results:
Among three marker compounds, momordin Ic, but not 20-hydroxyecdysone and oleanolic acid, had inhibitory effects on the production of inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in LPS-treated RAW264.7 macrophages. The effects of three marker compounds on prostaglandin E2(PGE2) were also evaluated. All three compounds significantly reduced PGE2 production in LPS-treated cells.
Conclusions:
We suggest that momordin Ic is the most potent phytochemical of the fructus of K. scoparia as an anti-inflammatory agent.
SUMMARY
Simultaneous analysis of three phenylpropanoids in the Kochia scoparia was established using HPLC-PDA system
The momordin Ic had inhibitory effects on production of inflammatory cytokines tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) in LPS-treated RAW264.7 macrophages
The momordin Ic, 20-hydroxyecdysone, and oleanolic acid significantly reduced PGE2 production in LPS-treated cells.
Abbreviations used: HPLC: High-performance liquid chromatography; TNF-α: Tumor necrosis factor alpha; IL-6: Interleukin-6; PGE2: Pro-inflammatory mediator prostaglandin E2; LPS: Lipopolysaccharide.
Keywords: Anti-inflammatory effect, Kochia scoparia, 20-hydroxyecdysone, momordin Ic, oleanolic acid, RAW264.7
INTRODUCTION
Inflammation is a biological process of response to various physical or chemical stimuli.[1] Inflammatory response is usually regulated by pro-inflammatory cytokines released from activated macrophages. Especially, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) have been considered as targets of molecular therapeutic intervention. for inflammatory disorders.[2] Prostaglain (PG) also plays an important role in the regulation of inflammation.[3] In addition, inflammation is a key event in different stages of cancer development.[4,5] Thus, targeting inflammation-related signaling molecules has been recognized as an attractive approach against inflammation-derived cancers.
The fructus of Kochia scoparia Schrader (Chenopodiaceae) is a well-known traditional herbal medicine and used for treatment of gonorrhea and dermatitis.[6] Phytochemical studies with K. scoparia have identified triterpenoids[6,7,8] and phytoecdysteroids.[9] Among these compounds, triterpenoid saponin and momordin Ic have been reported as the main constituents. Many investigations reported that K. scoparia has a variety of biological efficacies against cancer,[10] contact dermatitis,[11] asthma,[12] and rheumatoid arthritis[7] in vitro or in vivo. Phytochemicals from K. scoparia also have shown to have various biological activities. Momordin Ic reduced hepatotoxicity[13] and had antinociceptive,[14] allergic,[15] and rheumatoid arthritis[7] effects. Oleanolic acid had antipruritic effect[8] and inhibited carbon tetrachloride-induced hepatotoxicity.[13] Overall reports indicate strong activities of K. scoparia and its components against inflammatory diseases. Actually, Shin et al. reported K. scoparia extract had inhibitory effects on lipopolysaccharide (LPS)-mediated inflammation in macrophages.[16] Despite this observation, it remains to be established whether K. scoparia compounds exert anti-inflammatory in macrophages.
In the present study, we investigated the effects of compounds from the fructus of K. scoparia, including 20-hydroxyecdysone, momordin Ic, and oleanolic acid in LPS-stimulated murine macrophage RAW 264.7 cell line by measuring production of inflammatory cytokines TNF-α and IL-6 and PGE2. Moreover, we performed the simultaneous determination of three components 20-hydroxyecdysone, momordin Ic, and oleanolic acid for quality control of the fructus of K. scoparia using high-performance liquid chromatography (HPLC) coupled with a photodiode array (PDA) detector.
EXPERIMENTAL
Plant material
The dried fructus of K. scoparia used in this experiment was purchased from HMAX (Jecheon, Korea) in October 2009. Prof. Je-Hyun Lee (Dongguk University, Gyeongju, Republic of Korea) confirmed the origin of this plant taxonomically. A specimen (2009-ST23) has been deposited in the K-herb Research Center, Korea Institute of Oriental Medicine.
Chemicals and reagents
Momordin Ic (purity ≥ 98.0%) was purchased from Chengdu Biopurify Phytochemicals Ltd. (Sichuan, China). Oleanolic acid (purity 98.0%) and 20-hydroxyecdysone (purity 93.0%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The chemical structures of these compounds are shown in Figure 1. The HPLC-grade reagents methanol, acetonitrile, chloroform, and water were obtained from J.T. Baker (Phillipsburg, NJ, USA). Formic acid and sulfuric oxide, analytical grade, were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Junsei (Tokyo, Japan), respectively. Precoated HPTLC plate (Silica-gel 60 F254 GLP, 0.2 mm, 20 × 10 cm) was purchased from Merck KGaA (Darmstadt, Germany).
Figure 1.
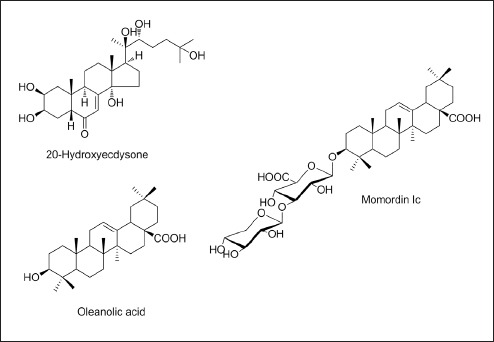
Chemical structures of three marker compounds in K. scoparia
Preparation of 70% ethanol extract of K. scoparia
The dried fructus of K. scoparia (200 g) was extracted three times with 70% ethanol (2 L) by sonication for 1 h. The extracted solution was filtered through filter paper, evaporated at 60°C using EYELA N-100 rotary evaporator (Tokyo, Japan) to dryness and freeze-dried (PVTFD100R, IlShinBioBase, Dongduchun, Korea). The yield of the freeze-dried 70% ethanol extract obtained was 28.75 g (14.38%).
HPLC analysis
The HPLC apparatus used for the simultaneous determination consisted of a Shimadzu Prominence LC-20A systems (Shimadzu Co., Kyoto, Japan) equipped with a pump (LC-20AT), online degasser (DGU-20A3R), column oven (CTO-20A), autosampler (SIL-20A), and ELSD (ELSD-LTII). Data acquisition was performed by LabSolution software (Version 5.54SP3, Shimadzu CO., Kyoto, Japan). The three compounds were separated on a SunFire C18 analytical column (4.6 × 150 mm, 5 μm, Waters, Milford, MA, USA). The chromatographic separation for the simultaneous analysis was conducted using a gradient elution of mobile phases (A) 0.1% v/v aqueous formic and (B) acetonitrile as follows: 0-3 min, 20% B; 3-10 min, 20-90% B; 10-13 min, 90% B; 13-15 min, 20% B. The re-equilibrium time was 5 min. The flow rate was kept constant at 1.0 mL/min, the column temperature was maintained at 40°C, and the injection volume was 10 μL. The drift tube temperature for ELSD was set at 40°C, the pressure of nebulizing gas (nitrogen) was 360 KPa, and gain value was 6. For the HPLC analysis of K. scoparia, the 70% ethanol extract (25 mg) was dissolved in 20 mL of 70% methanol and extracted by sonication for 20 min. The solution was filtered through a membrane filter (0.2 μm) before HPLC injection.
High-performance thin-layer chromatography analysis
High-performance thin-layer chromatography (HPTLC) was carried out using the CAMAG application system (Muttenz, Switzerland) consisting of CAMAG automatic TLC sampler 4, Reprostar 3, CAMAG ADC 2 automatic developing chamber, and a digital camera. The data were processed by WinCATS (CAMAG, Muttenz, Switzerland). The 20 μL loading of each standard and sample solution was spotted in bands of width 4 mm on the HPTLC plate by CAMAG application system. The samples were separated (migration distance 7 cm) using chloroform:methanol:water (7:3:0.5, v/v/v). The spots of migrated components were detected under white light using Reprostar 3 with a digital camera (CAMAG) after spraying with 10% H2SO4, followed by heating. For the HPTLC analysis, the lyophilized K. scoparia extract (50 mg) was transferred to 50 mL volumetric flask and dissolved up to the mark with 70% methanol.
Cell culture
The murine macrophage RAW 264.7 cell line was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in Dulbecco's modified Eagle's medium (Gibco Inc., Grand Island, NY, USA) supplemented with 5.5% heat-inactivated fetal bovine serum (Gibco Inc., Grand Island, NY, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL) in a 5% CO2 incubator at 37°C.
Cell viability
Cell viability was assessed with the CCK-8 assay (Cell Counting Kit-8, Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions. RAW264.7 cells were incubated in 96-well plates with various concentrations of the test materials for 24 h. CCK-8 reagent was added to each well and incubated for 4 h. The absorbance was measured at 450 nm with a Benchmark plus microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). The percentage of cell viability was calculated with the following formula: cell viability (%) = (mean absorbance in test wells/mean absorbance in control wells) × 100.
Measurement of TNF-α, IL-6, and PGE2 production
RAW264.7 cells were treated with various concentration of marker compounds from the fructus of K. scoparia for 4 h prior to LPS (1 μg/mL) stimulation. After incubation for 20 h, the supernatants were analyzed for the levels of TNF-α (BD Biosciences, Mountain View, CA, USA), IL-6 (BD Biosciences, Mountain View, CA, USA), and PGE2 (Cayman Chemical Co., Ann Arbor, MI, USA), according to manufacturers’ instructions.
Statistical analysis
All values are expressed as the mean ± SEM of three independent samples of each compound from the fructus of K. scoparia. Dunnett's test was used for multiple group comparisons. Differences were considered significant at P less than 0.05.
RESULTS
Determination of the three compounds in K. scoparia extract
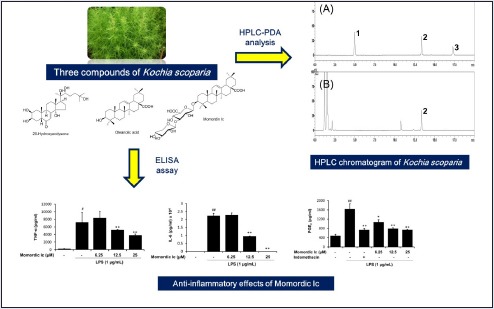
The established HPLC-ELSD analytical method was applied for simultaneous determination of three compounds in the K. scoparia extract. Consequently, three components were eluted within 20 min using the two mobile phases consisting of 0.1% (v/v) formic acid in water (A) and acetonitrile (B). The retention times of the 20-hydroxyecdysone, momordin Ic, and oleanolic acid were 5.00, 13.33, and 17.34 min, respectively [Figure 2]. Calibration curves plotted with three components showed good linearity, with correlation coefficients (r2) 0.9956 or higher in the tested concentration ranges (20-hydroxyecdysone and oeanolic acid, 125-500 μg/mL; momordin Ic, 125-1000 μg/mL). Among these components, momordin Ic was only detected as a concentration of 134.61 mg/g, whereas 20-hydroxyecdysone and oleanolic acid were not detected in K. scoparia extract.
Figure 2.

HPLC-ELSD chromatograms of the standard mixture (I) and K. scoparia extract (II). 20-Hydroxyecdysone (1), momordin Ic (2), and oleanolic acid (3)
HPTLC analysis of the three compounds in K. scoparia extract
Both the reference standards and the extracted sample were spotted on the TLC plates and run in different solvent systems. Initially, chloroform:methanol:water in various ratios (6:4:1, 6:4:0.5, 7:3:1, 7:3:0.5, v/v/v) was tried. Finally, the mobile phase chloroform:methanol:water (7:3:0.5, v/v/v) gave good separation of the three components. The Rf values of momordin Ic, oleanolic acid, and 20-hydroxyecdysone were 0.19, 0.91, and 0.60, respectively [Figure 3].
Figure 3.
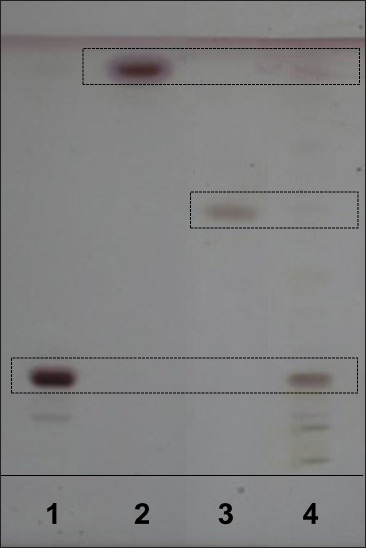
HPTLC chromatogram of K. scoparia extract. Momordin Ic (1), oleanolic acid (2), 20-hydroxyecdysone (3), and K. scoparia extract (4)
Cytotoxicity of 20-hydroxyecdysone, momordin Ic, and oleanolic acid from the fructus of K. scoparia in RAW264.7 cells
To evaluate the cytotoxicity of major components (20-hydroxyecdysone, momordin Ic, and oleanolic acid) from the fructus of K. scoparia in RAW264.7 cells, we performed CCK assay. Cells were exposed to various concentrations of each component for 24 h. As shown in Figure 4A 20-hydroxyecdysone had no cytotoxicity up to 20 μM and reduced the cell viability by 88.6 ± 5.63% and 55.0 ± 5.81% at 40 and 80 μM, respectively. Momordin Ic increased the cell proliferation up to 20 μM and dropped the cell viability to 1.30 ± 0.44% and 0.72 ± 0.23% at 40 and 80 μM, respectively [Figure 4B]. Oleanolic acid did not show any significant effect on the cell viability up to 100 μM [Figure 4C]. Nontoxic concentrations of tested three compounds were used for subsequent experiments.
Figure 4.
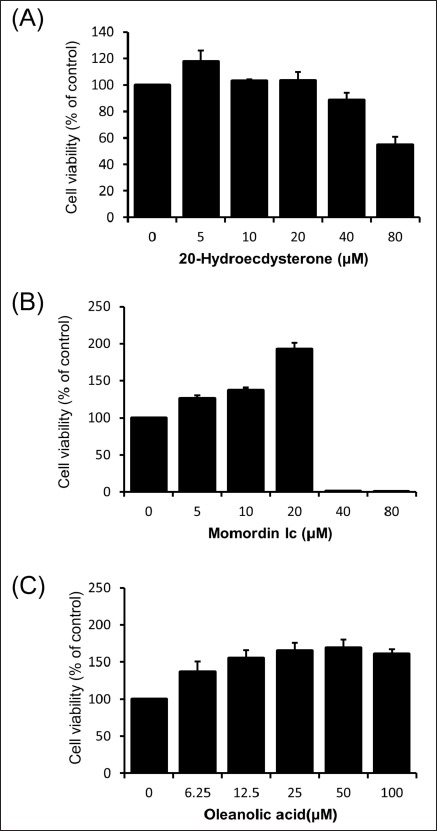
Cytotoxicity of 20-hydroxyecdysone, momordin Ic, and oleanolic acid from K. scoparia in RAW 264.7 cells. Cells were and treated with various concentration of 20-hydroxyecdysone, momordin Ic, or oleanolic acid for 24 h.
Effects of 20-hydroxyecdysone, momordin Ic, and oleanolic acid from the fructus of K. scoparia on TNF-α and IL-6 production in LPS-stimulated RAW264.7 cells
To investigate whether 20-hydroxyecdysone, momordin Ic, or oleanolic acid influence LPS-mediated TNF-α and IL-6 generation in macrophages, RAW264.7 cells were treated with various concentrations of each compounds and then stimulated with LPS. As expected, the amounts of secreted TNF-α and IL-6 were significantly increased by LPS stimulation [Figures 5 and 6]. Among three components from the fructus of K. scoparia, 20-hydroxyecdysone and oleanolic acid had no effect on LPS-induced TNF-α and IL-6 productions [Figures 5 and 6]. By contrast, momordin Ic significantly reduced LPS-stimulated productions of both TNF-α and IL-6 at 12.5 and 25 μM [Figures 5 and 6].
Figure 5.
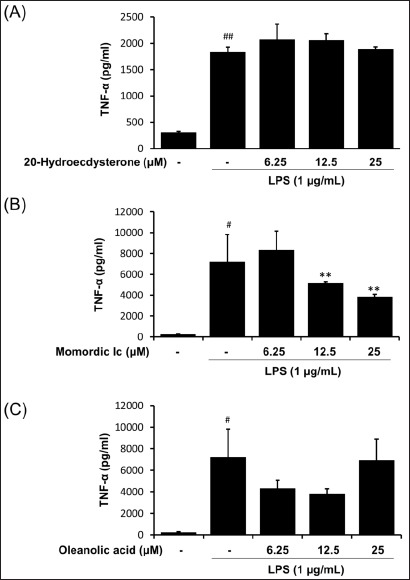
Effect of 20-hydroxyecdysone, momordin Ic, and oleanolic acid from K. scoparia on LPS-stimulated TNF-α production in RAW 264.7 cells. The production of TNF-α was measured in culture medium of cells pretreated with various concentrations (6.25, 12.5, or 25 μM) of 20-hydroxyecdysone, momordin Ic, or oleanolic acid for 4 h and then stimulated with LPS (1 μg/mL) for 20 h. Each bar represents the mean of three independent experiments. #P < 0.05 and ##P < 0.01 vs. untreated control; **P < 0.01 vs. LPS-treated cells
Figure 6.
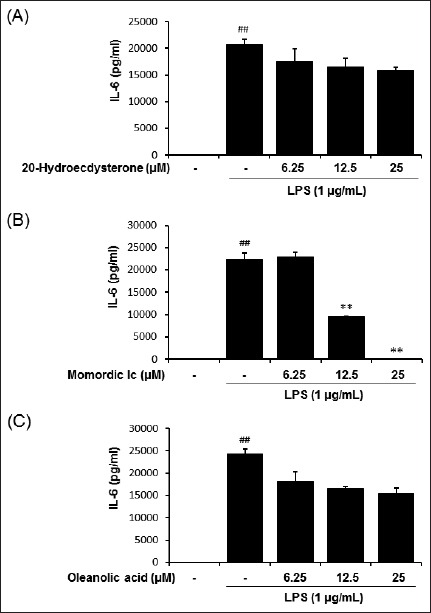
Effect of 20-hydroxyecdysone, momordin Ic, and oleanolic acid from K. scoparia on LPS-stimulated IL-6 production in RAW 264.7 cells. The production of IL-6 was measured in culture medium of cells pretreated with various concentrations (6.25, 12.5, or 25 μM) of ecdysterone, momordin Ic, or oleanolic acid for 4 h and then stimulated with LPS (1 μg/mL) for 20 h. Each bar represents the mean of three independent experiments. ##P < 0.01 vs. untreated control; **P < 0.01 vs. LPS-treated cells
Effects of 20-hydroxyecdysone, momordin Ic, and oleanolic acid from the fructus of K. scoparia on PGE2 production in LPS-stimulated RAW 264.7 cells
Next, we further analyzed anti-inflammatory effects of three compounds from the fructus of K. scoparia by measuring PGE2 production in LPS-stimulated RAW 264.7 cells. As shown in [Figure 7], LPS treatment significantly elevated level of the PGE2. On the contrary, all three compounds 20-hydroxyecdysone, momordin Ic, and oleanolic acid from the fructus of K. scoparia significantly inhibited LPS-mediated PGE2 generation in RAW264.7 cells, respectively.
Figure 7.
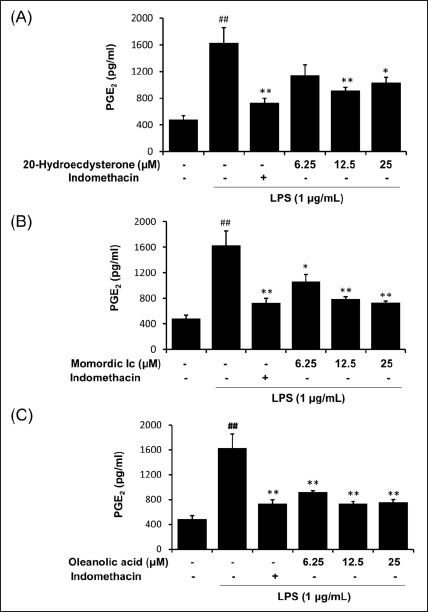
Effect of 20-hydroxyecdysone, momordin Ic, and oleanolic acid from K. scoparia on LPS-stimulated PGE2 production in RAW 264.7 cells. The production of PGE2 was measured in culture medium of cells pretreated with various concentrations (6.25, 12.5, or 25 μM) of 20-hydroxyecdysone, momordin Ic, or oleanolic acid for 4 h and then stimulated with LPS (1 μg/mL) for 20 h. Indomethacin (2.5 ng/mL) were used as positive control. Each bar represents the mean of three independent experiments. ##P < 0.01 vs. untreated control; *P < 0.05 and **P < 0.01 vs. LPS-treated cells
DISCUSSION
Inflammation is a self-defense reaction from infection or injury to pathogens and damaged cells.[1] Anti-inflammatory drugs include steroids such as corticosteroids and nonsteroids such as aspirin, ibuprofen, naproxen, and immune selective drugs. Nonsteroidal drugs are most popularly used to treat inflammatory diseases compared with steroidal agents. However, they have severe side effects such as gastric erosions[17] and myocardial infarction and stroke,[18] sometimes resulting in death. To overcome these problems, natural products including herbal medicines and phytochemicals have been suggested as potent anti-inflammatory materials.[19,20,21] In addition, they can be used for combinatorial therapy with synthetic drugs[22], or provided as health supplements or foods.[23]
In the present study, inhibitory effects of phytochemicals (20-hydroxyecdysone, momordin Ic, and oleanolic acid) from the fructus of K. scoparia were examined in RAW264.7 macrophages. Prior to investigate anti-inflammatory effect of the marker compounds, we analyzed the two triterpenoids (momordin Ic and oleanolic acid) and one steroid (20-hydroxyecdysone) using HPLC and HPTLC in K. scoparia. The result of experiment showed good separation in K. scoparia. These methods will be helpful in fingerprint analysis of K. scoparia.
Macrophages play an essential role in inflammatory process including initiation, maintenance, and resolution.[24] Several inflammatory stimuli can activate macrophages, such as TNF-α, interferon-γ, and LPS.[25] We also observed that LPS treatment significantly enhanced production of pro-inflammatory cytokines TNF-α and IL-6 in macrophages. Among three phytochemicals, momordin Ic significantly reduced LPS-stimulated productions of TNF-α and IL-6 [Figures 5 and 6]. PGE2 is also an important inflammation mediator and produced by COX-2 at inflammatory sites.[26]
As expected, LPS significantly increase PGE2 level, whereas three phytochemicals showed significant inhibitory action on PGE2 generation [Figure 7]. These results suggest that momordin Ic is a bioactive compound from the fructus of K. scoparia against inflammatory diseases by inhibiting inflammatory cytokines and PGE2 production.
In summary, HPLC and HPTLC method for the simultaneous determination of the three compounds in K. scoparia was established, which will help to improve quality control of K. scoparia. Furthermore, our findings demonstrate the anti-inflammatory effect of momordin Ic isolated from the fructus of K. scoparia in macrophages. We suggest the possible usage of momordin Ic as a anti-inflammatory drug.
Financial support and sponsorship
This work was supported by a Grant from the Korea Institute of Oriental Medicine (No. K16251).
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–35. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 3.Kang YJ, Mbonye UR, DeLong CJ, Wada M, Smith WL. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res. 2007;46:108–25. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whang WK, Hahn DR. Saponins from the fructus of Kochia scoparia. Arch Pharm Res. 1991;14:176–80. [Google Scholar]
- 7.Choi J, Lee KT, Jung H, Park HS, Park HJ. Anti-rheumatoid arthritis effect of the Kochia scoparia fruits and activity comparison of momordin Ic, its prosapogenin and sapogenin. Arch Pharm Res. 2002;25:336–42. doi: 10.1007/BF02976636. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda H, Dai Y, Ido Y, Murakami T, Matsuda H, Yoshikawa M, et al. Studies on Kochiae Fructus V. Antipruritic effects of oleanolic acid glycosides and the structure-requirement. Biol Pharm Bull. 1998;21:1231–3. doi: 10.1248/bpb.21.1231. [DOI] [PubMed] [Google Scholar]
- 9.Dian L. Phytoecdysteroids in Kochia scoparia (burning bush) J Chromatogra A. 1994;658:69–76. [Google Scholar]
- 10.Han HY, Kim H, Son YH, Lee G, Jeong SH, Ryu MH. Anti-cancer effects of Kochia scoparia fruit in human breast cancer cells. Pharmacogn Mag. 2014;10:S661–7. doi: 10.4103/0973-1296.139812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi YY, Kim MH, Lee JY, Hong J, Kim SH, Yang WM. Topical application of Kochia scoparia inhibits the development of contact dermatitis in mice. J Ethnopharmacol. 2014;154:380–5. doi: 10.1016/j.jep.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee MY, Shin IS, Lim HS, Seo CS, Ha H, Shin HK. Kochia scoparia fruit attenuates allergic airway inflammation in ovalbumin (OVA)-induced murine asthma model. Inhal Toxicol. 2011;23:938–46. doi: 10.3109/08958378.2011.627392. [DOI] [PubMed] [Google Scholar]
- 13.Kim NY, Lee MK, Park MJ, Kim SJ, Park HJ, Choi JW, et al. Momordin Ic and oleanolic acid from Kochiae Fructus reduce carbon tetrachloride-induced hepatotoxicity in rats. J Med Food. 2005;8:177–83. doi: 10.1089/jmf.2005.8.177. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda H, Dai Y, Ido Y, Ko S, Yoshikawa M, Kubo M. Studies on kochiae fructus. III. Antinociceptive and antiinflammatory effects of 70% ethanol extract and its component, momordin Ic from dried fruits of Kochia scoparia L. Biol Pharm Bull. 1997;20:1086–91. doi: 10.1248/bpb.20.1086. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda H, Dai Y, Ido Y, Yoshikawa M, Kubo M. Studies on kochiae fructus. IV. Anti-allergic effects of 70% ethanol extract and its component, momordin Ic from dried fruits of Kochia scoparia L. Biol Pharm Bull. 1997;20:1165–70. doi: 10.1248/bpb.20.1165. [DOI] [PubMed] [Google Scholar]
- 16.Shin KM, Kim YH, Park WS, Kang I, Ha J, Choi JW, et al. Inhibition of methanol extract from the fruits of Kochia scoparia on lipopolysaccharide-induced nitric oxide, prostaglandin [correction of prostagladin] E2, and tumor necrosis factor-alpha production from murine macrophage RAW 264.7 cells. Biol Pharm Bull. 2004;27:538–43. doi: 10.1248/bpb.27.538. [DOI] [PubMed] [Google Scholar]
- 17.Tamura A, Murakami K, Kadota J. Prevalence of gastroduodenal ulcers/erosions in patients taking low-dose aspirin with either 15 mg/day of lansoprazole or 40 mg/day of famotidine: the OITA-GF study 2. BMC Res Notes. 2013;6:116. doi: 10.1186/1756-0500-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macedo SB, Ferreira LR, Perazzo FF, Carvalho JC. Anti-inflammatory activity of Arnica montana 6cH: preclinical study in animals. Homeopathy. 2004;93:84–7. doi: 10.1016/j.homp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Alghaithy AA, El-Beshbishy HA, Abdel-Naim AB, Nagy AA, Abdel-Sattar EM. Anti-inflammatory effects of the chloroform extract of Pulicaria guestii ameliorated the neutrophil infiltration and nitric oxide generation in rats. Toxicol Ind Health. 2011;27:899–910. doi: 10.1177/0748233711399320. [DOI] [PubMed] [Google Scholar]
- 21.Wu SJ, Tam KW, Tsai YH, Chang CC, Chao JC. Curcumin and saikosaponin a inhibit chemical-induced liver inflammation and fibrosis in rats. Am J Chin Med. 2010;38:99–111. doi: 10.1142/S0192415X10007695. [DOI] [PubMed] [Google Scholar]
- 22.Bonaterra GA, Kelber O, Weiser D, Kinscherf R. Mechanisms of the anti-proliferative and anti-inflammatory effects of the herbal fixed combination STW 5 (Iberogast(R)) on colon adenocarcinoma (HT29) cells in vitro. Phytomedicine. 2013;20:691–8. doi: 10.1016/j.phymed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Alemi M, Sabouni F, Sanjarian F, Haghbeen K, Ansari S. Anti-inflammatory effect of seeds and callus of Nigella sativa L extracts on mix glial cells with regard to their thymoquinone content. AAPS Pharm Sci Tech. 2013;14:160–7. doi: 10.1208/s12249-012-9899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–6. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 25.Wright SD, Tobias PS, Ulevitch RJ, Ramos RA. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989;170:1231–41. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad N, Chen LC, Gordon MA, Laskin JD, Laskin DL. Regulation of cyclooxygenase-2 by nitric oxide in activated hepatic macrophages during acute endotoxemia. J Leukoc Biol. 2002;71:1005–11. [PubMed] [Google Scholar]


