Abstract
Background:
Fructus Aurantii (FA) derived from the dried, and unripe fruit of Citrus aurantium L. is one of the commonly used traditional Chinese medicines to treat gastrointestinal motility dysfunction diseases. According to the literature research, FA flavonoids (FAF) are important active ingredients of FA promoting gastrointestinal motility, but the exact material basis and mechanism of action are still not very clear.
Objective:
This experiment was designed to illustrate the material basis of FAF promoting gastrointestinal motility and explore the mechanism of action from an organic and inorganic combination point of view.
Materials and Methods:
In this experiment, high-performance liquid chromatography (HPLC) method was used to analyze the composition and content of FAF. Based on the prominent prokinetic effect of FAF on mice, the mechanism of action was speculated through a combination of HPLC coupled with quadrupole time-of-flight mass spectrometry (HPLC-QTOF-MS) and inductively coupled plasma mass spectrometry (ICP-MS).
Results:
With the method of HPLC, ten dominating components of FAF including neoeriocitrin, narirutin, rhoifolin, naringin, hesperidin, neohesperidin, neoponcirin, naringenin, hesperetin, and nobiletin accounting for more than 86% of FAF were identified. Combined HPLC-QTOF-MS with ICP-MS, the endogenous substances with difference in the blood of mice were analyzed, in which 4-dimethylallyltryptophan, corticosterone, phytosphingosine, sphinganine, LysoPC (20:4(5Z, 8Z, 11Z, 14Z)), LysoPC(18:2 (9Z, 12Z)), and Ca2+, Mg2+, Zn2+ metal ions had significant changes, involving tryptophan metabolism, corticosterone metabolism, sphingolipid metabolism, and other pathways.
Conclusion:
The results preliminarily elaborated the mechanism of FAF promoting gastrointestinal motility from an organic and inorganic point of view, which provide valuable information for researching and developing new multi-component Chinese medicine curing gastrointestinal underpower associated diseases.
SUMMARY
Fructus Aurantii flavonoids are one of the main components of Fructus Aurantii that possess prominent gastrointestinal motility promoting efficacy
The mainly material basis of Fructus Aurantii flavonoids promoting gastrointestinal motility were neoeriocitrin, narirutin, rhoifolin, naringin, hesperidin, neohesperidin, neoponcirin, naringenin, hesperetin, and nobiletin
Fructus Aurantii flavonoids can regulate the content of 4-dimethylallyltryptophan, corticosterone, phytosphingosine, sphinganine, LysoPC (20:4(5Z, 8Z, 11Z, 14Z)), LysoPC.(18:2(9Z, 12Z)) and Ca2+, Mg2+, Zn2+-metal ions, through tryptophan metabolism, corticosterone metabolism, sphingolipid metabolism, and other pathways to present its gastrointestinal motility promoting efficacy.
Abbreviations used: FA: Fructus Aurantii; FAF: Fructus Aurantii flavonoids; HPLC: High performance liquid chromatography; HPLC-QTOF-MS: High performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry; ICP-MS: Inductively coupled plasma mass spectrometry; PCA: Principal components analysis; CG: Control group; FAFLG: Low-dosage group of Fructus Aurantii flavonoids; FAFMG: Middle-dosage group of Fructus Aurantii flavonoids; FAFHG: High-dosage group of Fructus Aurantii flavonoids; DPG: Domperidone group.
Keywords: Fructus Aurantii flavones, gastrointestinal motility, high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry, inductively coupled plasma mass spectrometry, multi-component Chinese medicine
INTRODUCTION
According to the statistics, the incidence of gastrointestinal diseases accounted for about 12% of the population worldwide, the incidence rate of gastrointestinal under power in China was 20%–30%. Disorders of gastrointestinal motility are common, which include evidence of dysmotility include gastroparesis, gastroesophageal reflux disease, functional dyspepsia, irritable bowel syndrome and so on.[1,2,3,4] In clinical, dopamine receptor blocker-domperidone and 5-HT receptor agonist-cisapride are widely used in the treatment of this kind of diseases for promoting gastrointestinal motility. While relieving symptoms, due to their single acting targets, the drug resistance and side effects are inevitable.[5,6,7] Hence, it is meaningful to find relatively safe and effective alternative medicines with fewer side effects in clinical to improve general gastrointestinal function.
Fructus Aurantii (FA) is recorded in the 2015 edition of Chinese Pharmacopoeia and is one of the national drugs with more than 1000 years of using history. Flavonoids, volatile oils, and alkaloids are the main components of FA,[8,9,10,11] in which FA flavonoids (FAF) are kinds of important active components promoting gastrointestinal motility.[12,13,14] However, the exact chemical constitution and mechanism of action are still not clear.
In this study, based on the outstanding gastrointestinal motility promoting effect on mice, the chemical components of FAF were determinated with the method of high performance liquid chromatography (HPLC). Moreover, the mechanism of action was elaborated from an organic and inorganic point of view. Combined HPLC coupled with quadrupole time-of-flight mass spectrometry (HPLC-QTOF-MS) with inductively coupled plasma mass spectrometry (ICP-MS) approaches, the endogenous metabolites and metal ions with difference in blood of mice were detected and the relationship of FAF promoting gastrointestinal motility was indicated. It will lay a theoretical and experimental foundation for the research and development of multi-component Chinese medicine with explicit material basis and mechanism of action.
MATERIALS AND METHODS
Reagents and materials
FA was provided by Liaoning CR Benxi Third Pharmaceutical Co., Ltd., (Benxi, China) and authenticated. FAF were prepared according to our previous study.[15] Domperidone was purchased from Harbin Pharmaceutical Group (Heilongjiang, Chian). The standards of narirutin, rhoifolin and nobiletin were purchased from Sichuan Vic's biological Technology Co., Ltd., neoeriocitrin and neopon-cirin were purchased from Seema laboratory, naringin and hesperidin were purchased from National Institute for the Control of Pharmaceutical and Biological Products, naringenin and hesperetin were purchased from Guangzhou QiYun Biological Technology Co., Ltd., neohesperidin was purchased from Shanghai Forever Biotech Co. Ltd., MS grade acetonitrile was purchased from Merck (Darmstadt, Germany). MS grade formic acid was purchased from Fisher Scientific (MA, USA). Ultrapure water (18.2 MSZ) was prepared with a Milli-Q water purification system (Millipore, France). Nitric acid was purchased from Kermel Chemical Reagent Co., Ltd., (Tianjing, China). Mixed standard solution including 10−3 g/mL of Fe, K, Ca, Na, Mg and 10−5 g/mL of Ag, Al, As, Ba, Be, Cd, Co, Cr, Cu, Mn, Ni, Pb, Sb, Se, Ti, V, Zn, Th, U were purchased from Agilent (NJ, USA) and diluted to suitable concentration before the experiment.
Ethics statement
All experiments were performed in accordance with the approved animal protocols and guidelines established by Medicine Ethics Review Committee for animal experiments of Liaoning University of Traditional Chinese Medicine.
Animals
A 6-week-old male ICR mice weighing 25–30 g, were provided by Liaoning Immortality Biological Technology Co., Ltd., and housed in a climate-controlled room (humidity 55%–65% and temperature 20–25°C). The animals had free access to standard mouse food and drinking water during all the experiments.
Animals handling
The mice gastrointestinal underpower model was supported by the method of semi-solid paste carbon propelling.[16] Mice were allocated randomly to a control group (gavage semi-solid paste) and experimental groups, including FAF high-, middle-, low-dose groups (8.02 g/kg, 2.68 g/kg, 0.89 g/kg) and Domperidone group (3.90 g/kg). All the mice were orally administered the active group solution 0.4 mL (20 g) once daily (control group with saline) for 7 days, then prohibited any food for 20 h before the experiments, but were allowed access to water freely. On the last day, 1 h after the last administration, mice were administered semi-solid paste 0.8 mL. Twenty minutes later, mice were deeply anesthetized and sacrificed, plasma, and serum were collected, respectively, stomach and small intestine were collected, according to the following formula to evaluate the efficacy. The gastric residual rate (%) = (total weight of the stomach − stomach net weight)/semi-solid paste weight × 100%. Small intestinal propulsive rate (%) = distance from sphincter of pylorus to the carbon end/distance from sphincter of pylorus to the ileocecum ×100%.
Analysis conditions of samples
Fructus Aurantii flavonoids composition analysis conditions
The samples were separated on Agilent TC-C18 column (4.6 mm × 250 mm, 5 μm) with the column temperature at 30°C. The mobile phase consisted of solvent A (0.02% formic acid in water) and solvent B (acetonitrile). The gradient program was used as follows: 0–10 min, B 15%–20%, 10–20 min, B 20%–21%, 20–21 min, B 21%–22%, 21–25 min, B 22%–23%, 25–27 min, B 23%–32%, 27–32 min, B 32%–40%, 32–50 min, and B 40%–50%. The flow rate was 1 mL/min. Detection wavelengths were 283, 288 and 345 nm with full-time three-wavelength fusion method.[17]
Endogenous substances analysis conditions
Chromatography was performed on Agilent 1100 series HPLC system (Agilent Technologies, Inc., USA).[18] The separation was performed on a 4.6 mm × 100 mm ZORBAX SB-C18 column (Agilent Technologies, Inc., USA). The column temperature was maintained at 45°C. The gradient mobile phase consisted of a solvent A (0.1% formic acid in water), while mobile phase B (0.1% formic acid in acetonitrile), the flow rate was 1 mL/min. The gradient condition of the mobile phase was as follows: 0–5 min, B 65%–80%; 5–7 min, B 80%–94%; 7–10 min, B 94-100%. Mass spectrometry was performed on an Agilent 6220 Accurate-Mass Time-of-Flight mass spectrometer (Agilent Technologies, Inc., USA) equipped with an ESI source (Agilent Technologies, Inc., USA), the optimal conditions were as follows: Drying gas temperature at 350°C, drying gas flow rate of 9 L/min, fragmentor voltage of 175 V, nitrogen was used as the dry gas and nebulizer. MS data were collected in the full scan mode from m/z 50–1700. HPLC-QTOF-MS system (Agilent 6530 Accurate-Mass Q-TOF, Agilent Technologies, Inc., USA) was used to make further identification, the ESI ion source was set in the positive ion polarity mode for acquiring all mass spectrometry data. All data collected in centroid mode were acquired with MassHunter software (Agilent Technologies, Inc., USA).
Metal ion analysis conditions
An Agilent 7500a ICP-MS (Agilent Technologies, Inc., USA) was used for the determination of metal ions with a quantitative analysis. Agilent 7500a ICP-MS Chem Station was used for data acquisition. 50 μL serum was digested with 1 mL of nitric acid, and transferred to a 50 mL volumetric flask with ultrapure water. ICP-MS operating conditions were as follows: Sensitivity parameter, oxides, double charge, and mass shaft were tuned. RF power: 1280 w; plasma flow rate: 15.1 L·min−1; carrier gas flow rate: 1.13 L·min−1; sampling depth: 8.6 mm; fog room temperature: 2°C. In the best conditions of instrument, the content of elements in sample solution and blank solution were determinated.[19]
Multivariate data analysis, biomarker identification and pathway analysis
The raw MS data were exported by Agilent Mass Hunter Qualitative Analysis Software for peak detection, alignment and filtering (Agilent Technologies, Palo Alto, CA, USA). Then integrated information of the retention time and molecular mass were outputted from Agilent Mass Profiler Software (Ver B.02.00, Agilent Technologies, USA).[20,21,22] T-test and folder change were used to determine the statistical significance. Principal components analysis was used for multivariate analysis. Moreover, MS/MS data analysis highlighted neutral losses or productions, with the MS collision energy 20 eV, data were obtained in positive mode, software was used for data analysis. The identities of the specific metabolites were confirmed by elements information comparison of their mass spectra using the elemental composition information provided by the software. The identified endogenous metabolites with difference were compared with the accurate mass charge ratio in some databases, including HMDB, KEGG, METLIN, and PUBCHEM to excavate related pathways.
Data processing
The gastric residual rates, small intestinal propulsive rates and the content of metal ions were conducted by SPSS 19.0 for Windows statistical software, and the analysis of variance (one-way, ANOVA) was used to design the group data. All quantitative data were expressed as the means ± standard deviation as indicated.
RESULTS
Major composition of Fructus Aurantii flavonoids
The average extract rate of FAF is 8.26% (n = 3), the major compositions and the content of FAF were as follows: Neoeriocitrin (4.16%), narirutin (9.89%), naringin (33.87%), rhoifolin (0.59%), hesperidin (10.72%), neohesperidin (20.98%), neoponcirin (2.58%), naringenin (1.64%), hesperetin (0.75%), and nobiletin (1.01%) [Figure 1].[23,24,25] The purity of FAF extract can reach up to 86.19%, except rhoifolin and nobiletin, the content of dihydroflavones account for 98% of identified FAF extract, which basically explained the chemical material basis of FAF.
Figure 1.
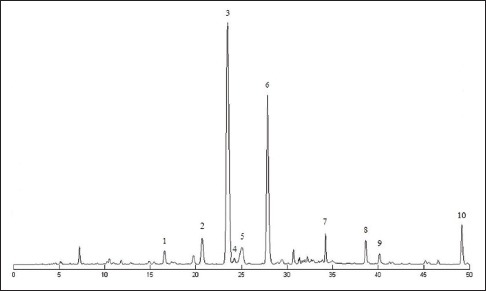
Chromatogram of Fructus Aurantii flavonoids ([1] neoeriocitrin,[2] narirutin,[3] naringin,[4] rhoifolin,[5] hesperidin,[6] neohesperidin,[7] neoponcirin,[8] naringenin,[9] hesperetin,[10] nobiletin)
Effect of Fructus Aurantii flavonoids on promoting gastrointestinal motility
The gastric residual rates of FAF groups were lower than that in control group and with significant differences (P < 0.05). Moreover, the small intestinal propulsive rates were higher (P < 0.05). The efficacy of promoting gastrointestinal motility was similar to domperidone [Figure 2].
Figure 2.
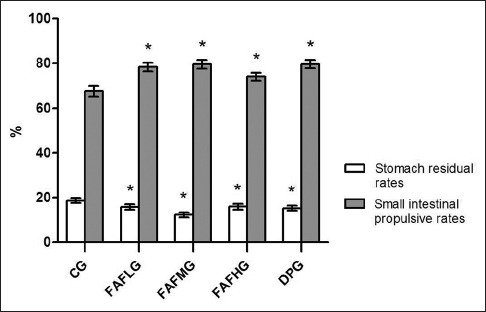
Effect of drugs on promoting gastrointestinal motility. Data are expressed as means ± standard deviation. *P < 0.05 compared to control group. CG: Control group; FAFLG: Low-dosage group of Fructus Aurantii flavonoids; FAFMG: Middle-dosage group of Fructus Aurantii flavonoids; FAFHG: High-dosage group of Fructus Aurantii flavonoids; DPG: Domperidone group
Principal components analysis
With pattern recognition methods, on the observation of three-dimensional plots, FAF high, middle, low groups and control group achieved a good separation, indicating that the endogenous metabolites control group was significantly different with drug groups [Figure 3].
Figure 3.
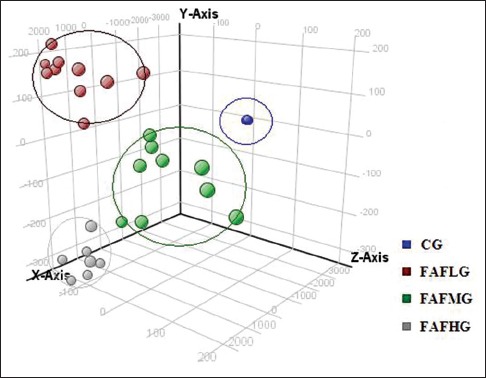
Principle component analysis of each group. Each colored point represents a sample. The first, second and third principal components are displayed on the X, Y and Z-axis, respectively. These three components represent the largest fraction of the overall variability. Blue ball: Control group; brown ball: Low dose group; green ball: Middle dose group; gray ball: High dose group
Endogenous metabolites with difference and pathways
The endogenous metabolites with difference in plasma of mice were speculated by using the “ID browser” in MPP software, which helps us analyze the small molecule metabolites of significant differences (t-test, P < 0.05), HMDB, Chemical Book, METLIN, KEGG, and other databases provided the information about these compounds. Finally, six endogenous metabolites related to the promoting gastrointestinal motility effect of FAF were identified [Table 1], involving tryptophan metabolism, corticosterone metabolism, sphingolipid metabolism, and other pathways. The connections between the various compounds are shown in Figure 4.
Table 1.
Mass spectrometry/mass spectrometry identification results of endogenous metabolites with difference
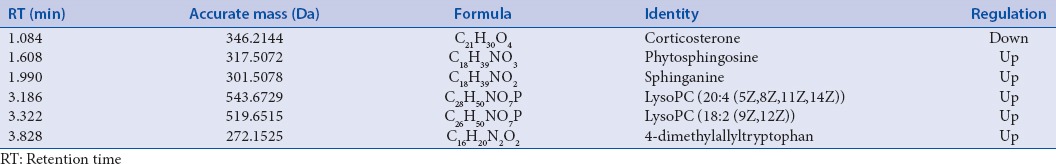
Figure 4.
Correlation networks of endogenous metabolites with difference
Determination of inorganic elements
The metal ions with difference in serum of mice were detected with ICP-MS. The content of Ca2+ and Mg2+ in FAF was lower than that in control group (P < 0.05), while Zn2+ was higher, and with significant differences (P < 0.05). Moreover, the trend of content change was the same as domperidone [Figure 5].
Figure 5.
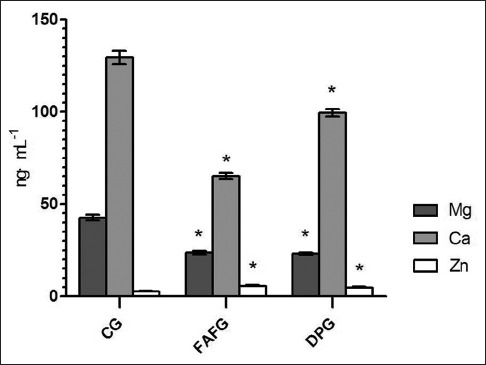
Results of inorganic elements in serum with significant differences. Data are expressed as means ± standard deviation. *P < 0.05 compared to control group. CG: Control group; FAFG: Fructus Aurantii flavonoids group; DPG: Domperidone group
DISCUSSION
In this study, based on the outstanding gastrointestinal motility promoting effect on mice, ten dominating components of FAF including neoeriocitrin, narirutin, rhoifolin, naringin, hesperidin, neohesperidin, neoponcirin, naringenin, hesperetin and nobiletin accounting for more than 86% of FAF were identified, which illustrated the material basis of FAF promoting gastrointestinal motility. Furthermore, the mechanism of action were speculated as follows:
4-Dimethylallyltryptophan is formed from tryptophan and dimethylallyl pyrophosphate by the action of dimethylallylpyrophosphate: L-tryptophan dimethylallyltransferase.[26] Moreover, the reaction between 4-Dimethylallyltryptophan and L-tryptophan is reversible. In this experiment, after orally administrated FAF, the content of 4-Dimethylallyltryptophan in serum was increased, according to the principle of chemical reaction, it was speculated that tryptophan can be up-regulated. In the presence of tryptophan hydroxylase, tryptophan can generate 5-HT, who is important neurotransmitter and adjacent secreted signaling molecule involves in both regulation of intestinal peristalsis and secretion function.[27] More than 95% of 5-HT present in the gastrointestinal tract, when 5-HT binds to 5-HT4 receptors on smooth muscle, it can cause gastrointestinal smooth muscle contraction through G protein coupling couplet of signal transduction.[28] FAF may through tryptophan metabolism pathway to perform its gastrointestinal prokinetic efficacy.
Besides that, it is reported that metal enzyme formed by tryptophan metabolite combining with metal ions possesses the ability of recognizing.[29,30] Therefore, ICP-MS method was applied in this experiment to determinate the inorganic ion content of mice serum in FAF groups. When compared with the control group, the content of Zn2+ was increased. High concentration of Zn2+ can accelerate the rate of gastric emptying,[31] which verified the tryptophan metabolism pathway of FAF promoting gastrointestinal dynamics. In our previous study,[32] with the same animal model after orally administrated FAF, the content of Ca2+ in the tissue of small intestine was increased significantly, while in this experiment, Ca2+ in serum was decreased sharply, which indicate that there exists distinction transforming the relationship between tissue and serum. The concentration of Ca2+ and Mg2+ in serum of FAF groups was lower than that in control group, which means higher concentration in small intestine tissue, activate Ca2+-ATPase and Mg2+-ATPase, influence distal gastric smooth muscle,[33,34] thus accelerate gastric bowel peristalsis, and the trend of regulation is the same as domperidone.
Corticosterone is an adrenocortical steroid that has modest but significant activities as a mineralocorticoid and a glucocorticoid. Stress/anxiety can activate hypothalamic-pituitary-adrenal axis, which induces the releasing of corticotropin-releasing factor in centrum and then increasing the content of corticosterone in blood.[35] FAF can down-regulate the content of corticosterone in serum, by influencing the mental state of mice to regulate gastrointestinal dysfunction.
Sphingolipids are important structural components of cellular membranes, during the metabolism of sphingolipids, there exists transforming the relationship between sphinganine, ceramides, sphingosine, sphingosine 1-phosphate and so on.[36] It is reported that altered sphingolipid composition can affect the contractility of gastric smooth muscle.[37] After taken FAF, the content of sphinganine and phytosphingosine in serum were up-regulated in mice, indicating FAF may regulate the potential biomarkers related to the sphingolipid metabolism pathway to affect the gastrointestinal peristalsis.
LysoPC has been proved to be a chemoattractant of T-lymphocytes.[38] In this experiment, LysoPC (20:4(5Z,8Z,11Z,14Z)) and LysoPC (18:2(9Z,12Z)) in serum of FAF groups were increased, from which can speculate that it may through acting on T-cells, to promote antibody formation and macrophages stimulus, therefore affects body's inflammatory state and enhances the immunity. That is helpful for the recovery of sick body.
Combined with the above discoveries, it is certificated that FAF can influence tryptophan metabolism, corticosterone metabolism, sphingolipid metabolism, and other pathways to present promoting gastrointestinal motility effect.
CONCLUSION
At present, disorders of gastrointestinal motility are common, gastroparesis, gastroesophageal reflux disease, functional dyspepsia, irritable bowel syndrome are all referring this kind of syndrome and bringing great bother to patients. FAFs are one of the main components of FA that possess gastrointestinal motility promoting efficacy. Herein, we illustrated the material basis of FAF containing neoeriocitrin, narirutin, rhoifolin, naringin, hesperidin, neohesperidin, neoponcirin, naringenin, hesperetin, and nobiletin. And from an organic and inorganic point of view, elaborated the mechanism of FAF promoting gastrointestinal motility preliminarily. The endogenous potential biomarkers in the blood of mice were analysed, in which 4-dimethylallyltryptophan, corticosterone, phytosphingosine, sphinganine, LysoPC (20:4(5Z, 8Z, 11Z, 14Z)), LysoPC (18:2(9Z, 12Z)), and Ca2+, Mg2+, Zn2+ metal ions had significant changes, involving tryptophan metabolism, corticosterone metabolism, sphingolipid metabolism and other pathways. This experiment provide valuable information for researching and developing new multi-component Chinese medicine curing gastrointestinal underpower associated diseases.
Financial support and sponsorship
The General Project of Liaoning Province Department of Education (No. L201605).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Valentin N, Acosta A, Camilleri M. Early investigational therapeutics for gastrointestinal motility disorders: From animal studies to phase II trials. Expert Opin Investig Drugs. 2015;24:769–79. doi: 10.1517/13543784.2015.1025132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikami DJ, Murayama KM. Physiology and pathogenesis of gastroesophageal reflux disease. Surg Clin North Am. 2015;95:515–25. doi: 10.1016/j.suc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Törnblom H, Simrén M, Abrahamsson H. Gastrointestinal motility and neurogastroenterology. Scand J Gastroenterol. 2015;50:685–97. doi: 10.3109/00365521.2015.1027265. [DOI] [PubMed] [Google Scholar]
- 4.Zorniak M, Waluga M, Hartleb M. Motility disorders, functional gastrointestinal disorders, inflammatory bowel disease and cardiac rhythm disturbances – is there a link? Review of literature. Curr Drug Targets. 2015;16:189–93. doi: 10.2174/1389450116666150304104453. [DOI] [PubMed] [Google Scholar]
- 5.Phan H, DeReese A, Day AJ, Carvalho M. The dual role of domperidone in gastroparesis and lactation. Int J Pharm Compd. 2014;18:203–7. [PubMed] [Google Scholar]
- 6.Buffery PJ, Strother RM. Domperidone safety: A mini-review of the science of QT prolongation and clinical implications of recent global regulatory recommendations. N Z Med J. 2015;128:66–74. [PubMed] [Google Scholar]
- 7.Cann PA, Read NW, Holdsworth CD. Galactorrhoea as side effect of domperidone. Br Med J (Clin Res Ed) 1983;286:1395–6. doi: 10.1136/bmj.286.6375.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen HF, Zhang WG, Yuan JB, Li YG, Yang SL, Yang WL. Simultaneous quantification of polymethoxylated flavones and coumarins in Fructus aurantii and Fructus aurantii immaturus using HPLC-ESI-MS/MS. J Pharm Biomed Anal. 2012;59:90–5. doi: 10.1016/j.jpba.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Pan MH, Lai CS, Lo CY, Dushenkov S, Ho CT. Isolation and syntheses of polymethoxyflavones and hydroxylated polymethoxyflavones as inhibitors of HL-60 cell lines. Bioorg Med Chem. 2007;15:3381–9. doi: 10.1016/j.bmc.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Dugo P, Mondello L, Dugo L, Stancanelli R, Dugo G. LC-MS for the identification of oxygen heterocyclic compounds in citrus essential oils. J Pharm Biomed Anal. 2000;24:147–54. doi: 10.1016/s0731-7085(00)00400-3. [DOI] [PubMed] [Google Scholar]
- 11.Li ZH, Chen HF, Luo LP, Yang B, Wei Y, Yuan JB, et al. Determination of the active constituents in aurantii fructus from Jiangxi province at different harvest time by HPLC. Zhong Yao Cai. 2013;36:28–31. [PubMed] [Google Scholar]
- 12.Jiang Y, Bai X, Zhu X, Li J. The effects of Fructus Aurantii extract on the 5-hydroxytryptamine and vasoactive intestinal peptide contents of the rat gastrointestinal tract. Pharm Biol. 2014;52:581–5. doi: 10.3109/13880209.2013.854396. [DOI] [PubMed] [Google Scholar]
- 13.Takase H, Yamamoto K, Hirano H, Saito Y, Yamashita A. Pharmacological profile of gastric mucosal protection by marmin and nobiletin from a traditional herbal medicine, Aurantii fructus immaturus. Jpn J Pharmacol. 1994;66:139–47. doi: 10.1254/jjp.66.139. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Chen J, Zhang J, Hu X, Zhou X, Lu Z, et al. Naringenin inhibits angiotensin II-induced vascular smooth muscle cells proliferation and migration and decreases neointimal hyperplasia in balloon injured rat carotid arteries through suppressing oxidative stress. Biol Pharm Bull. 2013;36:1549–55. doi: 10.1248/bpb.b13-00247. [DOI] [PubMed] [Google Scholar]
- 15.Teng JY, Meng XS, Han L, Pan Y, Bao YR, Guo XR. Study on extraction of naringin from Citrus aurantium. Chin J Exp Trad Med Formu (Chin) 2011;17:34–5. [Google Scholar]
- 16.Lin YS, Li S, Ho CT, Lo CY. Simultaneous analysis of six polymethoxyflavones and six 5-hydroxy-polymethoxyflavones by high performance liquid chromatography combined with linear ion trap mass spectrometry. J Agric Food Chem. 2012;60:12082–7. doi: 10.1021/jf303896q. [DOI] [PubMed] [Google Scholar]
- 17.Wu YP, Meng XS, Bao YR, Wang S, Kang TG. Simultaneous quantitative determination of nine active chemical compositions in traditional Chinese medicine Glycyrrhiza by RP-HPLC with full-time five-wavelength fusion method. Am J Chin Med. 2013;41:211–9. doi: 10.1142/S0192415X13500158. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Gao W, Hu X, Liu Z, Liu C. The influence of compatibility of traditional Chinese medicine on the pharmacokinetic of main components in Fructus aurantii. J Ethnopharmacol. 2012;144:277–83. doi: 10.1016/j.jep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Yong-Rui B, Xin-Xin Y, Shuai W, Xian-Sheng M, Rui-Qing Z, Yue-Ming X, et al. Study on the in vivo toxic mechanism of xixin based on trace elements determination by inductively coupled plasma-mass spectrometry. Pharmacogn Mag. 2014;10:141–6. doi: 10.4103/0973-1296.131025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nie H, Liu R, Yang Y, Bai Y, Guan Y, Qian D, et al. Lipid profiling of rat peritoneal surface layers by online normal- and reversed-phase 2D LC QToF-MS. J Lipid Res. 2010;51:2833–44. doi: 10.1194/jlr.D007567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin G, Liu C, Feng C, Fan Z, Dai Z, Lai C, et al. Metabolomic analysis reveals differences in umbilical vein plasma metabolites between normal and growth-restricted fetal pigs during late gestation. J Nutr. 2012;142:990–8. doi: 10.3945/jn.111.153411. [DOI] [PubMed] [Google Scholar]
- 22.Liu JL, Wang HL, Zhang LF, Xu YF, Deng W, Zhu H, et al. Metabonomics study of brain-specific human S100B transgenic mice by using high-performance liquid chromatography coupled with quadrupole time of flight mass spectrometry. Biol Pharm Bull. 2011;34:871–6. doi: 10.1248/bpb.34.871. [DOI] [PubMed] [Google Scholar]
- 23.Tong L, Zhou D, Gao J, Zhu Y, Sun H, Bi K. Simultaneous determination of naringin, hesperidin, neohesperidin, naringenin and hesperetin of Fractus aurantii extract in rat plasma by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal. 2012;58:58–64. doi: 10.1016/j.jpba.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Falkén Y, Webb DL, Abraham-Nordling M, Kressner U, Hellström PM, Näslund E. Intravenous ghrelin accelerates postoperative gastric emptying and time to first bowel movement in humans. Neurogastroenterol Motil. 2013;25:474–80. doi: 10.1111/nmo.12098. [DOI] [PubMed] [Google Scholar]
- 25.Hong L, Jiang W, Zheng W, Zeng S. HPLC analysis of para-aminosalicylic acid and its metabolite in plasma, cerebrospinal fluid and brain tissues. J Pharm Biomed Anal. 2011;54:1101–9. doi: 10.1016/j.jpba.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rilling HC. Dimethylallylpyrophosphate: L-tryptophan dimethylallyltransferase. Methods Enzymol. 1985;110:335–40. doi: 10.1016/s0076-6879(85)10091-1. [DOI] [PubMed] [Google Scholar]
- 27.Raybould HE, Cooke HJ, Christofi FL. Sensory mechanisms: Transmitters, modulators and reflexes. Neurogastroenterol Motil. 2004;16(Suppl 1):60–3. doi: 10.1111/j.1743-3150.2004.00477.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen BS, Liu SY. Effects of 5-hydroxytryptamine on smooth muscles. J Henan Univ Sci Tech Med Sci. 2004;22:312–4. [Google Scholar]
- 29.Sgambati SA, Zarif A, Basson MD. Octreotide differentially modulates human Caco-2 intestinal epithelial cell proliferation and differentiation by decreasing intracellular cAMP. Regul Pept. 1996;61:219–27. doi: 10.1016/0167-0115(95)00163-8. [DOI] [PubMed] [Google Scholar]
- 30.Matyska Liskova P, Fiser R, Macek P, Chmelik J, Sykora J, Bednarova L, et al. Probing the Ca(2+)-assisted p-p interaction during Ca(2+)-dependent protein folding. Soft Matter. 2016;12:531–41. doi: 10.1039/c5sm01796c. [DOI] [PubMed] [Google Scholar]
- 31.Broad J, Góralczyk A, Mannur K, Dukes GE, Sanger GJ. Drugs acting at 5-HT4, D2, motilin, and ghrelin receptors differ markedly in how they affect neuromuscular functions in human isolated stomach. Neurogastroenterol Motil. 2014;26:851–61. doi: 10.1111/nmo.12338. [DOI] [PubMed] [Google Scholar]
- 32.Cui YL, Meng XS, Bao YR, Wang S, Pan Y, Han L. Synergy effect of effective substances group and mechanism of Qi-Zhi Wei-Tong granules in promoting gastrointestinal dynamic effect. World Sci Tech. 2014;16:52–7. [Google Scholar]
- 33.Ai B, Yang CM. Synthesis and recognition of tryptophan-based receptor of transition metal ion. J Univ Jinan Nat Sci Ed. 2011;25:55–8. [Google Scholar]
- 34.Ogunbayo OA, Michelangeli F. Related flavonoids cause cooperative inhibition of the sarcoplasmic reticulum Ca2+ ATPase by multimode mechanisms. FEBS J. 2014;281:766–77. doi: 10.1111/febs.12621. [DOI] [PubMed] [Google Scholar]
- 35.Ao HQ, Xu ZW, Yan C, Su JF, Wang WZ. Effect of Chaihu Shugan powder and xiaoyao powder on serum corticosterone and gastrointestinal hormones of chronic multi-stress rats. Tradit Chin Drug Res Clin Pharm. 2007;18:288–291. [Google Scholar]
- 36.Blachnio-Zabielska A, Baranowski M, Wójcik B, Górski J. Reduction of ceramide de novo synthesis in solid tissues changes sphingolipid levels in rat plasma, erythrocytes and platelets. Adv Med Sci. 2016;61:72–7. doi: 10.1016/j.advms.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Choi S, Kim JA, Kim TH, Li HY, Shin KO, Lee YM, et al. Altering sphingolipid composition with aging induces contractile dysfunction of gastric smooth muscle via KCa1.1 upregulation. Aging Cell. 2015;14:982–94. doi: 10.1111/acel.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding X, Hu J, Li J, Zhang Y, Shui B, Ding Z, et al. Metabolomics analysis of collagen-induced arthritis in rats and interventional effects of oral tolerance. Anal Biochem. 2014;458:49–57. doi: 10.1016/j.ab.2014.04.035. [DOI] [PubMed] [Google Scholar]


