Abstract
Background:
Sepsis plays an important role in acute gastrointestinal injury (AGI). Our research was designed to determine the effects of omega-3 fish oil (FO) in patients suffering from severe sepsis combined with AGI III, and the ability of FO to modulate immune function.
Methods:
Seventy-eight patients diagnosed with severe sepsis with AGI III and a need for mechanical ventilation were randomized to two groups. In the FO group, 50 g of long chain fatty acid soybean oil (n = 6) and 10 g of FO (n = 3) were administered as total parenteral nutrition (TPN). The control group was treated with 50 g of long chain fatty acid soybean oil without addition of FO to TPN.
Results:
At baseline, there were no significant differences between the two groups. The 60-day mortality was lower in the FO group. Multiple factor logistic regression analysis revealed that intra-abdominal pressure (IAP) and abdominal infection were correlated with the FO intervention. The patients with abdominal infection demonstrated a lower mortality rate, fewer CD3 T lymphocytes, and fewer helper/inducer T lymphocytes in the FO group compared with the control group. After 7 days, the Marshall Score was lower in the FO group than in the control group.
Conclusion:
FO has positive effects in terms of improving the long-term prognosis of patients with severe sepsis with AGI III. Patients with a high IAP and abdominal infection might experience greater benefit from FO. This effect might be due, in part, to immunomodulation.
SUMMARY
Fish oil (FO) has positive effects in terms of improving the long-term prognosis of patients with severe sepsis with acute gastrointestinal injury Grade III
Patients with a high intra-abdominal pressure and abdominal infection might experience greater benefit from FO
This effect might be due, in part, to immunomodulation.
Abbreviations used: AGI: Acute gastrointestinal injury; FO: Fish oil; TPN: Total parenteral nutrition; IAP: Intra-abdominal pressure; ICU: Intensive Care Unit; MODS: Multiple organ dysfunction syndrome; TLR4: Toll-like receptor 4; DNR: Do Not Resuscitate; WGAP: Working Group of Abdominal Problem; EN: Enteral nutrition; BP: Low blood pressure; CRI: Catheter-related infection; PBS: Phosphate-buffered saline; ELFA: Enzyme-linked fluorescent assay; SD: Standard deviation; PUFAs: Polyunsaturated fatty acids; EPA: Eicosapentenoic acid; DHA: Docosahexaenoic acid.
Keywords: Acute gastrointestinal injury, multiple organ dysfunction syndrome, omega-3 fish oil, severe sepsis
INTRODUCTION
Sepsis is a common clinical syndrome caused by severe injury, infection, or major surgery, and it is a leading cause of morbidity and mortality in the Intensive Care Unit (ICU).[1,2] The annual incidence of sepsis in developed countries has increased from 8% to 13% in the past decade, with 750 thousand cases in the United States and 13.5 thousand cases in Europe. In developing countries, the death rate from sepsis is extremely high due to poverty and malnutrition, among others.[3] The pathogenesis of sepsis is complicated, and studies have shown that some organs and tissues are damaged in the early stage of sepsis, whereas severe sepsis, septic shock, or multiple organ dysfunction syndrome (MODS) occurs during later stages of sepsis.[2,4]
Critically ill patients may suffer an acute gastrointestinal injury (AGI), in which the patient's acute disease causes gastrointestinal dysfunction.[5,6] Research has shown that the gastrointestinal tract plays an important role in the progression of sepsis. The gastrointestinal mucosal barrier is damaged by septic inflammation. A large number of toxins and bacteria are then translocated through the blood through the portal vein and lymphatic system, ultimately resulting in MODS.
Recent studies investigating the pathogenesis of sepsis have demonstrated that excessive levels of cytokines and inflammatory mediators are released, establishing a foundation for sepsis.[1,4] Therapies for sepsis typically include the control of infection, improved organ perfusion, and immunomodulation. Among all therapeutic strategies, immunomodulation through the use of omega-3 fish oil (FO) has received increasing attention. FO modulates the immunological response to sepsis through several pathways. It may also decrease the expression of inflammatory factors.[7] FO can inhibit Toll-like receptor 4 (TLR4) to block the TLR signaling pathway,[8] and it can also block the nuclear factor kappa B pathway. Tsou et al. have confirmed that the number of CD4 lymphocytes in peripheral blood and the ratio of CD4 to CD8 cells increased in response to FO pretreatment in a rat model of sepsis.[9] FO may also have organ-protective functions. FO has been shown to diminish the requirement for renal replacement and to improve the function of the liver and pancreas.[10,11]
In 2012, a working guideline was developed by the Working Group of Abdominal Problem (WGAP) to define and grade AGI.[6] In the ICU, the mortality rate of septic patients with intestinal dysfunction (AGI Grade III) is extremely high. Thus, in the present study, we aimed to determine whether omega-3 fatty acid can decrease the mortality of patients suffering from severe sepsis with AGI Grade III. This study is registered on the Chinese Clinical Trial Register Network (http://www.chictr.org/, number: ChiCTR-ONRC-12002464) in 2012.
MATERIALS AND METHODS
Study population
The inclusion criteria were as follows: (1) age of 18 years or older; (2) severe systemic inflammatory response syndrome (SIRS) induced by severe infection, with the need for mechanical ventilation; (3) Marshall score >3; (4) intestinal dysfunction with AGI Grade III, including abdominal infection, abdominal surgery, or intestinal surgery; and (5) severe infection requiring carbapenems after admission to the ICU. The exclusion criteria were as follows: (1) Marshall score ≥20; (2) life expectancy < 28 days due to chronic or incurable disease such as uncontrolled cancer; (3) life expectancy < 24 h or discharge; (4) ICU stay <7 days after meeting the inclusion criteria; (5) signing of the Do Not Resuscitate paper; (6) hematological or rheumatic disease; and (7) severe liver dysfunction (Child-Pugh score >10 or Grade C liver function).
Definition
The diagnostic criteria for sepsis were based on the definition of the International Sepsis Forum.[12] In accordance with the criteria of the Europe WGAP, AGI Grade III was defined as the loss and difficulty of recovering gastrointestinal function. Furthermore, the condition of the patient could not be improved despite general interventions. Patients are presented as intolerant to enteral nutrition, which can induce continued or even complete deterioration of MODS. Septic shock is defined as severe sepsis plus persistently low blood pressure (BP) despite the administration of intravenous fluids. Low BP was defined as follows: (1) systolic BP (SBP) <90 mm of mercury (mmHg) or diastolic BP <60 mmHg; (2) mean BP <70 mmHg; (3) a reduction of SBP >40 mmHg, or a decrease of >2 standard deviation (SD) by age; and (4) exclusion of the other causes of low BP.[13] Catheter-related infection (CRI) included definitive diagnosis, clinical diagnosis, and suspected diagnosis, according to the prevention and treatment guidelines of the inter-vessel CRI of China.[14] The criteria for definitive diagnosis included the following: (1) positive results for semi-quantitative blood cultures from the central venous catheter; (2) both positive blood cultures from the central venous and peripheral catheters, with a positive central venous catheter blood culture obtained at least 2 h before the peripheral blood culture; and (3) the presence of the same microorganisms in both blood cultures. The criteria for the clinical diagnosis included the following: (1) a positive result for the catheter blood culture with a negative result for the peripheral blood culture and (2) the disappearance of infection symptoms 48 h after catheter removal without the use of additional antibiotics. A suspected diagnosis was defined as new symptoms of infection that disappeared after catheter removal.
Treatment protocols and intervention
All patients received early goal-directed fluid resuscitation, mechanical ventilation, and carbapenem medication. Antibiotics were adjusted according to the drug resistance test results. Carbapenems included imipenem/cilastatin (1.0 g, q8h), meropenem (2 g, q8h), or biapenem (0.3 g q6h). Glycopeptides included vancomycin (1.0 g, q12h), teicoplanin (initial dose of 0.4 g, q12h × 2 times, followed by 0.4 g, qd; or linezolid 600 mg, q12h). Anti-fungal drugs included fluconazole (0.6 g, qd), voriconazole (initial dose of 0.4 g, q12h 2 times, followed by 0.2 g, q12h), or caspofungin (initial dose of 70 mg, followed by 50 mg qd). The dose was adjusted based on the creatinine clearance rate. Blood glucose was controlled between 8 and 10 mmol/L. Other complications were precluded by the therapies. Patients were treated according to the SSC guidelines[13] and randomized into two groups. In the FO group, 100 mL/day of FO (n = 3, containing 10 g refined FO) was continuously injected in the vein over 24 h as total parenteral nutrition (TPN). The FO was the product of Fresenius Kabi Austria GmbH, and it was repacked by Sino-Swed Pharmaceutical Corp., Ltd. To the TPN, 50 g of long-chain fatty acid soybean oil (n = 6) was added. The proportion of n = 6/n = 3 was 5:1. All patients received 20 kcal/kg each day during the first 7 days, following admission to the ICU. After stabilization of the circulation, the caloric count was slowly increased to 30 kcal/kg/day after 7 days, but n = 6/n = 3 remained unchanged. As the control group, 50 g of long-chain fatty acid soybean oil without FO was added to the TPN. The caloric intake was calculated according to the body weight of the patient.
Randomization
Patients were assigned based on a random number table. Designers, researchers, and patients were blinded. Nurses who did not participate in the data assessment were not blinded to the grouping and medication.
Study outcomes
Age, gender, and inter-abdominal pressure (IAP) were recorded. Values were documented on the 1st and 7th day for the 28-day and 60-day mortality, mechanical ventilation time (day), length of stay in the ICU (days), and APACHE II and Marshall scores. Laboratory outcomes included the number of leukocytes (white blood cells [WBCs]) and the levels of hypersensitive C-reactive protein (hsCRP), serum procalcitonin (PCT), immunoglobulin (IgG, IgA, IgM), and complement (C3 and C4) on the 1st and 7th day of treatment. The percentages of CD3 lymphocytes, ratio of helper to inducer T-lymphocytes, ratio of CD3 to CD19 B lymphocytes, and CD3, CD19, and CD56 natural killer cells (NK cells) on the 1st and the 7th day of treatment were measured by flow cytometry. The ratio of CD4 to CD8 cells was also calculated automatically by flow cytometry.
Measurement of lymphocyte subsets
A BD FACSCanto™ II flow cytometer was used. Two milliliters of the blood sample was anticoagulated with heparin, from which 50 μL was used for flow cytometry analysis. Twenty microliters of fluorescent monoclonal antibody was added to the sample, mixed uniformly, and incubated for 20 min at room temperature. Two milliliters of hemolysin was added to the sample and incubated for 10 min. The sample was then centrifuged for 5 min at 1000 rpm, and then, the serum was discarded. Next, the sample was resuspended in 2 mL of phosphate-buffered saline (PBS) and then centrifuged for 5 min at 1000 rpm. This process was repeated twice. The sample was then resuspended in 500 μL of PBS and assessed by flow cytometry.
Measurement of procalcitonin
PCT was measured using a PCT kit from bioMerieux Industry. A bioMerieux-mini-VIDAS immunological analysis meter was used for detection. The surface plasmon resonance (SPR) fluid was used immediately, and it contained the marine monoclonal Ig antibody PCT.
Procalcitonin contrast solution
Two kinds of contrast solution (C1 and C2, freeze-dried) were provided in the kit. They were resuspended in 2 mL of distilled water and combined after 5–10 min.
Procalcitonin calibration solution
Two kinds of calibration solution (S1 and S2, freeze drying) were provided in the kit. They were resuspended in 2 mL of distilled water and combined after 5–10 min. The blood samples (3 mL) were anticoagulated with heparin lithium. The blood samples, PCT contrast solution, and calibration solution were added to a PCT slice and a solid phase pipette. In the machine, “PCT” was selected as the detection code. Two hundred milliliters of calibration solution, contrast solution, and sample were added to the sample, and then, the SPRs and slices were immunostained before automatic PCT measurement. The one-stage immunological sandwich assay and enzyme-linked fluorescent assay were used together for the assessment.
Ethics
This study was approved by the Medical Ethics Committee of Shenzhen People's Hospital. The trial is registered at the Chinese Clinical Trial Registry (http://www.chictr.org/) under ChiCTR-ONRC-12002464. This study was conducted according to the Declaration of Helsinki, and written informed consent was obtained from all patients.
Sample size and statistical analysis
The total sample size required 60 cases (at least 30 cases per group), which was calculated according to our previous study. Quantitative data were reported as the mean ± SD or median and were compared using an independent sample Student's t-test. For data that were nonnormally distributed, means and medians were compared using Mann–Whitney U-test. Qualitative data, such as gender, cases with abdominal infection or abdominal surgery, treatments, and mortality, were compared using Chi-square test. Multiple factor logistic regression analyses were performed if the factor was closely correlated with the clinical prognosis or P < 0.05. Abdominal infection was analyzed as the subgroup. P < 0.05 was considered statistically significant. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) for Windows was used to analyze the data.
RESULTS
There were no significant differences in the main diagnostic indexes, clinical characteristics, or baseline laboratory results between the fish oil and control group
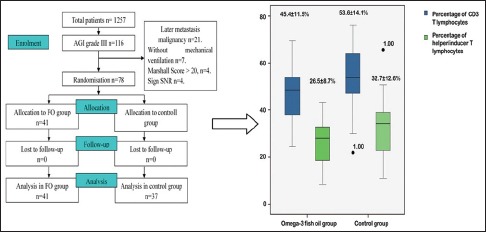
Seventy-eight cases were recruited for the study from August 2012 to July 2014. The patients were included in the FO group (41 cases) or the control group (37 cases) [Figure 1]. The main diagnoses are listed in Table 1. Gender, age, abdominal infection, abdominal surgery, IAP, and antibiotics did not differ between the groups [Table 2]. Laboratory results were also comparable between the groups [Table 3]. The positions and locations of the identified microbes are shown in the supplementary file.
Figure 1.
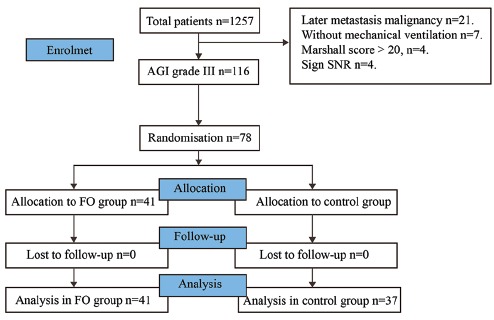
Patients included in the analysis
Table 1.
There were no significant differences in main diagnosis indexes between fish oil and control group
Table 2.
There were no significant differences in clinical characteristics between fish oil and control group
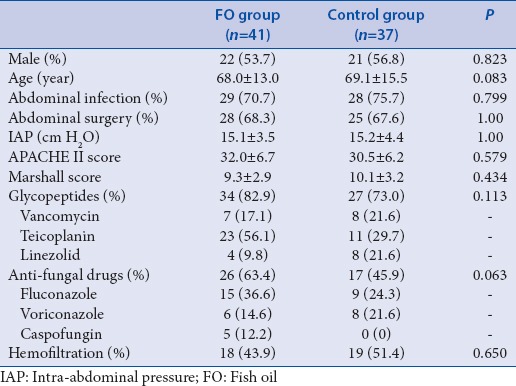
Table 3.
There were no significant differences in baseline laboratory results between fish oil and control group
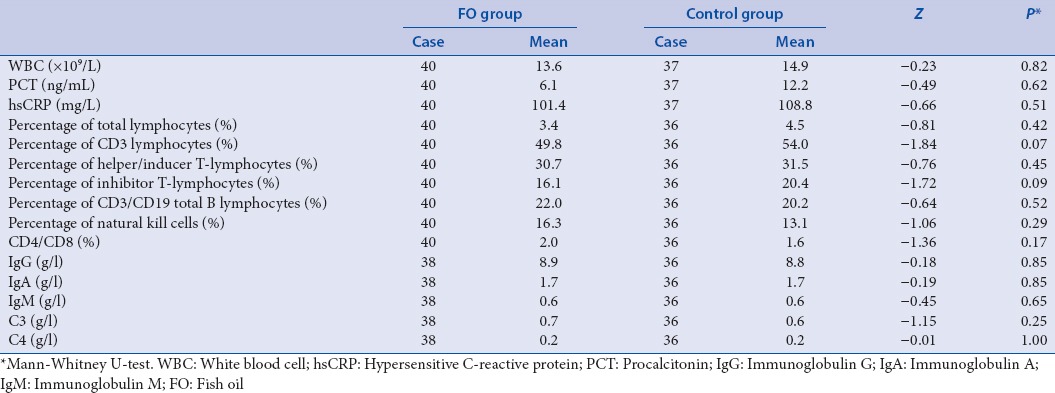
The 60-day mortality rate in the fish oil group was significantly improved compared with the control group
In the 28-day mortality study, 10 cases (of 41) in the FO and 15 cases (of 37) in the control group died. In the 60-day mortality study, 11 cases (of 41) in the FO group and 18 cases (of 37) in the control group died. The 60-day mortality rate was significantly improved in the FO group compared with the control group [Table 4]. The relationship between 60-day mortality and FO was analyzed by multiple factor logistic regression. The result revealed a correlation among IAP, abdominal infection, and FO intervention [Table 5].
Table 4.
The 60-day mortality rate of fish oil group was significantly lower than the control group
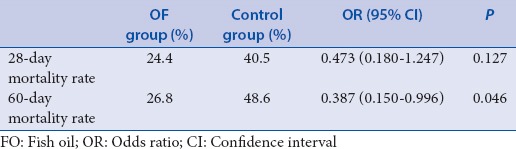
Table 5.
Intra-abdominal pressure, abdominal infection were relevant to the statistical grouping
The clinical outcomes were not significantly different between the fish oil and control group
The length of stay in the ICU and days of mechanical ventilation were similar in the two groups. In addition, the APACHE II Scores and Marshall scores on the 1st and 7th day were not significantly different. On day 7, there were no differences in total lymphocytes, CD3 T-lymphocytes, helper/inducer T-lymphocytes, inhibit T-lymphocytes, CD3/CD19 total B lymphocytes, NK cell, CD4/CD8, serum IgG, IgA, or IgM, serum complement C3 or C4, WBC, or hsCRP (P > 0.05, two independent sample t-test). The PCT levels in the two groups on day 7 were also similar (z = −0.83, P = 0.40, Mann–Whitney U-test).
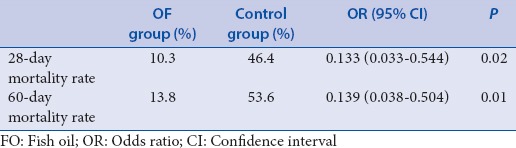
The mortality rate in the fish oil group was significantly improved compared with that in the control group in patients with abdominal infection
Fifty-seven cases (73%) suffered abdominal infection, of whom 29 were in the FO group and the remaining 28 in the control group. In 28 days, 3 patients died in the FO group compared with the 13 patients in the control group. In the 60-day mortality test, 4 patients died in the FO group compared with 13 in the control group. These differences were statistically significant [Table 6].
Table 6.
The 28-days and 60-day mortality rate of fish oil group was significantly lower than the control group in patients with abdominal infection
T-lymphocytes and helper/inducer T-lymphocyte counts and the Marshall score were significantly lower in the fish oil group compared with the control group
The length of stay in the ICU and the days of mechanical ventilation were similar between the two groups (P = 0.79 and P = 0.67, respectively, Mann–Whitney U-test). There were no statistical differences in lymphocytes, immunology, or complement (P > 0.05). The numbers of T-lymphocytes and helper/inducer T-lymphocytes were lower in the FO group compared with the control group [Figure 2]. The difference in APACHE II Scores on the 7th day was not significant (P = 0.19, t-test). Other baseline outcomes were similar (all P > 0.05).
Figure 2.
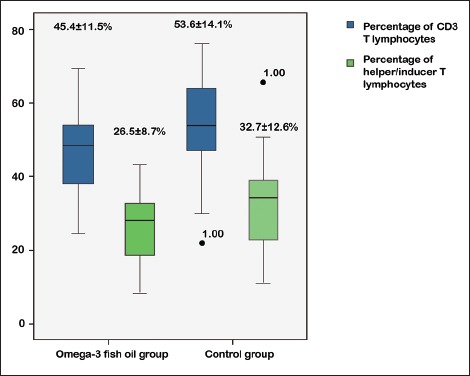
Subgroup analysis showing fewer baseline CD3 T-lymphocytes (mean different −8.21, 95% confidence interval [−15.11 to −1.31]) and helper/inducer T-lymphocytes (mean different −6.22, 95% confidence interval [−12.02 to −0.42]) in patients with abdominal infection in the fish oil group compared with the control group
However, the Marshall score was significantly lower in the FO group than in the control group (3.9 ± 2.7 vs. 6.1 ± 4.4, mean different − 2.22 [−4.14 ~ −0.29]).
DISCUSSION
Multiple factors could underlie and exacerbate the progression of AGI,[15] of which one of the most important factors is sepsis. Sepsis is a specific kind of SIRS that is induced in response to severe infection. When SIRS is uncontrolled, abnormal tissue perfusion occurs, which ultimately leads to MODS. Data have shown that MODS scores parallel the severity of SIRS.[16] Previous research has also demonstrated that thrombus embolism in the gastrointestinal microcirculation can be induced by endotoxemia.[17] In return, AGI can result in deterioration of the ectopic intestinal flora and lymphocyte apoptosis, inhibiting the immune functions of lymphocytes.[18]
FO contains abundance of polyunsaturated fatty acids (PUFAs), including eicosapentaenoic acid and docosahexaenoic acid. It is a nutritional substrate and also an important inhibitor of inflammation. FO can modify membrane lipid rafts by replacing n = 6 with n = 3 PUFA in the cell membrane, which in turn reduces the production of the n = 6 PUFA source to alkanoic acid. Thus, the fluidity of the lymphocyte membrane is protected and stabilized. A certain proportion of n = 3 and n = 6 is considered to be anti-inflammatory. Previous research has suggested that 1:2 ~ 1:4 would be a better proportion for preventing inflammatory reactions.[19] In our study, a proportion of 1:5 was used. A positive clinical effect has also been reported in patients with severe sepsis with AGI Grade III. However, there is no evidence demonstrating that enteral supplements of FO improve the prognosis of severe inflammatory reactions.[20] Because of the design of our study, parenteral nutrition was the only choice to supply calories, and therefore, we estimated the effects of the FO supplied by parenteral supplementation. The results obtained for the FO group revealed a better 60-day mortality rate compared with the control group. Patients, who are suffering from high IAP combined with abdominal infection, may obtain a greater benefit from the addition of FO parenteral therapy. Recent animal experiments have demonstrated that FO parenteral supplementation reduces Bax mRNA expression, increases Bcl2 mRNA expression, promotes the proliferation of intestinal epidermal cells, lengthens the villus, and inhibits apoptosis.[21] Thus, among all administration routes, patients are most likely to benefit from parenteral supplementation with FO.
In the present study, we observed a better 28- and 60-day mortality rate in the subgroup with abdominal infection that received FO. On day 7, the Marshall score was also lower in the FO group. These findings suggested that FO could either prevent organ failure or protect organs against failure. This result is consistent with the findings of a previous study.[22] Different degrees of abnormal T-lymphocytes were observed before FO supplementation, but the difference between the FO and control groups disappeared on day 7, which suggested that FO could improve the function of T-lymphocytes, potentially by modifying lipid rafts and affecting the fluidity of the lymphocyte plasma membrane.[23] FO supplementation has demonstrated negative clinical outcomes in previous cohort studies, but the gastrointestinal functions were not clearly graded,[24] which could have a significant influence on prognosis according to our previous study.
Previous studies have suggested that FO can reduce the levels of some inflammatory factors; however, we did not detect significant differences in leukocytes, hsCRP, or PCT between the two groups. Serum PCT levels have also been shown to be reduced by FO in children who underwent cardiac surgery or esophageal carcinoma surgery. This finding indicates that FO may have a beneficial effect on the control of infection and reduce CRP and the sequential organ failure assessment score[22] in patients with severe sepsis. However, a meta-analysis did not demonstrate an effect of FO on the levels of interleukin-6 or tumor necrosis factor-α, both of which are overexpressed during sepsis.[25] Thus, additional trials are needed to confirm whether FO can inhibit the production of inflammatory factors.[26]
CONCLUSION
In this study, we discovered that omega-3 FO fatty acid can improve the long-term prognosis of patients suffering from severe sepsis with AGI Grade III. Patients with high IAP and abdominal infection might obtain a greater benefit from FO. The effects of FO may be due, in part, to the modulation of immune functions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Jastrzebski J, Zlotorowicz M. Severe sepsis and septic shock. Przegl Epidemiol. 2001;55(Suppl 3):10–4. [PubMed] [Google Scholar]
- 3.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: An increasing global concern. Lancet. 2013;382:170–9. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 4.Adib-Conquy M, Cavaillon JM. Stress molecules in sepsis and systemic inflammatory response syndrome. FEBS Lett. 2007;581:3723–33. doi: 10.1016/j.febslet.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 5.Puleo F, Arvanitakis M, Van Gossum A, Preiser JC. Gut failure in the ICU. Semin Respir Crit Care Med. 2011;32:626–38. doi: 10.1055/s-0031-1287871. [DOI] [PubMed] [Google Scholar]
- 6.Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, et al. Gastrointestinal function in intensive care patients: Terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384–94. doi: 10.1007/s00134-011-2459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer K, Gokorsch S, Fegbeutel C, Hattar K, Rosseau S, Walmrath D, et al. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am J Respir Crit Care Med. 2003;167:1321–8. doi: 10.1164/rccm.200207-674OC. [DOI] [PubMed] [Google Scholar]
- 8.Singer P, Shapiro H, Theilla M, Anbar R, Singer J, Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: Novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34:1580–92. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 9.Tsou SS, Chiu WC, Yeh CL, Hou YC, Yeh SL. Effects of omega-3 fatty acids on inflammatory mediators and splenocyte cytokine mRNA expressions in rats with polymicrobial sepsis. Nutrition. 2008;24:484–91. doi: 10.1016/j.nut.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Fassett RG, Gobe GC, Peake JM, Coombes JS. Omega-3 polyunsaturated fatty acids in the treatment of kidney disease. Am J Kidney Dis. 2010;56:728–42. doi: 10.1053/j.ajkd.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Heller AR, Rössel T, Gottschlich B, Tiebel O, Menschikowski M, Litz RJ, et al. Omega-3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int J Cancer. 2004;111:611–6. doi: 10.1002/ijc.20291. [DOI] [PubMed] [Google Scholar]
- 12.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, et al. The Surviving Sepsis Campaign: Results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36:222–31. doi: 10.1007/s00134-009-1738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group; CMAoRDBAS, Branch; CMAoGM. Chinese guideline for prevention and treatment of intravascular catheter-related infection. Chin J Pract Intern Med. 2013;36:615–22. [Google Scholar]
- 15.Stefaniak J, Baron DM, Metnitz PG, Kramer L. Disturbances of gastrointestinal motility in Intensive Care Units. Anasthesiol Intensivmed Notfallmed Schmerzther. 2010;45:696–706. doi: 10.1055/s-0030-1268871. [DOI] [PubMed] [Google Scholar]
- 16.Talmor M, Hydo L, Barie PS. Relationship of systemic inflammatory response syndrome to organ dysfunction, length of stay, and mortality in critical surgical illness: Effect of Intensive Care Unit resuscitation. Arch Surg. 1999;134:81–7. doi: 10.1001/archsurg.134.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Li A, Weng YB, Duan ML, Wang BE, Zhang SW. Changes in intestinal mucosal immune barrier in rats with endotoxemia. World J Gastroenterol. 2009;15:5843–50. doi: 10.3748/wjg.15.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin JM, Stapleton RD. Omega-3 fatty acids in critical illness. Nutr Rev. 2010;68:531–41. doi: 10.1111/j.1753-4887.2010.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer K, Seeger W. Fish oil in critical illness. Curr Opin Clin Nutr Metab Care. 2008;11:121–7. doi: 10.1097/MCO.0b013e3282f4cdc6. [DOI] [PubMed] [Google Scholar]
- 20.Zhu D, Zhang Y, Li S, Gan L, Feng H, Nie W. Enteral omega-3 fatty acid supplementation in adult patients with acute respiratory distress syndrome: A systematic review of randomized controlled trials with meta-analysis and trial sequential analysis. Intensive Care Med. 2014;40:504–12. doi: 10.1007/s00134-014-3244-5. [DOI] [PubMed] [Google Scholar]
- 21.Sukhotnik I, Shany A, Bashenko Y, Hayari L, Chemodanov E, Mogilner J, et al. Parenteral but not enteral omega-3 fatty acids (Omegaven) modulate intestinal regrowth after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr. 2010;34:503–12. doi: 10.1177/0148607110362586. [DOI] [PubMed] [Google Scholar]
- 22.Hall TC, Bilku DK, Al-Leswas D, Neal CP, Horst C, Cooke J, et al. A randomized controlled trial investigating the effects of parenteral fish oil on survival outcomes in critically ill patients with sepsis: A pilot study. JPEN J Parenter Enteral Nutr. 2015;39:301–12. doi: 10.1177/0148607113518945. [DOI] [PubMed] [Google Scholar]
- 23.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–8. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Wohlmuth C, Dünser MW, Wurzinger B, Deutinger M, Ulmer H, Torgersen C, et al. Early fish oil supplementation and organ failure in patients with septic shock from abdominal infections: A propensity-matched cohort study. JPEN J Parenter Enteral Nutr. 2010;34:431–7. doi: 10.1177/0148607110362764. [DOI] [PubMed] [Google Scholar]
- 25.Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–70. doi: 10.1017/S0007114512001559. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhry H, Zhou J, Zhong Y, Ali MM, McGuire F, Nagarkatti PS, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo. 2013;27:669–84. [PMC free article] [PubMed] [Google Scholar]


