Abstract
Background:
Fuzi–Gancao herb couple is one of the commonly used herb couples involved in the traditional Chinese medicine formulations, with radix aconiti lateralis (Fuzi in Chinese) and radix glycyrrhizae (Gancao in Chinese) as a ratio of 1:1. Alkaloids and flavonoids were considered as the main active ingredients of Fuzi–Gancao herb couple. However, no analytical methods have been reported to quantitatively analyze these activity ingredients in Fuzi–Gancao herb couple simultaneously.
Objective:
To develop a simple, rapid, and sensitive UHPLC-MS/MS method for simultaneous quantitation of six alkaloid and three flavonoid compounds, namely, aconitine (AC), mesaconitine (MA), hypaconitine (HA), benzoylaconitine (BAC), benzoylmesaconitine (BMA), benzoylhypaconitine (BHA), liquiritin, isoliquiritin, and licochalcone A (LCA) in Fuzi–Gancao herb couple.
Materials and Methods:
The chromatographic separation was achieved using a reversed phase C18 column and a mobile phase consisted of water (0.1% formic acid in water, v/v) and acetonitrile. The detection was achieved in multiple reaction monitoring modes. The optimal mass transition ion pairs (m/z) for quantitation were 646.3/586.5 for AC, 632.4/572.5 for MA, 616.3/556.4 for HA, 604.5/572.5 for BAC, 590.4/540.4 for BMA, 574.1/542.5 for BHA, 419.4/257.1 for liquiritin, 419.4/257.1 for isoliquiritin, and 339.2/121.1 for LCA.
Results:
Good linearity was observed in validated concentration range for each analyte (r > 0.9992), and the intra- and inter-day precisions were <3.12% and 3.65%, respectively. The recovery ranged from 85.36% to 110.14%.
Conclusions:
The results demonstrated that the method developed was reliable, rapid, and specific. Moreover, this method was successfully applied to control the quality of Fuzi–Gancao herb couple.
SUMMARY
A simple, rapid, and sensitive UHPLC-MS/MS method for simultaneous quantitation of six alkaloid and three flavonoid compounds in Fuzi–Gancao herb couple has been developed.
Abbreviations used: UHPLC-ESI-MS/MS: Ultra High Performance Liquid Chromatography-electrospray ionization-mass spectrometry; RSD: Relative standard deviations; LOD: Limit of detection; LOQ: Limit of quantification; MRM: Multiple reaction monitor; TCMs: Traditional Chinese medicines.
Keywords: Alkaloid, flavonoid, radix aconiti lateralis, radix glycyrrhizae, UHPLC-MS/MS
INTRODUCTION
Herbal formula is the basis of traditional Chinese medicine (TCM), and multi-herb is the characteristic of formula which used as one component to treat disease according to the TCM theory.[1,2] However, quality control of the TCM formula is difficult because of a large amount of the ingredients, especially of the unknown components.[3] In the recent research, a special group named herb couple has been proposed based on the TCM theory. Herb couple is the unique combinations of two relatively fixed herbs in the clinic, and the two herbs in herb couple used together to increase efficacy and decrease toxicity.[4]
The combination of radix aconiti lateralis (Fuzi in Chinese) and radix glycyrrhizae (Gancao in Chinese), Fuzi–Gancao herb couple, is the commonly used herb couple involved in many TCM formulations such as Gancao-Fuzi decoction, Si-Ni decoction, and Fuzi-Lizhong pills. This herb couple, composed of two herbs as a ratio of 1:1 in weight, has been used for about 1700 years to treat rheumatoid arthritis and relieve joint pain.
The main active components found in Fuzi–Gancao herb couple are aconitum alkaloids in Fuzi and flavonoids in Gancao. Alkaloids were considered as the main ingredients in radix aconiti lateralis. Up to now, over 200 alkaloids had been isolated and identified from the plant which could be classified into four major categories as nonester alkaloids, monoester diterpene alkaloids (MDAs), diester diterpene alkaloids (DDAs), and lipoalkaloids, according to their chemical structure. Among of these four groups, both DDAs and MDAs exhibit excellent pharmacological effects, including anti-inflammatory, anti-allergic, analgesic, cardiotonic, and hepatoprotective activities.[5] Flavonoids were the main bioactive components in radix glycyrrhizae with activity of anti-inflammation, antibiosis, antioxidation, and anticancer.[6] In Chinese pharmacopoeia (2010), three DDAs, aconitine (AC), mesaconitine (MA), and hypaconitine (HA), and three MDAs, benzoylaconitine (BAC), benzoylmesaconitine (BMA), and benzoylhypaconitine (BHA), are used as the phytochemical markers for the quality control of radix aconiti lateralis. Liquiritin is the marker of radix glycyrrhizae with the same mass spectra properties but different in retention time in liquid chromatography of its isomer, isoliquiritin. In addition, licochalcone A (LCA) is also an important bioactivity flavonoid ingredient in radix glycyrrhizae. The above ingredients could also be used as major phytochemical markers to evaluate the quality of TCM prescription[7,8] [Figure 1].
Figure 1.
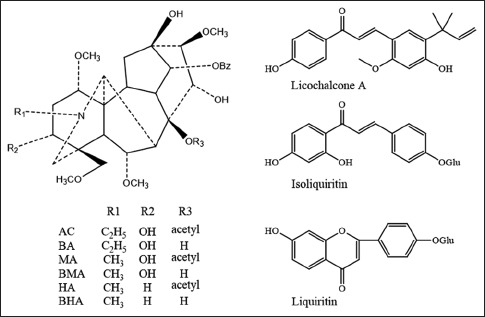
Chemical structures of aconitum alkaloids and flavonoids analyzed in Fuzi–Gancao herb couple
To minimize the variability of active ingredients in the herb couple and ensure repeatable and reproducible therapeutic effects, it is very important to establish quality control methodology for the herb couple; however, to the best of our knowledge, there is no report on determination of aconitum alkaloids and flavonoids in Fuzi–Gancao herb couple simultaneously. In this study, we describe a UHPLC-MS/MS assay with multiple reaction monitoring (MRM) for the simultaneously quantitative determination of three MDAs, three DDAs, and three flavonoids compounds in Fuzi–Gancao herb couple for the first time. It could help to evaluate the quality of this prescription and also provide quantification to ensure accuracy in the clinical application of Fuzi–Gancao herb couple.
MATERIALS AND METHODS
Chemicals and reagents
Acetonitrile (HPLC grade) was purchased from Merck (Germany); acetic acid (MS grade) and ammonium acetate were from Sigma-Aldrich. All other chemicals and solvents were of an analytical grade. Ultrapure water (18.2 MΩ) was prepared with a Milli-Q water purification system (Millipore, Bedford, MA, USA). The standard reference samples of AC, MA, HA, BAC, BMA, BHA, LCA, liquiritin, and isoliquiritin were purchased from the National Institute for Control of Pharmaceutical and Biological Products (Beijing, China). The purity of the standards was relatively high at no <98%. Radix aconiti lateralis was purchased from Huanggang Jingui Chinese Medicine industry development Co., Ltd., P.R. China. Radix glycyrrhizae was purchased from Anhui Yishengyuan Traditional Chinese Medicine Pellets Co., Ltd., P. R. China, and all the materials were identified by Dr. Xiao Fu, Department of Traditional Chinese Medicine, the First Affiliated Hospital of Liaoning Medical University.
Reference standards solutions
Stock solutions of AC, MA, HA, BAC, BMA, BHA, LCA, liquiritin, and isoliquiritin were prepared by dissolving the reference standards in methanol to give stock solutions at the concentration of 0.800, 0.724, 1.092, 0.916, 0.926, 0.256, 1.116, 0.542, and 0.780 mg/ml, respectively. All the stock solutions were stored at 4°C.
Extracts of Fuzi–Gancao herb couple
Radix aconiti lateralis (10.0 g) and radix glycyrrhizae (10.0 g) were ground and refluxed twice with distilled water (1:8, v/w) for 2 h and 1 h, respectively. After cooling to room temperature, the aqueous solution was obtained by filtration and concentrated under reduced pressure at 60°C to give 3.02 g of residue. The residue was dissolved and precipitated with 50 mL methanol-water solution (70%, v/v) and allowed to sit for 24 h at 4°C as the reported method with minor modification. The sample solution was centrifuged for 10 min at 3000 rpm to obtain the supernatant. 1 mL of the sample solution was filtered through a 0.22 μm membrane filter before injection.
UHPLC-MS analysis
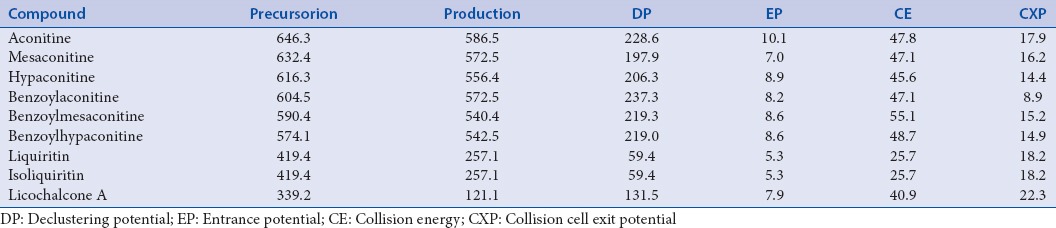
A Waters ACQUITY UPLC system coupled to an AB Sciex API 4000 triple quadrupole mass spectrometer system equipped with an electrospray ionization source and operated in positive ion mode was used for simultaneous determination of six aconitum alkaloids and three flavonoids in Fuzi–Gancao herb couple. Analyst 1.6.2 software (AB Sciex Pte. Ltd) was used for system control and data acquisition. The analytical was performed on an ACQUITY UPLC BEH C18 reverse phase column (2.1 × 100 mm, 1.7 μm). The gradient mobile phases consisted of water containing 0.1% formic acid (A) and acetonitrile (B) for gradient elution. The optimal gradient conditions were as follows: 0–5 min, 20%–40% B; 5–10 min, 40–80% B; 10–13 min, 80% B; 13–15 min, 80%–20% B. The column temperature was 30°C at the flow rate of 0.35 ml/min and injection volume of 10 μl. The optimized conditions for MS analysis were as follows: Capillary voltage 4000 V, drying gas flow rate 11 L/min, gas temperature 300°C, and nebulizer pressure 15 psi. MRM scan mode was selected for quantification. The precursor-to-product ion pair, declustering potential (DP), entrance potential (EP), collision energy (CE), and collision cell exit potential (CXP) for each analyte are listed in Table 1.
Table 1.
Mass spectra properties of nine phytochemical markers in Fuzi–Gancao herb couple
Method validation
The linear calibration curves of nine reference standards were constructed by external calibration method, plotting the peak areas versus the concentration of the analytes in triplicate. The limit of detection (LOD) and limit of quantification were determined in accordance to the baseline noise, considering a signal-to-noise ratio at 3 and 10, respectively. The precision and accuracy were determined by replicate analysis (n = 6) at three concentration levels on the same day (intra-day) and within three consecutive days (inter-day). Accuracy and precision were expressed by relative error and relative standard deviation (RSD), respectively. In the repeatability test, five independent sample solutions were prepared by the procedures noted in Fuzi–Gancao herb couple. The recovery tests were carried out by adding a known amount (approximately equivalent to 0.8, 1.0, and 1.2 times of the concentration of the original amount in the matrix) of mixed standards into a certain amount (0.5 g) of Fuzi–Gancao herb couple. Six replicates were performed for the test. The recoveries were calculated by the following equation: Recovery (%) = (total amount detected − amount original)/amount spiked × 100%.
RESULTS AND DISCUSSION
UHPLC-MS/MS analysis of marker chemicals in Fuzi–Gancao herb couple
The optimized resolution and peak shape were obtained with a mixture of consisted of water containing 0.1% formic acid (A) and acetonitrile (B) for gradient elution in 15 min after different mobile phases were selected such as water with different contents of formic acid, acetic acid, and ammonium formate. The typical UHPLC-MS/MS chromatograms of the nine phytochemical markers in the Fuzi–Gancao herb couple is shown in Figure 2. The detection was achieved in MRM mode.
Figure 2.
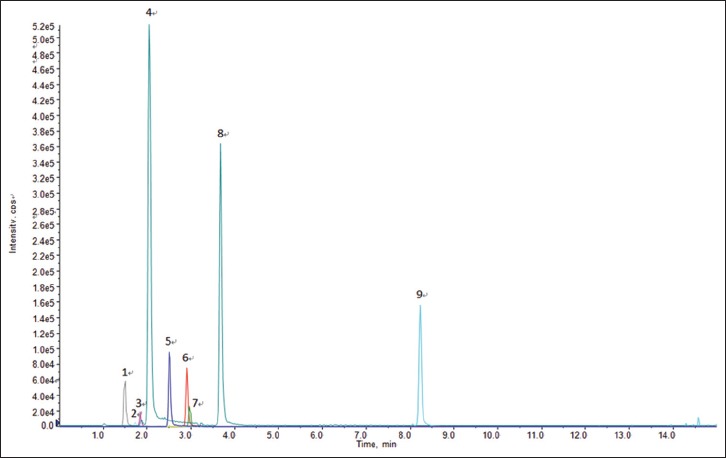
Typical MS/MS chromatogram of marker chemicals in Fuzi–Gancao herb couple. 1. Benzoylmesaconitine, 2. Benzoylaconitine, 3. Benzoylhypaconitine, 4. Isoliquiritin, 5. Mesaconitine, 6. Hypaconitine, 7. Aconitine, 8. Liquiritin, 9. Licochalcone A
Validation of UHPLC-MS/MS assay
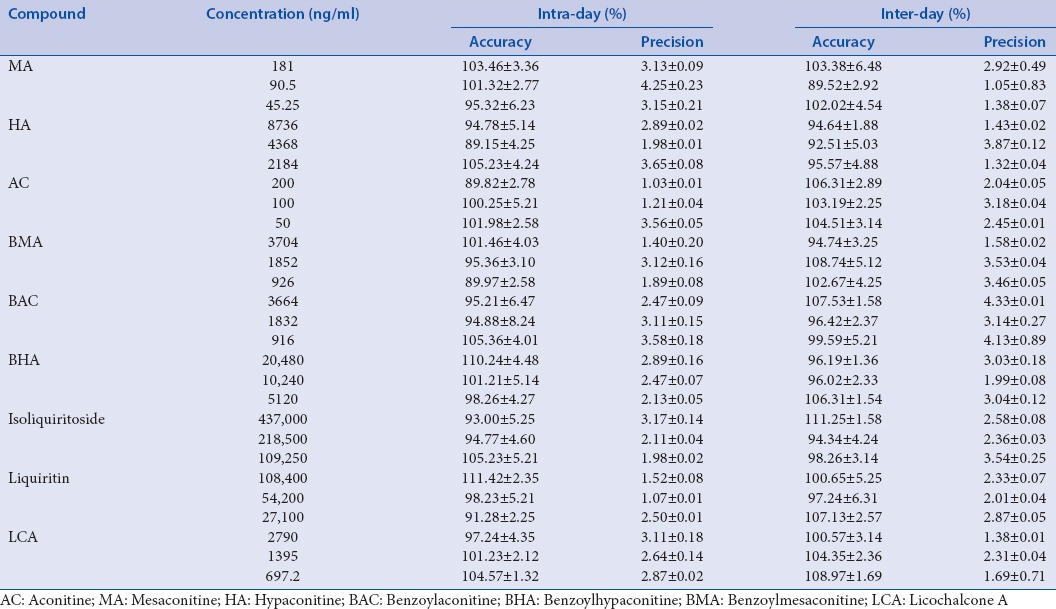
Method validations including linearity, repeatability, precisions (intra-day and inter-day), and recovery test were achieved before determining the nine phytochemical markers in Fuzi–Gancao herb couple. The standard calibration curves of all the ingredients are shown in Table 2 with satisfactory linearity (r > 0.9992). The LOD ranged from 0.02 ng/mL to 0.46 ng/mL for all the analytes. The precisions of intra-day and inter-day were <3.12% and 3.65%, respectively. All the ingredients show good repeatability with RSD <4.89%. The recovery of all the ingredients ranged from 85.36% to 110.14%. The recoveries of three concentrations indicated that there is no difference in the percent yield with different concentrations of the ingredients. Hence, the nine phytochemical markers in Fuzi–Gancao herb couple can be quantitatively analyzed simultaneously [Tables 1–4].
Table 2.
Calibration curve, R2, and concentration range of nine compounds in Fuzi–Gancao herb
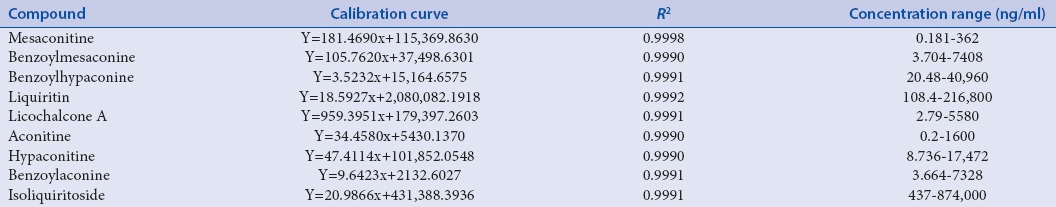
Table 4.
Analysis of the recovery of nine compounds in Fuzi–Gancao herb
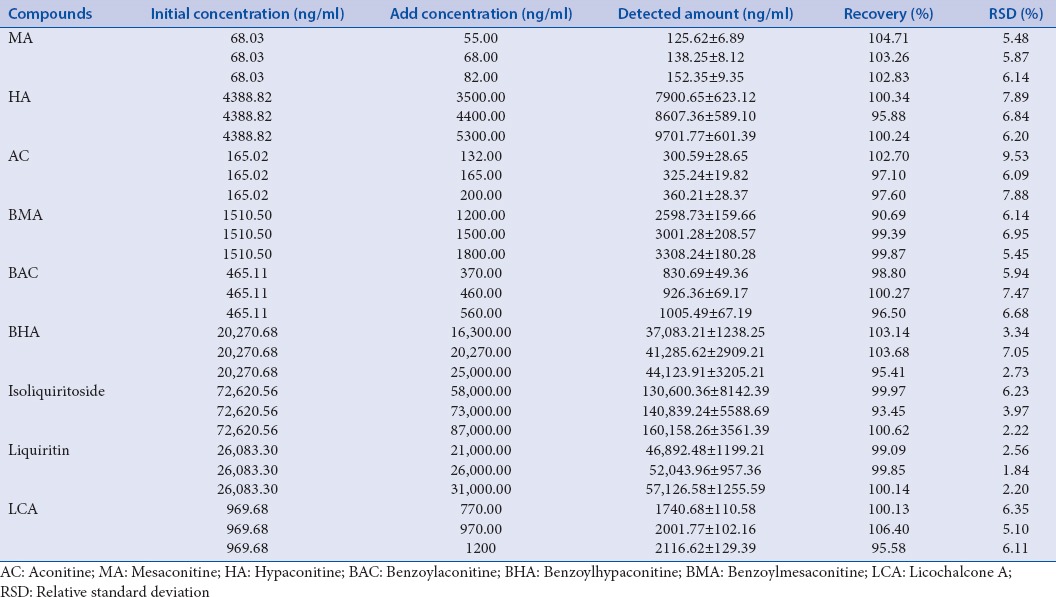
Table 3.
Analysis of the precision of nine compounds in Fuzi–Gancao herb
Sample analysis
The analytical method established was applied to determine of six aconitum alkaloids and three flavonoids in the herb couple of Fuzi–Gancao. The repeatability range of the nine chemical constituents is shown in Table 5. Isoliquiritoside was the most abundant. Conversely, aconitine was shown to be the least abundant of the nine compounds in Fuzi–Gancao herb couple. Therefore, the Fuzi–Gancao herb couple showed higher amounts of flavonoids than alkaloids. Aconitine, hypacoitine, and mesaconitine are known for their high toxicity and pharmacological activity; this result meant that the Fuzi–Gancao herb couple may have very low toxicity [Table 5].
Table 5.
Contents of nine compounds in Fuzi–Gancao herb couple (μg/g, n=3)

CONCLUSION
In the present study, a rapid, simple, and reliable UHPLC-MS/MS method was established for the first time to analyzing the nine chemical markers (BAC, BMA, BHA, aconitine, mesaconitine, hypaconitine, liquiritin, isoliquiritin, and LCA) in Fuzi–Gancao herb couple simultaneously. The developed LC-MS/MS method could be used for the quantitative assessment and quality control of Fuzi–Gancao herb couple in the future research. Fuzi and Gancao are the most common herbs in Chinese Medicine, so the analysis method for the quality control of Fuzi–Gancao herbal couple could also be used for the quality control of the other formulas containing these two herbs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zhao FR, Mao HP, Zhang H, Hu LM, Wang H, Wang YF, et al. Antagonistic effects of two herbs in Zuojin Wan, a traditional Chinese medicine formula, on catecholamine secretion in bovine adrenal medullary cells. Phytomedicine. 2010;17:659–68. doi: 10.1016/j.phymed.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–8. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 3.Tang DC. Science of Chinese Materia Medica. Shanghai: Publishing house of Shanghai University of Traditional Chinese Medicine; 2003. [Google Scholar]
- 4.Wang S, Hu Y, Tan W, Wu X, Chen R, Cao J, et al. Compatibility art of traditional Chinese medicine: From the perspective of herb pairs. J Ethnopharmacol. 2012;143:412–23. doi: 10.1016/j.jep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Shaw LH, Lin LC, Tsai TH. HPLC-MS/MS analysis of a traditional Chinese medical formulation of Bu-Yang-Huan-Wu-Tang and its pharmacokinetics after oral administration to rats. PLoS One. 2012;7:e43848. doi: 10.1371/journal.pone.0043848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton E. Biological properties of plant flavonoids: An overview. Int J Pharm. 1996;34:344–8. [Google Scholar]
- 7.Suo ZR, Xu M, Qin HY. LC-MS derermination of three disaster diterpenoid alkaloids in Fuzilizhong Pills. Chin J Pharm Anal. 2010;12:2279–86. [Google Scholar]
- 8.Guo N, Liu M, Yang D, Huang Y, Niu X, Wu R, et al. Quantitative LC-MS/MS analysis of seven ginsenosides and three aconitum alkaloids in Shen-Fu decoction. Chem Cent J. 2013;7:165. doi: 10.1186/1752-153X-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]


