Abstract
Background:
Rattan tea is a medicinal plant that has been used for many years for the treatment of inflammation, fatty liver, tumor, diabetes, and hyperlipidemia.
Objective:
A green and novel approach based on surfactant-mediated, ultrasonic-assisted extraction (SM-UAE) was developed for the extraction of antioxidant polyphenols from Rattan tea. A nonionic surfactant Tween-80 was selected as extraction solvent. The antioxidant activity was measured by total phenolic content (TPC) and ferric-reducing/antioxidant capacity (FRAC) assay.
Materials and Methods:
Optimization of extraction parameters including concentration of solvent, ultrasonic time, and temperature were investigated by response surface methodology. The antioxidant activity was measured by TPC and FRAC assay.
Results:
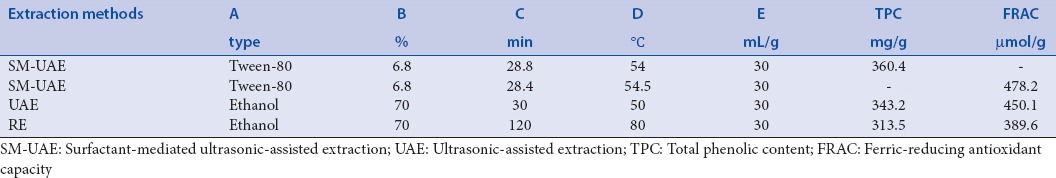
The optimal extraction conditions were determined as 6.8% (v/v) of aqueous Tween-80, ultrasonic temperature of 54°C, and ultrasonic time of 28.8 min. Under these conditions, the highest TPC value of 360.4 mg gallic acid equivalent per gram of dry weight material (GAE/g DW) was recorded. Moreover, 6.8% (v/v) of aqueous Tween-80, ultrasonic temperature of 54.5°C, and ultrasonic time of 28.4 min were determined for the highest FRAC value of 478.2 μmol of Fe2+/g of weight material (μmol Fe2+/g DW). Compared with other methods, the TPC and FRAC values of 313.5 mg GAE/g DW and 389.6 μmol Fe2+/g DW were obtained by heat reflux extraction using ethanol as solvent, respectively, and 343.2 mg GAE/g DW and 450.1 μmol Fe2+/g DW were obtained by UAE using ethanol as solvent, respectively.
Conclusion:
The application of SM-UAE markedly decreased extraction time or extraction cost and improved the extraction efficiency, compared with the other methods.
SUMMARY
Surfactant-mediated ultrasonic-assisted extraction of antioxidant polyphenols from Rattan Tea
Response surface methodology used to optimize parameters and study combined effects
Optimized surfactant-mediated ultrasonic-assisted extraction process enhances the antioxidant phenolics extraction in less time.
Abbreviations used: SM-UAE: Surfactant-mediated ultrasonic-assisted extraction; TPC: total phenolic content; FRAC: Ferric reducing antioxidant capacity; RSM: Response surface methodology; BBD: Box-Behnken design.
Keywords: Antioxidant polyphenols, Rattan tea, response surface methodology, Tween-80 mediated extraction, ultrasonic-assisted extraction
INTRODUCTION
Ampelopsis grossedentata (Hand-Mazz) W.T. Wang, a famous traditional Chinese herbal medicine, grows wildly in the southern region of China. The tea known as Rattan tea or vine tea is prepared from its stems and leaves.[1] It has been used for many years for the treatment of inflammation, fatty liver, tumor, diabetes, and hyperlipidemia.[2,3,4] Several pharmacological effects are mainly due to the antioxidant activity of Rattan tea. Recently, many active phenolic compounds have been isolated from Rattan tea such as dihydromyricetin, quercetin, myricetin, taxifolin, kaempferol, dihydrokaempferol, ampelopsin, resveratrol, and 5,7-dihydroxycoumarin.[5] Among them, dihydromyricetin [Figure 1] is a main bioactive compound with a high level >30% in Rattan tea.[6] All of these phenolic compounds are responsible for the antioxidant activity of Rattan tea. Therefore, it is important to develop a simple, efficient, and economical extraction method to extract antioxidant polyphenols from Rattan tea.
Figure 1.
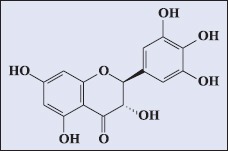
Chemical structure of dihydromyricetin
Several conventional extraction methods such as heating reflux and Soxhlet extraction were used to extract antioxidant polyphenols from different medicinal plants. However, these conventional extraction techniques have several disadvantages such as the consumption of lots of time, energy, and organic solvent but low recovery rate and loss of target compounds due to degradation because these techniques required long time and high temperature.[7,8] Recently, various novel extraction techniques have been developed for the extraction of antioxidant polyphenols from different medicinal plants including ultrasonic-assisted extraction (UAE), microwave-assisted extraction, supercritical fluid extraction, and pressurized solvent extraction.[9,10,11,12] Among these techniques, UAE is an inexpensive, simple, and more efficient alternative to conventional extraction methods.[13,14] However, the expensive and toxic organic solvents, such as methanol and ethanol, are also used as extraction solvent. Therefore, a high green, more accurate and efficient alternative extraction solvent system is of great interest for the enhancement of recovery rate and health protection nowadays. Surfactant-mediated extraction has been recognized as a novel method to extract bioactive compounds.[15,16] Surfactants, amphiphilic molecules with a hydrophilic head and hydrophobic tail, are also known as surface active agents.[17] When the concentration of surfactant is present at or above its critical micelle concentration, the micelles will be formed.[18] Moreover, these micelles can soluble different type of hydrophilic and lipophilic compounds and enhance the transfer of target material into micelle which results in a high extraction yield and recovery.[19,20] Surfactant-mediated extraction could be assumed as a cheap, rapid, and green method.
The main purpose of this study was to develop a rapid and highly accurate surfactant-mediated extraction method combined with UAE for the extraction of antioxidant polyphenols from Rattan tea. Tween-80, a nonionic, nontoxic, and edible surface active agent was preferred as extraction solvent. The influence of different parameters on the extraction efficiency such as Tween-80 concentration, liquid to material ratio, ultrasonic time, and temperature were investigated and optimized by response surface methodology (RSM) with Box–Behnken design (BBD).
MATERIALS AND METHODS
Chemical reagents and plant material
The compounds gallic acid, sodium carbonate, iron sulfate heptahydrate, iron chloride hexahydrate, Folin-Ciocalteu's phenol reagent were obtained from Sigma Chemicals Co. (St. Louis, MO, USA). 1,10-phenanthroline, emulsifier (OP-10), detergent (NP-10), polysorbate 80 (Tween 80), Triton-114 were provided from TianKe Co., Ltd. (Suzhou, China). All other chemical reagents used in experiments were of analytical grade and doubly distilled water was used throughout the experiment.
Sample preparation
Rattan tea (stems and leaves of A. grossedentata) was purchased from Wangsheng Commercial Co., Ltd. (Zhangjiajie, China). Samples were ground into about 40-mesh powder.
Tween-80 mediated ultrasonic-assisted extraction
The ultrasonic-assisted Tween-80-mediated extraction was performed in a ultrasonic device (KQ-250DB, Kunshan Ultrasonic Co. Ltd., China) with a fixed frequency of 40 kHz, electric power of 200 W equipped with a temperature controller, and a digital timer. For extraction using RSM, fixed liquid to material ratio 30 mL/g was used. Sample (1.0 g) was extracted under these various conditions: Tween-80 concentration ranging from 5% to 9%, ultrasonic time from 25 to 35 min, and ultrasonic temperature from 40°C to 60°C. After extraction, the mixtures were centrifuged at 10,000 rpm for 10 min to remove the insoluble materials, and the supernatants were collected for the antioxidant activity and phenolic content determination.
For comparison, in UAE, 2.0 g sample was extracted using 60 mL of 70% ethanol, at 50°C for 30 min. In heating reflux method, 2.0 g sample was extracted with 60 mL of 70% at 80°C for 120 min.
Determination of total phenolic content
The amount of total phenolics content was determined using Folin–Ciocalteu method with gallic acid as a standard.[21] Briefly, 200 μL of each suitable diluted extract was added to 0.5 mL of the Folin–Ciocalteu reagent, the mixture was shaken and allowed to stand. After 5 min, 2 mL of 20% sodium carbonate solution was added. The reaction solution was then adjusted with distilled water up to a final volume of 10 mL. After 120 min of incubation in dark at room temperature, the absorbance was measured at 760 nm. The total phenolic content (TPC) was expressed as milligram of gallic acid equivalent per gram of dry weight material (mg GAE/g DW).
Ferric-reducing antioxidant capacity
In the recent years, a lot of methods have been developed for evaluation of the antioxidant capacity of phenolics such as ABTS, DPPH, FRAP, CUPRAC, and total antioxidant activity assay.[22,23,24] In contrast to other methods, ferric-reducing antioxidant capacity (FRAC) method has been confirmed to be a cheap, simple, precise, stable, and closely correlated method with the TPC.[25]
The FRAC of extract was determined according to the method of 1,10-phenanthroline[22] with minor modification. In this case, 200 μL of each suitable diluted extract was mixed with 0.5 mL of 0.5% 1, 10-phenanthroline solution in methanol, and 1 mL of 0.2% FeCl3 solution in methanol. The reaction solution was then adjusted with methanol up to a final volume of 10 mL. The reaction mixture was kept at room temperature in dark for 20 min, and then absorbance was measured at 510 nm. The result was expressed as μmol of Fe2+/g of weight material (μmol Fe2+/g DW).
Recovery of antioxidant polyphenols by cloud-point preconcentration
The antioxidant polyphenols phenolics from Rattan Tea could be recovered by cloud-point preconcentration method.[26] In this method, 10 mL of extracted supernatant was transferred into a 25 mL centrifuge tube. Then, the 5 g sodium chloride was added and mixed violently for 5 min. The aqueous solution was kept in a controlled temperature bath for 20 min at 70°C. Separation of the two phases was achieved by centrifuging for 10 min at 4000 rpm. The upper phase was obtained and diluted with methanol for determination of TPC and FRAC.
Experimental design
RSM was employed to optimize the extraction conditions for antioxidant polyphenols from Rattan tea. Tween-80 concentration (X1, %), ultrasonic time (X2, min), and ultrasonic temperature (X3, °C) were preferred for independent variables related to two response values of TPC and FRAC. A three-level three-variables BBD was applied in this study. The variables and their levels, with both coded and natural values are presented in Table 1. Regression analysis was performed based on experimental data from BBD and fitted to a second-order polynomial model (Equation 1).
Table 1.
Coded and uncoded levels of independent variables used for Box-Behnken design (BBD)

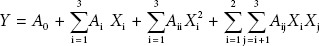
Where Y is response, A0 is intercept, Ai is coefficient of variable for linear, Aii is coefficient of variable for quadratic and Aij is coefficient of variable for interaction term. Xi and Xj are independent variables.
The experimental data were analyzed using a statistical package, Design-Expert version 8.0.5b, (Stat-Ease Inc., Minneapolis, MN, USA). The adequacy of the model was evaluated by the lack of fit, coefficient of determination (R2), Fisher's test value (F-value) generated from the analysis of variance (ANOVA) analysis (P < 0.05).
RESULTS AND DISCUSSION
Single-factor optimization for extraction
In this study, five key parameters including effect of extraction solvents, Tween-80 concentration, ultrasonic time, liquid to material ratio, and ultrasonic temperature were picked out for investigation. The results are shown in Figure 2. The effect of extraction solvents on the TPC and FRAC values is shown in Figure 2a. It was observed that Tween 80 as extraction solvent showed highest TPC and FRAC values as compared to water, OP-10, NP-10, and Triton-114., Moreover, It is well versed that Tween-80 is a nontoxic reagent and is considered to be edible by the USFDA.[27] The high hydrophilic-lipophilic balance (HLB) value of Tween-80 (HLB = 15.0) signified its high water solubility and high dissolving capacity of target compounds. Therefore, Tween-80 was chosen as extraction solvent for further experiment.[28] From Figure 2b, it was revealed that 7% of Tween-80 resulted in highest TPC and FRAC values. Moreover, the concentration of Tween-80 above 7% resulted in a decrease of TPC and FRAC values. This could be due to the higher viscosity that creates a difficulty for transferability of the antioxidant polyphenols from Rattan tea to surfactant phase.[29] The effect of liquid to material ratio on the TPC and FRAC values are shown in Figure 2c. When the liquid to material ratio increased from 15 mL/g to 35 mL/g, the TPC and FRAC values increased and then decreased slowly. The maximum of TPC and TAC values were obtained at 30 mL/g, but had no significant difference compared with 25 mL/g or 35 mL/g. This result indicating that there was no significant influence of liquid to material ratio on TPC and FRAC values. The effect of ultrasonic time on the TPC and FRAC values is shown in Figure 2d. It was observed that the TPC and FRAC values reached to a maximum level at 30 min and then declined. Thus, the optimal ultrasonic time was chosen as 30 min. The effect of ultrasonic temperature on the TPC and FRAC values is shown in Figure 2e. Enhancing the ultrasonic temperature from 20°C to 60°C, the TPC and FRAC values increased and then declined rapidly. The highest TPC and FRAC values were obtained at 50°C. According to the single factor results, the effects of Tween-80 concentration, ultrasonic time, and ultrasonic temperature were more significant on the TPC and FRAC values. Hence, these three parameters were preferred for optimizing the Tween-80-mediated UAE of antioxidant polyphenols from Rattan tea using RSM [Table 1].
Figure 2.
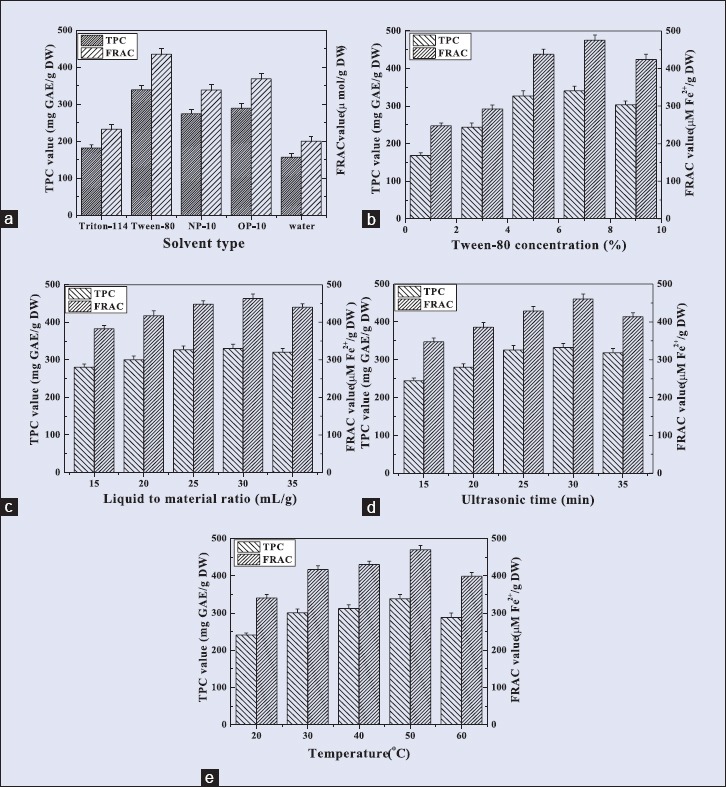
Effect of Tween-80 concentration, Liquid to material ratio, ultrasonic time and temperature on the responses. Extraction conditions were set as follows: (a) 25 mL/g, 25 min, (b) 25 mL/g, 25 min, 50°; (c) 7 % Tween-80, 25 min, 50°; (d) 7 % Tween-80, 25 mL/g, 50°; and (e) 7 % Tween-80, 25 mL/g, 25 min
Fitting the models
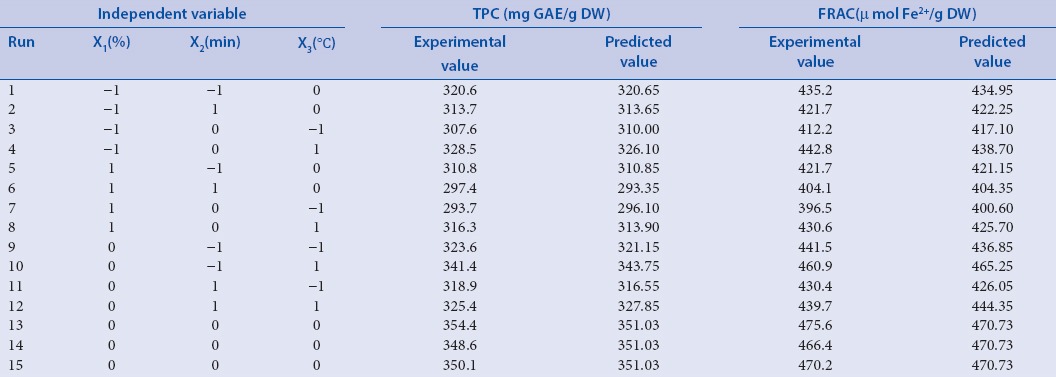
The two different response values of TPC and FRAC obtained from experimental design set are shown in Table 2. The TPC and FRAC values of surfactant-mediated extract of Rattan tea varied from 293.7 to 354.4 mg GAE/g DW and from 396.5 to 475.6 μmol Fe2+/g DW, respectively. Among these 15 experiments, experiment 13 (Tween-80 concentration 7%, ultrasonic time 30 min, ultrasonic temperature 50°C) represented the highest TPC and FRAC values while experiment 7 (Tween-80 concentration 9%, ultrasonic time 30 min, ultrasonic temperature 40°C) produced the least TPC and FRAC values.
Table 2.
Experiment design of three levels, three variables Box–Behnken experimental design and their responses, including Total Phenol Contents (TPC) and Ferric-Reducing Antioxidant Capacity (FRAC)
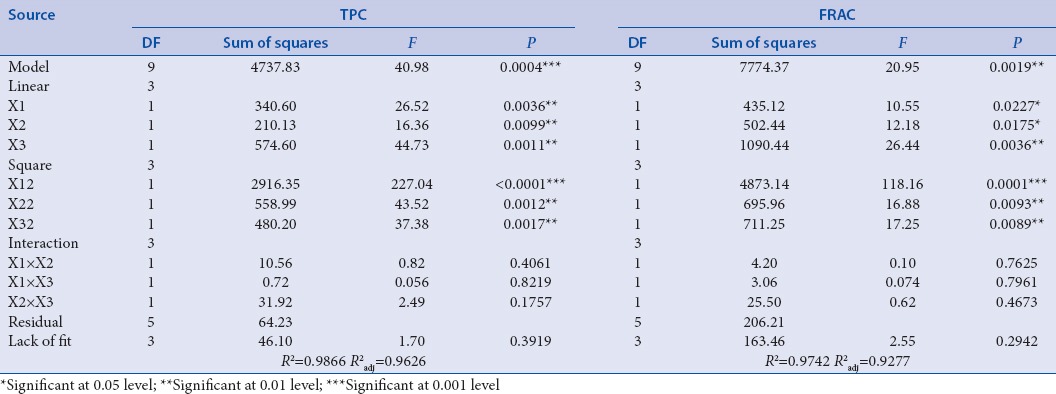
The ANOVA is shown in Table 3. According to the results, it was observed that quadratic polynomial models were highly significant with the low P values of 0.0004 and 0.0019 (both P < 0.01) for TPC and FRAC, respectively. The F values of 40.98 for TPC and 20.95 for FRAC suggested that the significant of the models were higher than 95% of confidence level. The high value of coefficient of determination (R2) for TPC and FRAC (0.9866 and 0.9742, respectively) as well as R2adj (0.9626 and 0.9277, respectively) indicated that there is a good correlation between the experimental and predicted values. Hence, there was only 3.74% and 7.23% of the total variation for TPC and FRAC, respectively. Furthermore, no significant of lack of fit (P = 0.3919 and 0.2972 > 0.05) for the two models indicated the well reproducibility of the experimental data.[30]
Table 3.
Analysis of variance (ANOVA) of the quadratic model and lack of fit
Response surface analysis of total phenolic content
The experimental data were analyzed through multiple regression analysis. The regression equation asserting the relationship between Tween-80 concentration, ultrasonic time, and ultrasonic temperature. TPC value was obtained according to (Equation 2) as given below:
YTPC = 351.03 − 6.52 X1 − 5.13 X2 + 8.47 X3 − 1.63 X1 X2 + 0.43 X1 X3 − 2.82 X2 X3 − 28.10 X12 − 12.30 X22 − 11.40 X32 (2)
The significant of each coefficient was checked by F values and P values and the results were shown in Table 3. The TPC value was significantly influenced by three linear (X1, X2, X3) and three quadratic (X12, X22, X32) parameters as P < 0.01. However, the interaction effect of X1X2, X1X3, and X2X3 were not significantly influenced on the TPC value due to higher P value (P > 0.05).
To investigate the interactive effects of variables for the TPC value, three-dimensional surface plots were constructed according to Equation 2. Figure 3 showed the effect of Tween-80 concentration, ultrasonic time, ultrasonic temperature, and their mutual interaction on TPC value. These three variables exhibited significant quadratic effects in response of TPC value, which indicated that an increase in the effects near the midpoint resulted in the highest TPC value. However, the TPC value was negatively affected when the Tween-80 concentration and ultrasonic time were increase beyond the midpoint (Equation 2). The TPC value was positively affected when the ultrasonic temperature was increased beyond the midpoint up to about 55°C. These effects showed that the higher concentration of Tween-80 inhibits the transfer of antioxidant polyphenols from Tween-80-mediated extract because of the increase in viscosity. Moreover, due to longer ultrasonic time, impurities dissolve into solvent leading to the decrease of TPC value. The increase in extraction temperature to an appropriate level accelerates mass transfer and thus improves the TPC value.
Figure 3.
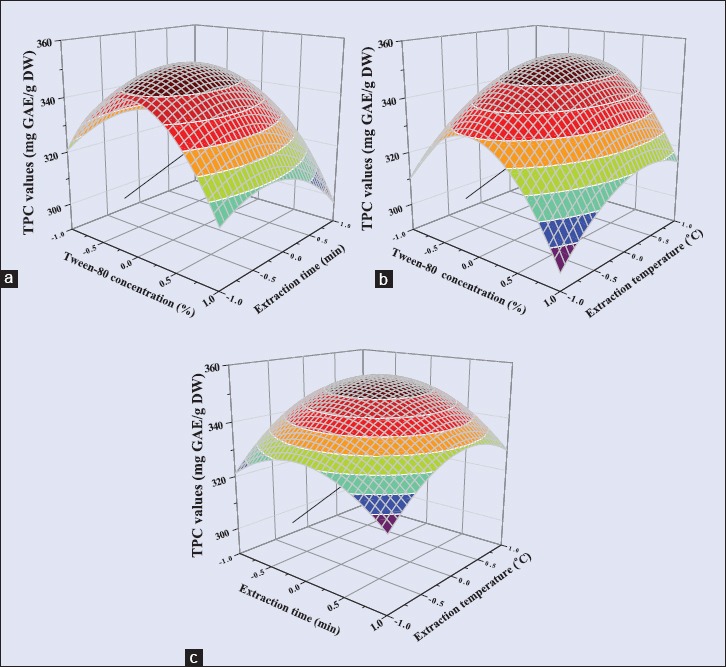
Response surface plots of Rattan tea showing the effects of (a) Tween-80 concentration and ultrasonic time, (b) Tween-80 concentration and ultrasonic temperature, (c) ultrasonic time and ultrasonic temperature on TPC
Response surface analysis of ferric-reducing antioxidant capacity
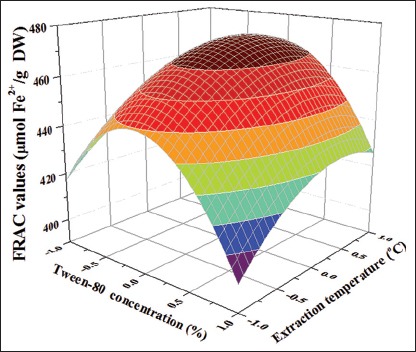
Equation 3 showed the relationship between Tween-80 concentration, ultrasonic time, ultrasonic temperature, and FRAC value. Figure 3 showed the effect of the three variables on FRAC value. The FRAC value [Table 2] was significantly influenced by linear effect of ultrasonic temperature (P < 0.01) while significantly influenced by Tween-80 concentration and ultrasonic time (P < 0.05). The FRAC value was significantly influenced by quadratic effects of three variables. Similar to the TPC value of extract, the interactive effects of the three variables were not significantly influenced on the FRAC value (P > 0.05).
The three-dimensional surface plots of FRAC value [Figure 4] are similar to the TPC. The FRAC value increased up to the highest level by increasing the Tween-80 concentration and ultrasonic time up to midpoint but subsequently decreased quickly. This suggested that the interaction between FRAC value, Tween-80 concentration, and ultrasonic time was suitable at a set of conditions. An increase in temperature from about 40 to 55°C induced a consistent increase in the FRAC value. This phenomenon was due to improvement in mass transfer rate.[7] A further increase in temperature resulting in decreased value of FRAC due to the thermal degradation of antioxidant polyphenols.[31,32]
Figure 4.
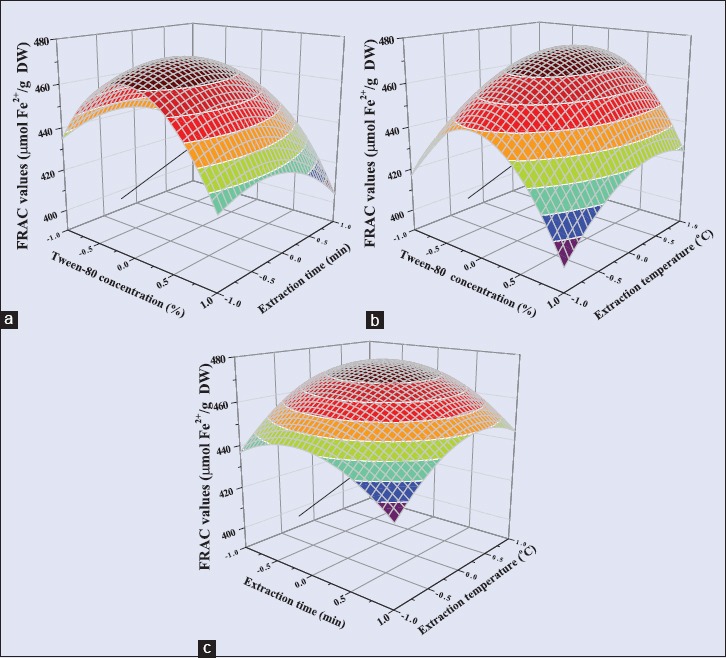
Response surface plots of Rattan tea showing the effects of (a) Tween-80 concentration and ultrasonic time, (b) Tween-80 concentration and ultrasonic temperature, (c) ultrasonic time and ultrasonic temperature on FRAC
YFRAC = 470.73 − 7.37X1 − 7.93X2 + 11.68X3 − 1.03X1X2 + 0.87X1X3 − 2.52 X2X3 − 36.33X12 − 13.73X22 − 13.88X32 (3)
Verification of predictive models and optimization of process
The object of this study was to optimize the Tween-80-mediated UAE conditions with maximum TPC and FRAC values from Rattan tea. The optimum extraction conditions obtained from the models were as follows: 6.8% (v/v) aqueous Tween-80, ultrasonic temperature 54°C, and ultrasonic time 28.8 min for the TPC value and 6.8% (v/v) aqueous Tween-80, ultrasonic temperature 54.5°C, ultrasonic time 28.4 min for the FRAC value. The predicted values (TPC 353.708 mg GAE/g DW and FRAC 474.976 μmol Fe2+/g DW) agreed well with the experimental values (TPC 360.4 mg GAE/g DW and FRAC 478.2 μmol Fe2+/g DW), which validated the suitability of the fitted response surface model. Hence, a good correlation between these results showed well adequacy of the response surface model with expected optimization [Table 4]. Furthermore, when compared with other methods such as UAE and heating reflux method [Table 4], the application of Tween-80-mediated UAE of antioxidants markedly improved the extraction efficiency.
Table 4.
Comparison of experimental results with predicted results obtained by the fitted model and the results obtained by the other common methods
Recovery of antioxidant polyphenols by cloud-point preconcentration
The antioxidant polyphenols from Rattan tea could be recovered by cloud-point preconcentration method. The results were shown in Figure 5. Under the condition of 20%(m/v) NaCl and equilibration at 70°C for 20 min, the high recovery of 82.9% and 83.2% for TPC and FRAC values were obtained, respectively.
Figure 5.
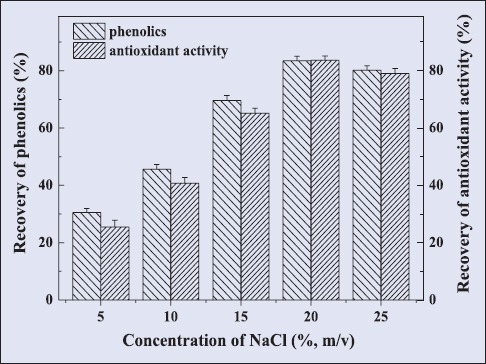
Effect of cloud point preconcentration conditions on the recovery of antioxidant phenolics
CONCLUSION
Surfactant-mediated extraction has been proved to be a low cost and eco-friendly method offering a beneficial alternative to the conventional extraction method. In this paper, a Tween-80-mediated UAE method has been applied for the extraction of antioxidant polyphenols from Rattan tea. The three key parameters including Tween-80 concentration, ultrasonic time, and ultrasonic temperature extraction were successfully optimized by response surface methodology. The R2 values of the second order polynomial models for TPC and FRAC were 0.9866 and 0.9742, respectively, which represented a well fit to the quadratic models. The optimal conditions for obtaining maximal TPC value of 360.4 mg GAE/g DW, defined to be 6.8% of Tween-80 concentration, 54°C of ultrasonic temperature, and 28.8 min of ultrasonic time. Similarly, 6.8% of Tween-80 concentration, 54.5°C of ultrasonic temperature, and 28.4 min ultrasonic time were optimal conditions to obtain maximal FARC value of 478.2 μmol Fe2+/g DW. Under the condition of 20% (m/v) NaCl and equilibration at 70°C for 20 min, the high recovery of 82.9% and 83.2% for TPC and FRAC values were obtained, respectively. These results confirmed that Tween-80-mediated UAE is a green, fast, reliable, and economical tool for the extraction of phenolic antioxidants from Rattan tea.
Financial support and sponsorship
The financial support for this investigation given by Natural Science Foundation of Jiangsu Province administration of traditional Chinese, China (LZ13244), Doctoral Innovation Project of Jiangsu Province, China (CXLX13-686), and Undergraduate Student Innovation Project (201410299084X) of Jiangsu Province, China.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kou X, Chen N. Pharmacological potential of ampelopsin in rattan tea. Food Sci Hum Wellness. 2012;1:14–8. [Google Scholar]
- 2.Chen S, Zhao X, Wan J, Ran L, Qin Y, Wang X, et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol Res. 2015;99:74–81. doi: 10.1016/j.phrs.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XJ, Xiao H, Zeng Z, Sun ZW, Lei C, Dong JZ, et al. Composition and serum antioxidation of the main flavonoids from fermented vine tea (Ampelopsis grossedentata) J Funct Foods. 2014;9:290–4. [Google Scholar]
- 4.Sangiovanni E, Di Lorenzo C, Colombo E, Colombo F, Fumagalli M, Frigerio G, et al. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015;6:2453–63. doi: 10.1039/c5fo00410a. [DOI] [PubMed] [Google Scholar]
- 5.Du Q, Chen P, Jerz G, Winterhalter P. Preparative separation of flavonoid glycosides in leaves extract of Ampelopsis grossedentata using high-speed counter-current chromatography. J Chromatogr A. 2004;1040:147–9. doi: 10.1016/j.chroma.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 6.Wang DY, Zheng ZZ, Xu SY, Zheng SZ. Four new isoflavones from Ampelopsis grossedentata. J Asian Nat Prod Res. 2002;4:303–8. doi: 10.1080/1028602021000049104. [DOI] [PubMed] [Google Scholar]
- 7.Chen M, Zhao Y, Yu S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015;172:543–50. doi: 10.1016/j.foodchem.2014.09.110. [DOI] [PubMed] [Google Scholar]
- 8.Fang X, Wang J, Wang Y, Li X, Zhou H, Zhu L. Optimization of ultrasonic-assisted extraction of wedelolactone and antioxidant polyphenols from Eclipta prostrate L using response surface methodology. Sep Purif Technol. 2014;138:55–64. [Google Scholar]
- 9.Setyaningsih W, Saputro IE, Palma M, Barroso CG. Optimisation and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem. 2015;169:141–9. doi: 10.1016/j.foodchem.2014.07.128. [DOI] [PubMed] [Google Scholar]
- 10.Kuo CH, Chen BY, Liu YC, Chang CM, Deng TS, Chen JH, et al. Optimized ultrasound-assisted extraction of phenolic compounds from Polygonum cuspidatum. Molecules. 2013;19:67–77. doi: 10.3390/molecules19010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solana M, Mirofci S, Bertucco A. Production of phenolic and glucosinolate extracts from rocket salad by ssupercritical fluid extraction: Process design and cost benefits analysis. J Food Eng. 2016;168:35–41. [Google Scholar]
- 12.Kamali H, Khodaverdi E, Hadizadeh F, Ghaziaskar S. Optimization of phenolic and flavonoid content and antioxidants capacity of pressurized liquid extraction from Dracocephalum kotschyi via circumscribed central composite. J Supercrit Fluid. 2016;107:307–14. [Google Scholar]
- 13.Heleno SA, Diz P, Prieto MA, Barros L, Rodrigues A, Barreiro MF, et al. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016;197(Pt B):1054–63. doi: 10.1016/j.foodchem.2015.11.108. [DOI] [PubMed] [Google Scholar]
- 14.Zou TB, Xia EQ, He TP, Huang MY, Jia Q, Li HW. Ultrasound-assisted extraction of mangiferin from mango (Mangifera indica L.) leaves using response surface methodology. Molecules. 2014;19:1411–21. doi: 10.3390/molecules19021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S, Kori S, Parmar A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chem. 2015;185:284–8. doi: 10.1016/j.foodchem.2015.03.106. [DOI] [PubMed] [Google Scholar]
- 16.He S, Shi J, Walid E, Zhang H, Ma Y, Xue SJ. Reverse micellar extraction of lectin from black turtle bean (Phaseolus vulgaris): Optimisation of extraction conditions by response surface methodology. Food Chem. 2015;166:93–100. doi: 10.1016/j.foodchem.2014.05.156. [DOI] [PubMed] [Google Scholar]
- 17.Memon AA, Memon N, Bhanger MI. Micelle-mediated extraction of chlorogenic acid from Morus laevigata W. leaves. Sep Purif Technol. 2010;76:179–83. [Google Scholar]
- 18.Shi Z, He J, Chang W. Micelle-mediated extraction of tanshinones from Salvia miltiorrhiza bunge with analysis by high-performance liquid chromatography. Talanta. 2004;64:401–7. doi: 10.1016/j.talanta.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Zain NN, Bakar NA, Mohamad S, Saleh NM. Optimization of a greener method for removal phenol species by cloud point extraction and spectrophotometry. Spectrochim Acta A Mol Biomol Spectrosc. 2014;118:1121–8. doi: 10.1016/j.saa.2013.09.129. [DOI] [PubMed] [Google Scholar]
- 20.He S, Shi J, Walid E, Ma Y, Xue SJ. Extraction and purification of a lectin from small black kidney bean (Phaseolus vulgaris) using a reversed micellar system. Process Biochem. 2013;20:746–52. [Google Scholar]
- 21.Lee JH, Jeon JK, Kim SG, Kim SH, Chun T, Imm JY. Comparative analyses of total phenols, flavonoids, saponins and antioxidant activity in yellow soy beans and mung beans. Int J Food Sci Technol. 2011;46:2513–9. [Google Scholar]
- 22.Szydlowska-Czerniak A, Dianoczki C, Recseg K, Karlovits G, Szlyk E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta. 2008;76:899–905. doi: 10.1016/j.talanta.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 23.Sahin S, Aybastier O, Isik E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013;141:1361–8. doi: 10.1016/j.foodchem.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Ranic M, Nikolic M, Pavlovic M, Buntic A, Siler-Marinkovic S, Dimitrijevic-Brankovic S. Optimization of microwave-assisted extraction of natural antioxidants from spent espresso coffee grounds by response surface methodology. J Clean Prod. 2014;80:69–79. [Google Scholar]
- 25.Safari P, Rezaei M, Shaviklo AR. The optimum conditions for the extraction of antioxidant compounds from the Persian gulf green algae (Chaetomorpha sp.) using response surface methodology. J Food Sci Technol. 2015;52:2974–81. doi: 10.1007/s13197-014-1355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahugo Santana C, Sosa Ferrera Z, Santana Rodríquez JJ. Use of non-ionic surfactant solutions for the extraction and preconcentration of phenolic compounds in water prior to their HPLC-UV detection. Analyst. 2002;127:1031–7. doi: 10.1039/b202092k. [DOI] [PubMed] [Google Scholar]
- 27.Peng X, Xu H, Yuan X, Leng L, Meng Y. Mixed reverse micellar extraction and effect of surfactant chain length on extraction efficiency. Sep Purif Technol. 2016;160:117–22. [Google Scholar]
- 28.Hemavathi A, Hebbar HU, Raghavarao K. Mixed reverse micellar systems for extraction and purification of β-glucosidase. Sep Purif Technol. 2010;71:263–8. [Google Scholar]
- 29.Amiri-Rigi A, Abbasi S. Microemulsion-based lycopene extraction: Effect of surfactants, co-surfactants and pretreatments. Food Chem. 2016;197(Pt A):1002–7. doi: 10.1016/j.foodchem.2015.11.077. [DOI] [PubMed] [Google Scholar]
- 30.Ren J, Zhao M, Shi J, Wang J, Jiang Y, Cui C, et al. Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT Food Sci Technol. 2008;41:1624–32. [Google Scholar]
- 31.Tabaraki R, Nateghi A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason Sonochem. 2011;18:1279–86. doi: 10.1016/j.ultsonch.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Asnin L, Assefa AD, Ko EY, Sharma K, Park SW. Extraction of antioxidants from Aloe vera leaf gel: A response surface methodology study. Food Anal Methods. 2014;7:1804–15. [Google Scholar]


