Abstract
Background:
The purple sweet potato, Ipomoea batatas, belongs to the family Convolvulaceae. It is one of the most widely consumed tubers in Asia and is found in many dishes. Many people with diabetes eat purple sweet potato tubers to help reduce blood glucose in China.
Objective:
To predict the ultrasonic conditions for getting the optimal in vitro antioxidant and antiglycated activity of ultrasonic extracted polysaccharides from purple sweet potato (I. batatas) tubers, the artificial neural network (ANN) regression models was used in this study.
Materials and Methods:
The antioxidant activity of polysaccharides was quantified by evaluating the hydroxyl radical scavenging effect after ultrasonic extraction, and the data were used in conjunction with optimized extraction conditions to train the predictive ANN models.
Results:
The following conditions were predicted to yield optimal hydroxyl scavenging activity: 200 W, 22°C, and 40 min. In contrast, conditions of 230 W, 22°C, and 50 min yielded the greatest inhibitory effect on albumin nonenzymatic glycosylation. The accuracy and predictive ability of the models ranged from good to excellent, as indicated by R2 values ranging from 0.953 to 0.998.
Conclusion:
The results of the present study showed that ANN predictive models are useful in ultrasonic processing, which can rapidly and accurately predict the optimum extraction conditions for polysaccharides based on their antioxidant and antiglycated activities. In addition, the results of the present study suggest that the consumption of sweet potatoes may help reduce free radicals in the body and prevent or treat diabetes.
SUMMARY
Ultrasonic extraction conditions were simulated and optimized using artificial neural network
Bioactivities showed nonlinear relationship with ultrasonic conditions
The optimal extraction conditions were 200 W, 22°C, and 40 min for the highest antioxidant capacity
The optimal extraction conditions were 230 W, 22°C, and 50 min for the highest antiglycated effect.
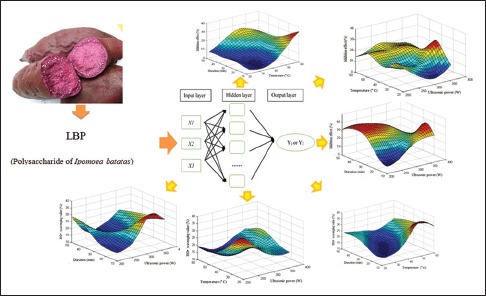
Abbreviations used: IBP: Polysaccharide of Ipomoea batatas; RSM: response surface methodology; ANN: Artificial neural network; BSA: Bovine serum albumin.
Keywords: Antioxidant, Ipomoea batatas (L.) Lam, nonenzymatic glycosylation, polysaccharide, purple sweet potato
INTRODUCTION
The purple sweet potato, Ipomoea batatas, known as “zishu” in mainland China, belongs to the family Convolvulaceae. It is one of the most widely consumed tubers in Asia and is commonly included in various dishes. The international project called Flora of China[1] describes that “zishu” is a carbohydrate-rich food resource. The tubers of I. batatas Satsuma are purple and contain high levels of polysaccharides, anthocyanins, carotenoids, and polyphenols, in addition to dietary fiber, vitamins, and protein. Many of these compounds are regarded as antioxidants that eradicate free radicals.[2,3,4] In China, eating purple sweet potato tubers could help reduce blood glucose levels in diabetes.
Previous studies have shown that components in the aqueous extracts of fruits and plants, including polysaccharides, have antioxidant, antiglycated, and antitumor activity.[5,6,7,8] Recent epidemiological studies have associated the antioxidant ingredients in fruits, vegetables, and plants with a reduced risk for life-threatening diseases such as diabetes, cancer, and stroke.[9,10,11,12] The reasonable explanation is that most oxygen-based molecules from the atmosphere undergo oxidation to produce energy through the respiratory chain in the mitochondria; however, they often lose electrons and produce free radicals during metabolism, and make damage to cell and tissue; the levels of reactive oxygen species can significantly increase due to environmental stress.[13,14] The hydroxyl molecule (•OH) is one of the free radicals which can react with important biomolecules, resulting in cellular damage or death. Boligon et al.[15] reported that the removal of •OH protects the brain and prevent diseases.
Central to our present understanding of diabetic hyperglycemia is the idea that high sugar levels lead to the covalent crosslinking of glucose, glycosylated protein, and lysine. Such crosslinks occur on the surface of proteins, influencing its normal function.[16] Nonenzymatic glycosylation is the main biochemical reaction that generates these crosslinks. Studies by Brownlee et al.,[17,18] inhibition of nonenzymatic glycosylation was shown to reduce the occurrence of protein glycosylation. Therefore, polysaccharides that inhibit glycosylation may have clinical applications that may potentially benefit patients with diabetic hyperglycemia.
Ultrasonication is commonly used technique for the extraction of natural products. With its unique advantages of mild extraction temperature, high extraction efficiency, this method can be applied to the extraction of compounds from a variety of animal tissues and plants. For these reasons, ultrasonication methods have to be used in extracting polysaccharides from sweet potato tubers. The main variable parameters in ultrasonic extraction are ultrasonication power, temperature, and duration; most of these parameters are associated with nonlinear, irregular effects,[19] thus making it difficult to establish optimal conditions of extraction.
For solving this difficulty, we selected response surface methodology (RSM). The superior performance of RSM is reflected in the exploration of nonlinear or less systematic data. By training with several actual values obtained from varied conditions of ultrasonic extraction, the structured initial artificial neural network (ANN) model modified by genetic algorithms, it will transfer into a high-fitting class function that can accurately predict the outcomes of extraction conditions.[20] Based on the self-adaptive, self-organizing, and self-learning characteristics of the ANNs of Wasserman,[21] this model can analyze data with nonlinear or discrete distributions such as interactions between ultrasonic extraction conditions and bioactivities, thus improving data analysis and optimization of polysaccharide extraction conditions. Therefore, predicting the optimal ultrasonic conditions by genetic algorithms adjusted ANN model could solve the difficult to establish optimal conditions of extraction.
One folklore activity involves the consumption of sweet potatoes to reduce blood sugar levels of individuals with diabetes, which in turn has prompted researchers to determine the antioxidant effects of sweet potato extracts.[22] The present study aimed to optimize the extraction of polysaccharides from purple sweet potatoes and to analyze the in vitro activities of extracted polysaccharides. The findings of the present study may serve as reference for future studies on sweet potato polysaccharides in relation to pharmacological issues.
MATERIALS AND METHODS
Materials
Raw tubers of the purple sweet potato (I. batatas) were purchased from a market at the Higher Education Mega Center (Guangzhou, China). Vitamin C, sodium azide (NaN3), glucose standard, bovine serum albumin (BSA), and amino guanidine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents were of analytical grade and were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China).
Preparation of purple sweet potato flour
Purple sweet potato tubers were cut into strips and completely dried in a 60°C air dryer. Dried samples were then pounded into a powder for use in subsequent experiments.
Extraction of polysaccharides from purple sweet potato tubers
Polysaccharides were extracted from 10 g of purple sweet potato powder dissolved distilled water (150 mL) in an ultrasonic cleaner (SB-4200DTD, Cosette Huai Instrument Co., Shanghai, China, 40 kHz) using different ultrasonication powers (200–360 W), temperatures (20–60°C), and duration (10–50 min), as described in Table 1. Each extract was filtered through a filter paper using a vacuum pump. Then, the filtrate was evaporated under vacuum in a rotary evaporator at 100 rpm and 65°C up to a volume of about 30 mL. Removal of protein from the filtrate was then conducted using the method of Sevag.[19] The Sevag reagent (80 mL of CHCl3 and 20 mL of butanol) was added to the concentrated extract, which was then shaken for 20 min at room temperature. After centrifugation at 3000 rpm for 20 min, the supernatant was collected. The Sevag reagent was then removed through dialysis, and 95% alcohol was added to yield a solution with an alcohol strength of up to 75%, as determined by a YH109 instrument (YanHe Instrument Co., Henan, China). The solution was then incubated overnight at 4°C to allow precipitation of polysaccharides. The resulting slurries were centrifuged at 3,000 rpm for 20 min, and the precipitates were collected. The precipitates were then lyophilized for 72 h at −47°C. Each lyophilized sample was weighed and placed in an individual Ziploc plastic bag until analysis. Polysaccharide content of the lyophilized purple sweet potato samples was determined by using the phenol-sulfuric acid method,[4,23] using glucose as standard, and the results were expressed as glucose equivalents. The antioxidant capacity and inhibitory effects of the extracts on albumin nonenzymatic glycosylation in vitro were determined by conducting hydroxyl radical scavenging assays[24] and the method of Brownlee et al.[17,18] The optimal conditions for antioxidant and antiglycated activities were selected as described in the RSM illustration section.
Table 1.
The three selected levels of three independent variables in this experiment

Determination of three levels of three independent variables (X1, X2, and X3)
Three levels for each independent variable were selected using the procedure described. Three levels for each independent variable were determined by conducting a series of extractions in 150 mL of distilled water using different durations (10 min–50 min; variable X1), ultrasonication power (200 W–360 W; variable X2), and temperature (20°C–60°C; variable X3), as shown in Table 1. The full factorial design for the three variables (referred to as the 23 design) that describes the interactions in a total of 27 experimental groups is shown in Table 2.
Table 2.
Experiments for polysaccharide extraction in a 3×3×3 full factorial design
Characterization of the extracted polysaccharides from sweet potato tubers.
Content analysis of each polysaccharide
The following glucose standards were used in the analysis: 0.02, 0.04, 0.05, 0.06, 0.07, 0.08, 0.10, and 0.12 mg/mL. Next, 1 mL of each standard was transferred into a tube. For conditions using ice water, 1 mL of a 5% phenol solution was slowly added, and the sample was mixed. Next, 5 mL of concentrated sulfuric acid was rapidly added, and the solution was shaken and incubated for 20 min at room temperature. For the blank control, 1 mL of distilled water was added using the same procedure. The absorbance was measured at a wavelength of 490 nm using a spectrophotometer. Glucose content was determined using Equation 1, which was derived from the standard curve of glucose:
Abs = 10.0425c + 0.0018 R2 = 0.9989 (1)
Approximately, 10 mg of the lyophilized polysaccharide powder was mixed with water to a total volume of 100 mL to yield a final concentration of 0.1 mg/mL. Next, 1.0 mL of the solution was transferred into a tube, and the same procedure was performed as earlier described. Glucose concentration was calculated by using the absorbance of the solution and Equation 1. Percent glucose content was calculated using Equation 2:
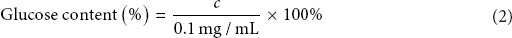
where c is the concentration of glucose calculated by the glucose standard curve equation above.
Hydroxyl radical scavenging activity assay
The hydroxyl radical scavenging activity of each extract was measured by using a modified Cumbes and Smironoff method and the Fenton reaction.[25] The reaction mixture (10 mL) contained 2 mL of FeSO4 (9 mM), 2 mL of salicylic acid (9 mM), and 2 mL of the sample (0.5 mg/mL). For positive control, 2 mL of 0.5 mg/mL ascorbic acid was used; and for blank solvent control, 2 mL of distilled water was employed. Approximately, 2 mL of H2O2 (8 mM) was added to activate the reaction, followed by incubation of the solution at 37°C for 0.5 h. The absorbance of each solution was measured at a wavelength of 510 nm using an ultraviolet spectrophotometer. The hydroxyl radical scavenging capacity was calculated using Equation 3:

where Acontrol is the absorbance of the control, and Asample is the absorbance of the samples.
Analysis of the inhibitory effects of sweet potato tuber extracts on albumin nonenzymatic glycosylation in vitro.
The inhibitory effects of the sweet potato tuber extracts were examined by using a modified version of the method of Brownlee et al.[17,18,26] A glucose mixture containing 5% (w/v) BSA, 1 M glucose, and 0.1% (w/v) NaN3 (pH 7.4) in phosphate-buffered saline (PBS) was used. Each lyophilized polysaccharide extract was dissolved to yield a 0.5 mg/mL solution. (i) The same amount of distilled water was added to the blank control (o), or the same concentration of amino guanidine was used as positive control.
All of the solutions were filtered through a 0.22 μm Millipore filter before transferring into Eppendorf tubes. Each sample was incubated in the dark at 37°C for 4 weeks, and the fluorescence intensity was measured each week with a fluorospectrophotometer at an excitation wavelength (Ex) of 370 nm and an emission wavelength (Em) of 440 nm. The inhibitory effect was calculated by using Equation 4, and the value at the 4th week was used for RSM analysis.
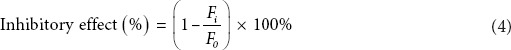
where Fi is the fluorescence intensity of the lyophilized polysaccharide extract, and Fo is the fluorescence intensity of the blank control.
Design of the artificial neural network
Modeling of the artificial neural network
By using the back-propagation algorithm,[27,28] the multilayer feed-forward and feedback neural network has been proven to be an excellent universal approximator of nonlinear functions.[29] The architecture of this model represents a fully-connected structure, and each experimental group is considered as a neuron. In addition, each neuron is connected to all the neurons in the hidden layer.[30] Figure 1 shows that in the present study, a feed-forward neural network trained with an error back-propagation algorithm was employed using MATLAB Neural Network Toolbox (Version 7.11, Mathworks, Natick, MA, USA) to model the hydroxyl radical scavenging value and glycosylation inhibition of independent variables used in the ultrasonic treatments. The input parameters chosen in the present study were ultrasonication power, extraction time, and temperature, and the output parameters were the hydroxyl radical scavenging value and the inhibitory effect on glycosylation value (Y1 and Y2, respectively). The neurons in the input layer introduced the normalized input data to the hidden layer via weights. The neurons in the hidden layer summed up the weighted inputs to neurons, including bias, based on Equations 5–7:
Figure 1.
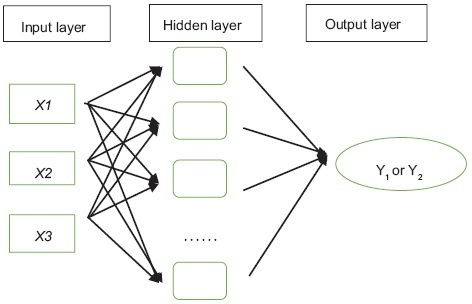
Diagram of the architecture of the artificial neural network model (not all the factors in the hidden layer are shown). Showing the internal connections among extraction time (X1), ultrasonic power (X2), and extraction temperature (X3) with hydroxyl radical scavenging activity (Y1) and nonenzymatic glycosylation inhibition (Y2)

where wi (i = 1, n) wi (i = 1, n) is the connecting weight, θ is the bias, xi xi is the input data, and net is the activation.
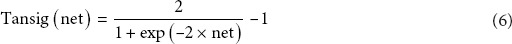
The weighted output was then activated using the hyperbolic tangent sigmoid transfer function, as shown in Equation 7.
Pureline (xi) = xi (7)
The output produced by the hidden layer became an input to output using this linear transfer function.
Supervised learning was used to train this network. The predicted output and desired output were compared to a virtual value and the errors were calculated. An error back propagation algorithm was used to adjust the weights to reduce the deviations.[31] Using this decreasing gradient, the weights were proportionally changed to decrease the error gradient. The training iterations were terminated when the validation error reached the set minimum.
Optimization of the artificial neural network
Using the genetic algorithms of Goldberg and Holland,[32] Yang et al.[19] solved linear and nonlinear problems by exploring all aspects of the network and scanning promising areas through selection, multiplication, and mutation operations applied to individuals in the population.[3] Genetic algorithms also reduce the number of times required for training iterations, thereby avoiding training to an extreme microregion. After the feed-forward ANN model was completely trained, genetic algorithms can identify the optimal ultrasonic extraction conditions that would yield the highest hydroxyl radical scavenging value or the strongest inhibitory effects on glycosylation.
Statistical analysis
All data were expressed as the mean of three replicates. Statistical calculations were performed using Microsoft Excel 2013 (Microsoft, Seattle, WA, USA) and MATLAB.
RESULTS AND DISCUSSION
Antioxidant activities, inhibition of glycosylation, and polysaccharide content of each sweet potato tuber extract.
Polysaccharides from purple sweet potato tubers contain a high number of hydroxyl groups that have the capacity of eradicate free radicals. According to Zhu et al.,[33] hydroxyl radicals are produced through several different reactions. Using the products of the Fenton reaction to produce free radicals in vitro, we measured the hydroxyl radical scavenging activity of the extracted polysaccharides. Table 3 shows the hydroxyl radical scavenging activity of extracts prepared using different extraction conditions. Ultrasonic treatment facilitates in the destruction of plant cells and influences the release of polysaccharides. Under the conditions tested in the present study, the scavenging rates of the extracts ranged from 11.22% to 30.89%. These findings suggest that the consumption of purple sweet potato tubers may reduce the amount of free radicals present in the body. Polysaccharides from purple sweet potatoes may also have some antitumor activities, similar to the polysaccharides isolated from sclerotia and mycelia of Pleurotus tuber-regium.[7]
Table 3.
The predicted and actual values of glycosylation inhibitory effects and hydroxyl radical scavenging activities of polysaccharides extracted from purple sweet potatoes prepared under different extraction conditions
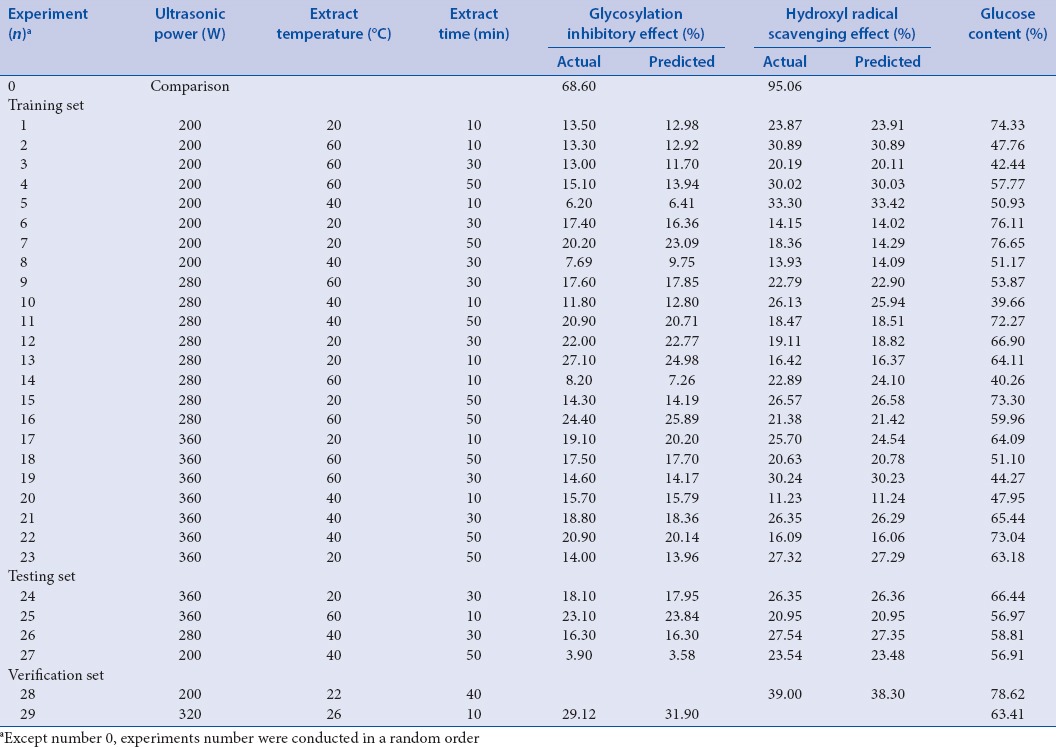
The Brownlee method is a conventional technique for the identification of AGE inhibitors that can block the production of early glycosylation products. Amino guanidine was used as a positive control in the inhibitory effects assay.[24,34] Table 3 shows that all lyophilized sweet potato samples inhibited glycosylation when used at a concentration of 0.5 mg/mL. Differences in the inhibitory effects among groups may have resulted from variations in extraction conditions, which in turn might have influenced polysaccharide dissolution. Nonetheless, the results showed that polysaccharides from purple sweet potatoes inhibited nonenzymatic glycosylation and may be potentially used for the prevention and/or treatment of diabetes.[35]
Polysaccharide content of the experimental groups was determined by using the phenol-sulfuric acid method, and each sample contained about 39.66%–78.62% deoxidized sugar. Further studies on the relationship between glucose content and antioxidant activity or glycosylation inhibitory activity are in progress. There may be other nonreducing sugars that affect the antioxidant and glycosylation inhibitory activities of these extracts.
All of these three assays yielded data that were nonlinear in nature and described its relationship with various ultrasonic extraction conditions. Thus, these assays, which used ANN through the RSM in MATLAB, provided significant improvement in this particular extraction methodology.
Optimization and prediction of ultrasonic extraction conditions by ANN according to hydroxyl radical scavenging activity and non-enzymatic glycosylation inhibition.
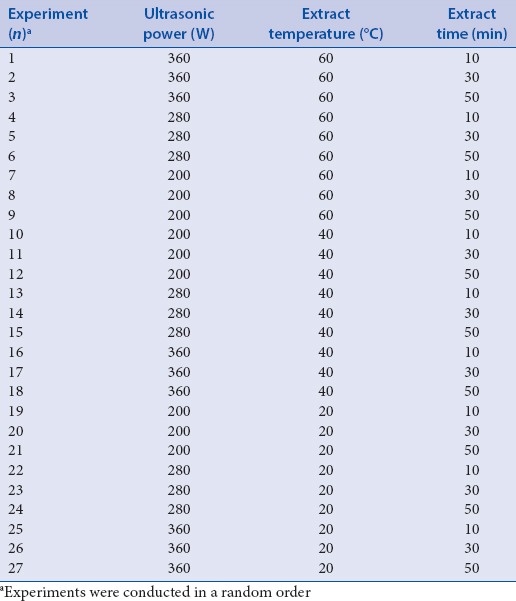
Table 3 and Figure 2 show the nonlinear relationship of ultrasonication temperature, time, power, and different combinations of these variables on hydroxyl radical scavenging activity. The optimum conditions for ultrasonic extraction were as follows: Ultrasonication power at 200 W, temperature at 22°C, and duration of 40 min. These conditions are predicted to result in extracts with 38.3% hydroxyl radical scavenging activity. Validation experiments showed an activity of 39.0% [verification set No. 28, Table 3].
Figure 2.
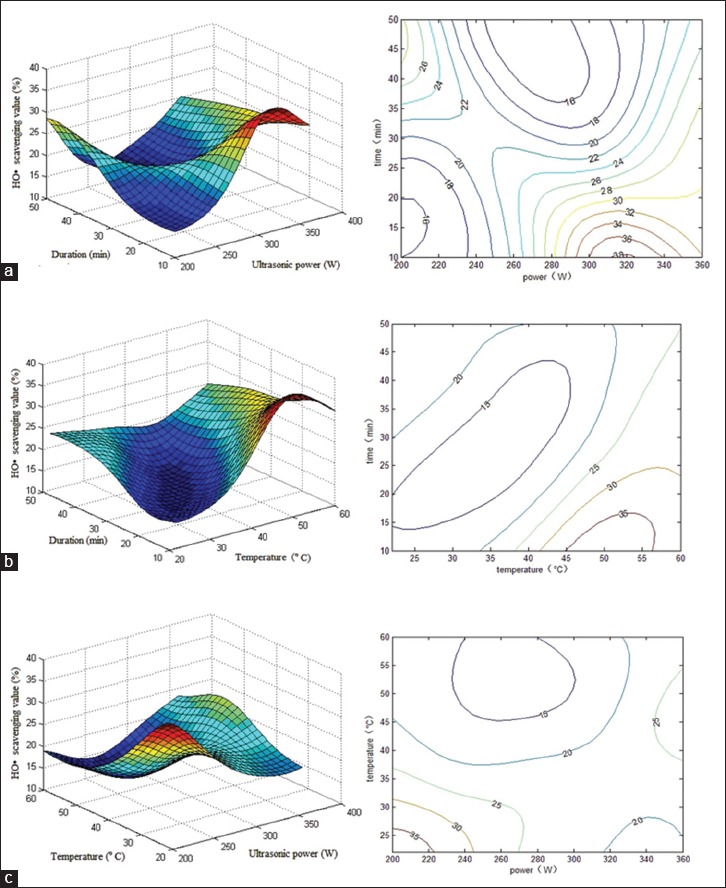
Response surface plot showing the effects of ultrasonication power and duration on hydroxyl radical scavenging activity. The corresponding two-dimensional elevation chart is shown on the left. Experimental and predicted data and conditions are shown in Table 3. (a) Ultrasonication temperature was kept constant at 22°C; (b) ultrasonication power was kept constant at 200 W; (c) ultrasonication temperature was kept constant at 40 min
Figure 2a–c shows that the highest free radical scavenging activity was obtained when the ultrasonication power and temperature were close to 200 W and 20°C, respectively. On the other hand, in terms of ultrasonication power and extraction time, the respective conditions of 320 W and 10 min showed the best results. In terms of extraction time and temperature, the optimal conditions were 50°C for 10–15 min. The more complicated interactions between two of the three factors were demonstrated in each three-dimensional graph. For example, when 40 min of ultrasonication at 50°C was performed, hydroxyl radical scavenging activity decreased with increasing ultrasonication power, and thereafter increased. On the other hand, lower scavenging activity was observed with increasing ultrasonication power for 40 min at 30°C. Similar relationships were observed between time and power, as well as between temperature and power.
Table 3 and Figure 3 show that the three factors interacted when the glycosylation inhibitory effect was utilized as output. The nonlinear nature of the relationships among the three conditions and the glycosylation inhibitory effect is shown in Figure 3a–c. Each of the three factors is presented in both the three-dimensional graph and its corresponding two-dimensional elevation chart. The optimal conditions for ultrasonic extraction, as computed through ANN, were as follows: Ultrasonication power at 230 W, temperature at 20°C, and duration of 50 min. The predicted hydroxyl radical scavenging activity was 34.5%. We predicted the powers of 200, 240, 280, 320, and 360, and the optimum condition of 320 W and 26°C for 10 min, to result in a 31.9% glycosylation inhibition, which was obtained from these five power values. Validation testing generated an inhibitory value of 29.1%, as shown in verification set No. 29 of Table 3.
Figure 3.
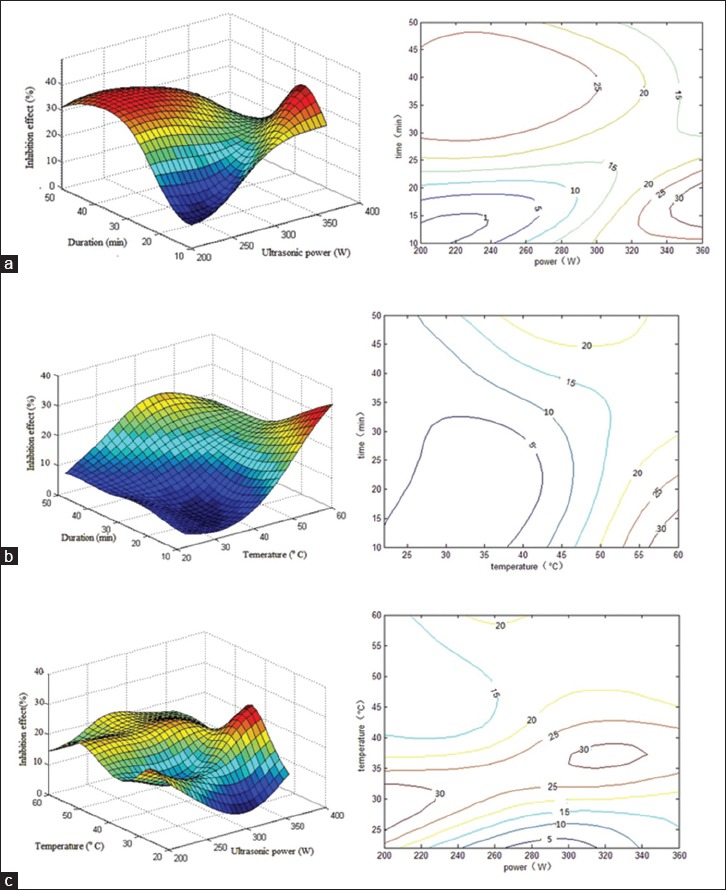
Response surface plot showing the effects of ultrasonication power and duration on the inhibition of nonenzymatic glycosylation. The corresponding two-dimensional elevation chart is shown on the left. Experimental and predicted data and conditions are shown in Table 3. (a) Ultrasonication temperature was kept constant at 22°C; (b) ultrasonication power was kept constant at 230 W; (c) temperature was kept constant at 50 min
Similarly, using the same ultrasonication power, a glycosylation inhibitory effect was observed, even when the variables of time and temperature were changed.
Overall, our data showed that at a certain ultrasonication power, the activity of antioxidants and the inhibition of glycosylation increased with higher temperatures (50°C–60°C) and shorter duration (10–25 min). Furthermore, these inhibitory effects increased with lower temperatures (20°C–40°C) and longer ultrasonication duration (30–50 min). We believe that similar results may be observed using other factors not included in the present study. These data are presented as two peak regions in the same figure.
Similar to the results of Li et al.,[36] changes in the inhibitory effects arise from ultrasonic treatment, which could modify polysaccharide solubility and yield during extraction. In addition, Wu et al.[37] reported that the activities of polysaccharides largely depend on the number of hydroxyl groups and the structure of the actual molecule.[20] In the present study, a higher ultrasonication power resulted in a greater reduction in molecular weight of the polysaccharide. However, at the same time, higher ultrasonication power increased molecular motion, thereby improving the solubility of the polysaccharide. In terms of extraction time, longer durations may result in more soluble polysaccharide extracts, but also increase the shearing effect of the ultrasonic waves. Higher temperatures also promote polysaccharide solubility, but again may accelerate the shearing effects of the ultrasonic waves. Therefore, because of the complicated relationships among ultrasonic extraction factors, RSM has facilitated in the optimization of extraction conditions for polysaccharides from sweet potato tubers.
Evaluation of model predictability
In the present study, RSM was employed to identify the optimal ultrasonication power, temperature, and time for achieving maximal hydroxyl radical scavenging activity and nonenzymatic glycosylation inhibition. The actual and predicted values are presented in Table 3.
The parameter R2 is defined as the ratio of the explained variation to the total data and reflects the degree of fitness. The model well fits the actual values when R2 approached 1. The performance of the ANN was statistically analyzed by using the R2 values obtained using Equation 8:

where n is the number of experimental groups, yp is the predicted value by using the ANN model, yo is the actual value, and ya is the average of the actual values.
The ANN effects of the three extraction factors within specific ranges (ultrasonication power: 200–360 W, duration: 10–50 min, and temperature: 20°C–60°C) were examined. To test the generalization capacity of the network, 27 experimental groups were divided into two sets, and four groups were randomly selected [Table 3] for testing. The rest of the sets was used for training. Data processing was performed using the least squares method in Microsoft Excel, and the results were analyzed as plots of the actual and predicted values of hydroxyl radical scavenging activities and R2 values [Figure 4]. The R2 values of the training and testing sets were 0.953 and 0.999, respectively. Using MATLAB, the trained network yielded an R2 of 0.957. Figure 5 depicts plots of the actual and predicted values of nonenzymatic glycosylation inhibition, as well as the R2 values. The R2 values of the training and testing sets were 0.996 and 0.999, respectively. The trained network yielded an R2 of 0.998 when using MATLAB.
Figure 4.

Correlation between the predicted values of the neural network and the actual values of the hydroxyl radical-scavenging activity. The training set and testing set were obtained from this chart. The fitting formula and its R2 were included. Experimental and predicted data and conditions are shown in Table 3
Figure 5.
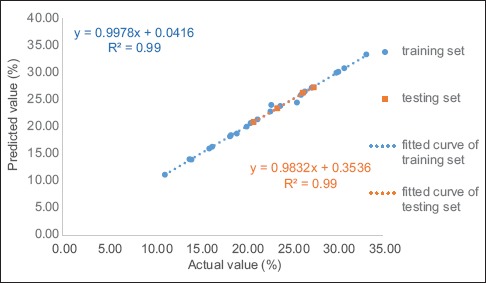
Correlation between the predicted values of the neural network and the actual values of the glycosylation inhibitory effect. The training set and testing set were obtained from this chart. The fitting formula and its R2 were included. Experimental and predicted data and conditions are shown in Table 3
Therefore, based on the MATLAB results, both ANNs predicted a value within the range of ± 0.8% of the actual value. These findings were in good agreement with the predicted value of the neural network model and the actual value in the two model predictions, thereby confirming that this ANN is generally applicable.
CONCLUSIONS
Polysaccharides extracted from purple sweet potato tubers showed free radical scavenging activity and inhibited albumin nonenzymatic glycosylation. Using RSM, we developed an ANN model, which predicted high-fitting experimental data. Ultrasonication conditions of 200 W, 22°C, and 40 min generated the highest hydroxyl scavenging activity, whereas conditions of 230 W, 22°C, and 50 min yielded the greatest inhibitory effects on albumin nonenzymatic glycosylation. Multiple regression analysis indicated that ultrasonication power, temperature, and duration largely influenced the antioxidant and glycosylation inhibitory activities of these extracts. However, these activities did not show an absolute relationship with polysaccharide content. Perhaps, these were affected by other nonreducing sugars in the extracts. The results suggested that frequent consumption of purple sweet potatoes may facilitate in the reduction of blood sugar levels in patients with diabetes, which also further strengthens the validity of this therapeutic folk diet. Further studies on the bioactivities of other polysaccharides in the purple sweet potato are in progress.
Financial support and sponsorship
This study was financially supported by the National Natural Science Foundation of China (No. 81102779), the Guangdong Natural Science Foundation (No. 9451022401003453), the Pearl River S&T Nova Program of Guangzhou (No. 2013J2200035), the Innovation Program of the University of Guangdong Province (No. 2014KTSCX118), the Science and Technology Program of Guangdong Province (No. 2014A050503067 and 2015A020211032) and the High-level Talents Program of the University of Guangdong Province.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.The Editorial Committee of the Administration Bureau of Flora of China. Flora of China (Zhongguo Zhiwuzhi) Vol. 64. Beijing, China: Beijing Science and Technology Press; 2005. pp. 88–90. [Google Scholar]
- 2.Oki T, Masuda M, Furuta S, Nishiba Y, Terahara N, Suda I. Involvement of anthocyanins and other phenolic compounds in radical-scavenging activity of purple-fleshed sweet potato cultivars. J Food Sci. 2002;67:1752–6. [Google Scholar]
- 3.Philpott M, Gould KS, Lim C, Ferguson LR. In situ and in vitro antioxidant activity of sweetpotato anthocyanins. J Agric Food Chem. 2004;52:1511–3. doi: 10.1021/jf034593j. [DOI] [PubMed] [Google Scholar]
- 4.Hou WC, Han CH, Chen HJ, Wen CL, Lin YH. Storage proteins of two cultivars of sweet potato (Ipomoea batatas L.) and their protease hydrolysates exhibited antioxidant activity in vitro. Plant Sci. 2005;168:449–56. [Google Scholar]
- 5.Nandita S, Rajini PS. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004;85:611–6. [Google Scholar]
- 6.Rupérez P, Ahrazem O, Leal JA. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J Agric Food Chem. 2002;50:840–5. doi: 10.1021/jf010908o. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Zhang LN, Cheung PC, Ooi VE. Molecular weight and anti-tumor activity of the water-soluble polysaccharides isolated by hot water and ultrasonic treatment from the sclerotia and mycelia of Pleurotus tuber-regium. Carbohydr Polym. 2004;56:123–8. [Google Scholar]
- 8.Cui CB, Jeong SK, Lee YS, Lee SO, Kang IJ, Lim SS. Inhibitory activity of caffeoylquinic acids from the aerial parts of Artemisia princes on rat lens aldose reductase and on the formation of advanced glycation end products. J Korean Soc Appl Biol Chem. 2009;52:655–62. [Google Scholar]
- 9.Negri E, La Vecchia C, Franceschi S, D’Avanzo B, Parazzini F. Vegetable and fruit consumption and cancer risk. Int J Cancer. 1991;48:350–4. doi: 10.1002/ijc.2910480307. [DOI] [PubMed] [Google Scholar]
- 10.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: The Zutphen study. Arch Intern Med. 1996;156:637–42. [PubMed] [Google Scholar]
- 11.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 12.Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. Am J Clin Nutr. 2003;78(3 Suppl):570S–8S. doi: 10.1093/ajcn/78.3.570S. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DL, Lehninger AL, Cox MM. Lehninger Principles of Biochemistry. New York: Palgrave Macmillan; 2004. pp. 488–599. [Google Scholar]
- 14.Ortiz de Montellano PR. Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd ed. New York: Kluwer Academic/Plenum Publishers; 2005. pp. 149–70. [Google Scholar]
- 15.Boligon AA, Pereira RP, Feltrin AC, Machado MM, Janovik V, Rocha JB, et al. Antioxidant activities of flavonol derivatives from the leaves and stem bark of Scutia buxifolia Reiss. Bioresour Technol. 2009;100:6592–8. doi: 10.1016/j.biortech.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 16.Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–42. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 17.Brownlee M, Vlassara H, Cerami A. Inhibition of heparin-catalyzed human antithrombin III activity by nonenzymatic glycosylation. Possible role in fibrin deposition in diabetes. Diabetes. 1984;33:532–5. doi: 10.2337/diab.33.6.532. [DOI] [PubMed] [Google Scholar]
- 18.Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527–37. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Zhao M, Jiang Y. Optimization of tyrosinase inhibition activity of ultrasonic-extracted polysaccharides from longan fruit pericarp. Food Chem. 2008;110:294–300. doi: 10.1016/j.foodchem.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Despagne F, Massart DL. Neural networks in multivariate calibration. Analyst. 1998;123:157R–78R. doi: 10.1039/a805562i. [DOI] [PubMed] [Google Scholar]
- 21.Wasserman PD. Advanced Methods in Neural Computing. New York: John Wiley & Sons, Inc; 1993. pp. 36–116. [Google Scholar]
- 22.Meira M, Silva EP, David JM, David JP. Review of the genus Ipomoea: Traditional uses, chemistry and biological activities. Rev Bras Farmacogn. 2012;22:682–713. [Google Scholar]
- 23.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6. [Google Scholar]
- 24.Yan C, Kong F, Zhang D, Cui J. Anti-glycated and antiradical activities in vitro of polysaccharides from Ganoderma capense. Pharmacogn Mag. 2013;9:23–7. doi: 10.4103/0973-1296.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smirnoff N, Cumbes QJ. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28:1057–60. [Google Scholar]
- 26.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–34. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 27.Rumelhart DE, McClelland JL PDP Research Group. Parallel Distributed Processing: Explorations in the Microstructure of Cognition. Vol. 1. Cambridge, MA: MIT Press; 1986. pp. 293–323. [Google Scholar]
- 28.Watrous RL. Learning Algorithms for Connectionist Networks: Applied Gradient Methods of Nonlinear Optimization. Technical Reports (CIS) 1988:1–33. [Google Scholar]
- 29.Zhang GQ, Patuwo BE, Hu MY. Forecasting with artificial neural networks: The state of the art. Int J Forecast. 1998;14:35–62. [Google Scholar]
- 30.Dreyfus C, Dreyfus G. A machine learning approach to the estimation of the liquidus temperature of glass-forming oxide blends. J Non Cryst Solids. 2003;318:63–78. [Google Scholar]
- 31.Hagan MT, Demuth HB, Beale M. Neural Network Design. Vol. 11. Boston, MA: PWS Publisher; 1996. pp. 7–11. [Google Scholar]
- 32.Goldberg DE, Holland JH. Genetic algorithms and machine learning. Mach Learn. 1988;3:95–9. [Google Scholar]
- 33.Zhu F, Cai YZ, Yang X, Ke J, Corke H. Anthocyanins, hydroxycinnamic acid derivatives, and antioxidant activity in roots of different Chinese purple-fleshed sweetpotato genotypes. J Agric Food Chem. 2010;58:7588–96. doi: 10.1021/jf101867t. [DOI] [PubMed] [Google Scholar]
- 34.Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–32. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy L, Baynes JW. Non-enzymatic glycosylation and the chronic complications of diabetes: An overview. Diabetologia. 1984;26:93–8. doi: 10.1007/BF00281113. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Guo SY, Li XN. Degradation kinetics of polystyrene and EPDM melts under ultrasonic irradiation. Polym Degrad Stab. 2005;89:6–14. [Google Scholar]
- 37.Wu Q, Zheng C, Ning ZX, Yang B. Modification of low molecular weight polysaccharides from Tremella fuciformis and their antioxidant activity in vitro. Int J Mol Sci. 2007;8:670–9. [Google Scholar]


