Abstract
Objective:
This work aimed to investigate the anti-epileptic effects of valepotriate isolated from Valeriana jatamansi Jones and studied its possible mechanisms.
Methods:
The anti-epileptic effects of valepotriate were studied using maximal electroshock-induced seizure (MES), pentylenetetrazole (PTZ)-induced epilepsy, and pentobarbital sodium-induced sleeping model in mice. The possible anti-epileptic mechanisms of valepotriate were investigated by analyzing the expressions of GABAA, GABAB, glutamic acid decarboxylase (GAD65), Bcl-2, and caspase-3 in the brain using Western blot assay.
Results:
The results indicated that valepotriate showed significant anti-epileptic activity against MES- and PTZ-induced epilepsy at doses of 5, 10, and 20 mg/kg, and ED50 values for MES- and PTZ-induced epilepsy were 7.84 and 7.19 mg/kg, respectively. Furthermore, valepotriate (10 and 20 mg/kg) can significantly prolong sleeping time and shorten the latency time on the pentobarbital sodium-induced sleeping time test. Furthermore, valepotriate (5, 10, and 20 mg/kg) could significantly up-regulate the expression of GABAA, GAD65, and Bcl-2 and down-regulate the expression of caspase-3, but had no significant effect on the expression of GABAB.
Conclusion:
The results indicated that valepotriate had anti-epileptic activity and the mechanisms might be associated with regulation of GABA and inhibition of neuronal apoptosis.
SUMMARY
Anti-epileptic effect of valepotriate was investigated for the 1st time
Valepotriate showed notable anti-epileptic activity
Valepotriate can significantly increase the expression of GABAA, glutamic acid decarboxylase 65, and Bcl-2 and reduce the expression of caspase-3.
Keywords: Anti-epileptic, apoptosis, GABA, valepotriate, Valeriana jatamansi jones
INTRODUCTION
Epilepsy is a chronic brain disease related to central nervous system dysfunction because of the abnormal discharge of neurons in the brain. It is clear that epilepsy cannot be cured completely. In addition, epilepsy patients need a lot of their family members to take care of them because epilepsy is a sudden and repetitive disease and can hinder their athletic ability, sense, behavior, autonomic nerve, etc.[1] When people suffered from epilepsy, anti-epileptic drug (AED) is a common measure to inhibit the abnormal discharge of neurons or prevent the abnormal discharge of neurons from diffusing.[2] Even so, there are many people with epilepsy all over the world, and over 30% people with epilepsy are still out of control.[3] Furthermore, these currently used AEDs could result in some serious side effects. Therefore, more new strategy or drugs with effective and less adverse effects are desperately needed.[4,5,6]
Traditional Chinese medicines (TCMs) were effective complementary and alternative medicines and played a huge role in the treatment of epilepsy.[7,8,9] In addition, a lot of researches reported that TCMs can alleviate epilepsy, such as Acorus gramineus,[10] Gastrodia elata,[11] Uncaria rhynchophylla,[12] Glycyrrhiza glaba,[13] Panax ginseng,[14] and Curcuma longa.[15] To evaluate the anti-epileptic effect of drugs, generally macroscopic pharmacological experiments, such as maximal electroshock-induced seizure (MES) model,[16] pentylenetetrazole (PTZ)-induced epilepsy model,[17] and pentobarbital sodium-induced sleeping time model,[18] were widely applied from macroscopic responding in animals. If these macroscopic pharmacological experiments show that drugs have a good anti-epileptic effect, further research should focus on anti-epileptic mechanism by microcosmic experiments, such as observing the change of neurotransmitter and receptor in the brain.[19,20] These results can provide a scientific basis for the development of drugs.
Valeriana jatamansi Jones (Valerianaceae) was the frequently used TCMs as anxiolytic, sedative, and hypnotic drug.[21,22,23] According to the existing pharmacologic action, it indicated that V. jatamansi could inhibit the excitability of nerve in the brain. The purpose of this paper was mainly to focus on investigating the anti-epileptic activity of valepotriate and explore the potential mechanism.
MATERIALS AND METHODS
Plant material
The roots of V. jatamansi were purchased from Chinese herbal medicine market located in Baoding city, Hebei, and plant material was identified by the Second Affiliated Hospital of Zhejiang University's Pharmacy Department, Hangzhou, China. A voucher specimen (voucher no. 201185/ZJUSM) was stored in the School of Medicine, Zhejiang University, for future reference.
Animals
All experiments involving animals in this research accorded with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were favored by the Second Affiliated Hospital of Zhejiang University, Zhejiang University (protocol ZJUSMEA2012). In the experiment, a series of measures were carried out to relieve the suffering of animals and reduce the amount of animals. Male ICR mice (20 ± 2 g) and Sprague Dawley (SD) rats (200 ± 20 g) were purchased from Shanghai laboratory animal center (Shanghai, China). Animals were bred with adjustable humidness (60–65%) and constant room temperature (25 ± 1°C). Animals had free access to food and water (n = 10).
Drugs and chemicals
The analytical reagents ethanol, ethyl acetate (EtOAc), petroleum ether, and ethyl ether and HPLC grade acetonitrile were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Silica gel (300–400 mesh) was obtained from Qingdao Haiyang Chemical Co., Ltd (Qingdao, China). Phenytoin sodium, diazepam, PTZ, and pentobarbital sodium were purchased from Sigma (St. Louis, MO) and Aladdin Reagent Inc., (Shanghai, China). GABAA, GABAB, Bcl-2, and caspase-3 monoclonal antibodies were purchased from Abcam Co., Ltd (Cambridge, UK). Goat anti-rabbit/ thorseradish peroxidase-conjugated antibodies was purchased from Shanghai Hao Yang Biotechnology Co., Ltd (Shanghai, China).
Preparation of valepotriate
The dried powders of the roots of V. jatamansi (40 kg) were extracted 5 times and refluxed with 90% ethanol. The ethanol extract was concentrated and partitioned with EtOAc. Then, the EtOAc-soluble extract was further separated by column chromatography on silica gel (300–400 mesh), which was eluted with ethyl ether-EtOAc (30:1, 15:1, 10:1, 6:1, 2:1, 1:1). Then, six sub-fractions were obtained (I-VI), and subsequently, the valepotriate (purity >90%) was isolated from the IV by repeated silica gel (300–400 mesh) column chromatography. The identification of the purity of valepotriate was performed on Bruker Avance DRX-500 nuclear magnetic resonance (NMR) spectrometer (Bruker, Germany) and SHIMADZU LC-20AT (Shimadzu, Japan). The chromatographic column was Ultimate AQ C18 (250 mm × 4.6 mm, 5 μm), the mobile phase was acetonitrile-water (70:30), and the detection wavelength was 254 nm.
Protocols
The anti-epileptic effect of valepotriate was evaluated by MES- and PTZ-induced epilepsy mice model, and pentobarbital sodium-induced sleeping model in mice. Further, a PTZ-induced chronic epileptic rat model was established, and the anti-epileptic mechanism of valepotriate was investigated by Western blot, which was used to test the expression of GABAA, GABAB, glutamic acid decarboxylase (GAD 65), Bcl-2, and caspase-3 in SD rats’ brains. The positive control dosage was in accordance with clinical dosage. The valepotriate dosages were 5, 10, and 20 mg/kg, and administered by intraperitoneal injection (ip).
Acute toxicity studies
Mice were divided into 7 groups randomly (n = 10), and mice in groups of 1–6 were administered 5, 10, 20, 40, 80, and 120 mg/kg of valepotriate (ip), respectively. In addition, the 7th group received 1% DMSO (20 mL/kg, ip). The mortality rates and abnormal neurobehaviors of the mice (including ataxia, motor coordination, etc) within 24 h were observed and recorded.
In our present study, neither death nor any abnormal neurobehaviors were observed, and the 50% lethal dose was not obtained due to lack of observable toxicity of all the testing dosages.
Maximal electroshock-induced seizure model in mice
The MES experiment was carried out according to the existing method[24] with minor modification and the evaluation index was Hind Limb Tonic Extension (HLTE).[25,26] ICR mice were pretreated with phenytoin sodium (20 mg/kg, ip), valepotriate (5, 10 and 20 mg/kg, ip), and 1% DMSO (20 mL/kg, ip, control). After 30 min, the alternating current stimulus (50 mA, 50 Hz, 1 s duration) was used to stimulate mice. The HLTE action of the animal was observed within 10 s after electrical stimulation. If valepotriate had anti-epileptic activity, the HLTE rate of animals would be significantly lower than the control group.
Pentylenetetrazole-induced epilepsy model in mice
The PTZ-induced epilepsy assay was performed according to previously reported method,[17] which was suitably modified and the evaluation index was HLTE.[24,25] ICR mice were pretreated with diazepam (4 mg/kg, ip), valepotriate (5, 10 and 20 mg/kg, ip), and 1% DMSO (20 mL/kg, ip, control). After 30 min, ICR mice in each group were treated by PTZ (90 mg/kg, ip). Animals were observed within 30 min. If valepotriate had anti-epileptic activity, the HLTE rate of animals would be significantly lower than the control group.
Pentobarbital sodium-induced sleeping time model in mice
The pentobarbital sodium-induced sleeping time assay was performed according to the previous method,[27] which was suitably modified. ICR mice were pretreated with diazepam (4 mg/kg, ip), valepotriate (5, 10 and 20 mg/kg, ip), and 1% DMSO (20 mL/kg, ip, control). After 30 min, ICR mice in each group were treated by pentobarbital sodium (50 mg/kg, ip). In this assay, the “righting reflex,” the animals recover normal position autonomously when they were in abnormal position was an important index. The latency time was from intraperitoneal injection of pentobarbital sodium to the loss of the righting reflex, and the sleeping time was from the loss of the righting reflex to the recovery of the righting reflex.
Measurement of GABAA, GABAB, glutamic acid decarboxylase 65, Bcl-2, and caspase-3 expressions
According to the existing literature,[28] a PTZ-induced chronic epileptic rat model was established for futhre Western blot assay. Briefly, 40 SD rats were divided into four groups: Control group (1% DMSO, control) and three treatment groups (valepotriate, 5, 10, 20 mg/kg, ip). All SD rats of PTZ-induced chronic epileptic model was prepared by administering PTZ (32 mg/kg, ip) once per day in the morning for 28 days. “Intense jumping, shouting, and jerking” was the assessment criteria for the PTZ-induced chronic epileptic mouse model. SD rat with PTZ-induced chronic epilepsy were pretreated with valepotriate (5, 10, 20 mg/kg/d, ip) and 1% DMSO (20 mL/kg/d, ip) for 3 weeks. Afterward, SD rats were sacrificed after anesthesia. Whole and total brain of every group were collected to extract total protein. Equal amounts of protein (35 μg) were separated by SDS-PAGE, blotted on polyvinylidene difluoride (PVDF) membrane. Further, PVDF membranes with target protein were probed with anti-GABAA, GABAB, GAD65, Bcl-2, and caspase-3 rabbit polyclonal IgG and where after with goat anti-rabbit/HRP, and detected with chemiluminescence. Antibody directed against β-actin was used to measure protein loading.
Statistical analysis
All data were expressed as mean ± SD, and analyzed on SPSS 22.0 (SPSS Inc., USA). The Students t- test and one-way ANOVA were used to analyze the differences between two groups and among 3 or more groups separately. The differences were recognized as statistically significant when P < 0.05.
RESULTS
Qualitative identification of valepotriate
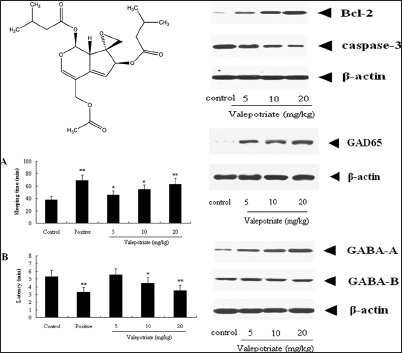
The 1H NMR and 13C NMR of yellow oil obtained from V. jatamansi were as follows: 1H NMR (600 MHz, CDCl3): 6.71 (1H, s, H-3), 5.92 (1H, d, J = 10.3, H-1), 5.85 (1H, m, H-7), 5.43 (1H, d, J = 2.9, H-6), 4.51, 4.72 (2H, d, J = 13.2, H-11), 3.53 (1H, dd, J = 9.3, 2.8, H-9), 2.86, 3.11 (2H, d, J = 4.7, H-10), 2.08–2.24 (6H, m, H-17, 18, 23, 24), 2.13 (3H, s, H-14), 1.12 (3H, s, H-26), 1.02 (3H, s, H-25), 0.99 (3H, s, H-20), 0.89 (3H, s, H-19); 13C NMR (150 MHz, CDCl3): 173.9 (C-13), 171.7 (C-22),169.8 (C-16), 151.5 (C-3), 145.7 (C-5),119.5 (C-6), 111.6 (C-4), 92.7 (C-1), 82.2 (C-7), 65.3 (C-8), 62.0 (C-11), 49.3 (C-10), 42.5 (C-17), 41.3 (C-9, C-23), 26.2 (C-18), 25.7 (C-24), 23.1 (C-19, C-20), 22.5 (C-25, C-26), 20.2 (C-14). According to the 1H NMR and 13C NMR data compared with the reference,[29] this compound was identified as valepotriate [Figure 1].
Figure 1.
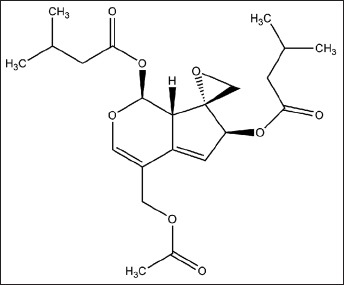
Chemical structure of valepotriate
Results of maximal electroshock-induced seizure and pentylenetetrazole-induced epilepsy assays
As can be seen from Tables 1 and 2, compared with control group, valepotriate at the doses of 5, 10, and 20 mg/kg significantly inhibited the convulsions induced by both MES and PTZ compared with the control mice (P < 0.05) with a dose-dependent manner, and the ED50 values for MES- and PTZ-induced epilepsy were 7.84 and 7.19 mg/kg, respectively.
Table 1.
Effects of valepotriate on maximal electroshock-induced seizures in mice

Table 2.
Effects of valepotriate on pentylenetetrazole-induced seizures in mice
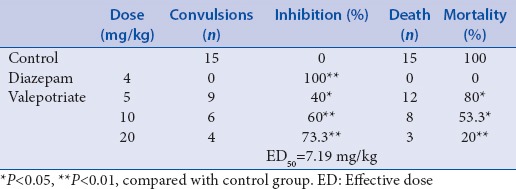
In addition, the valepotriate (5, 10, and 20 mg/kg) can also decrease the mortalities of mice suffered from MES and PTZ compared with the control mice (P < 0.05), with a dose-dependent manner.
Results of pentobarbital sodium-induced sleeping time assay
From our results presented in Figure 2, valepotriate at doses of 5, 10, and 20 mg/kg can significantly prolong the sleeping time (P < 0.05, P < 0.05, and P < 0.01, respectively).
Figure 2.
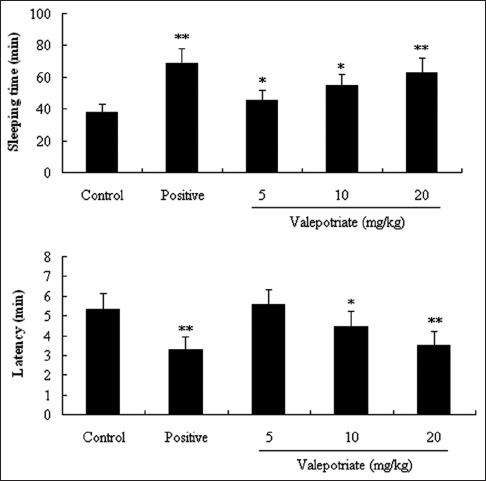
Effects of valepotriate and diazepam on pentobarbital sodium-induced sleeping time model in mice (n = 10). Positive and control groups were diazepam (4 mg/kg) and 1% DMSO. *P < 0.05, **P < 0.01, compared with control group
In addition, the latency time could be also shortened by valepotriate at the doses of 10 and 20 mg/kg compared with control group (P < 0.05, P < 0.01).
Results of Western blot assay
To explore the possible mechanisms of anti-epileptic effects of valepotriate, Western blot assay was performed. In the results of our present investigation, valepotriate can significantly up-regulate the expressions of GABAA, an important inhibitory receptor, at the doses of 5, 10, and 20 mg/kg compared with the control rats (P < 0.01), with an obvious dose-dependent manner. However, no obvious difference was observed in the expressions of GABAB (P > 0.05) [Figure 3].
Figure 3.
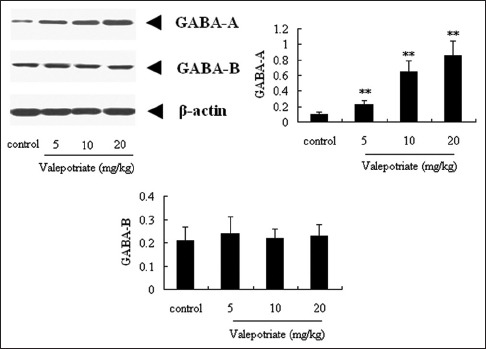
Effects of valepotriate on the expression of GABAA and GABAB proteins in the brain of chronic epileptic rats (n = 10). Control group was 1% DMSO. **P < 0.01, compared with control group
Similarly, after treatment with valepotriate (5, 10 and 20 mg/kg), the GAD65 expressions were significantly up-regulated (P < 0.01) compared with the control rats, with a dose-dependent manner during 5–20 mg/kg [Figure 4].
Figure 4.
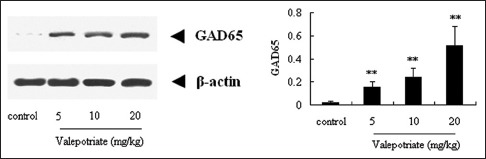
Effects of valepotriate on the expression of glutamic acid decarboxylase65 protein in the brain of chronic epileptic rats (n = 10). Control group was 1% DMSO. **P < 0.01, compared with control group
Result of Western blot assay of apoptosis-related proteins
As shown in Figure 5, compared with control group, valepotriate at the doses of 5, 10, and 20 mg/kg can significantly up-regulate the expressions of Bcl-2 in brain (P < 0.01), whereas down-regulate the expression of caspase-3 (P < 0.01) compared with the control rats, with a dose-dependent manner.
Figure 5.
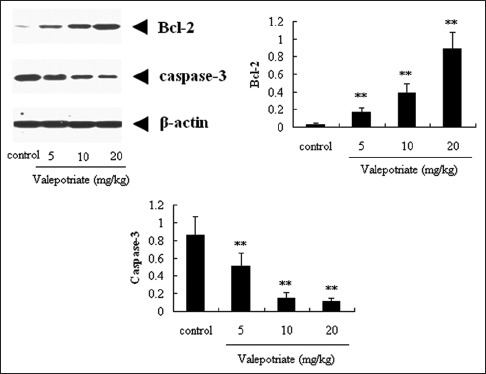
Effects of valepotriate on the expression of Bcl-2 and caspase-3 proteins in the brain of chronic epileptic rats (n = 10). Control group was 1% DMSO. **P < 0.01, compared with control group
DISCUSSION
In our study, the anti-epileptic activity of valepotriate was investigated for the first time. Our present results demonstrated that valepotriate isolated from the V. jatamansi possessed notable anti-epileptic activity via up-regulating the expressions of inhibitory receptor and down-regulating apoptosis-related proteins.
MES and PTZ can be used to simulate the abnormal discharge of neurons in the brain.[16,17] The MES assay is considered to be similar to generalized tonic-clonic seizures, and the PTZ test is recognized as a valid model for human generalized myoclonic and also absence seizures.[24] Therefore, the two animal models are commonly used to screen the potential AEDs via determining the inhibitory rates of convulsion and mortality. Pentobarbital sodium-induced sleeping time model was used to inhibit the normal nervous excitation in the brain by pentobarbital sodium.[18] The sleeping time and latency time were used to evaluate the anti-epileptic effect of candidate drugs.
GABAA protein was an ionotropic receptor and related to the chloridion. When the GABAA protein had high expression affected by drug, the exoteric frequency of chloridion channel would be indirectly increased, leading to hyperpolarization of nerve cell. GABAB protein was a metabotropic receptor and related to calcium ion in nerve endings and potassium ion in soma or dendrite. GABAB protein can inhibit calcium ion channel to reduce the neurotransmitter release and open potassium ion channel to generate late inhibitory postsynaptic potential (late IPSP) for inhibiting the generation of action potential. The GAD is the rate-limiting enzyme for the synthesis of GABA, and GAD is reported to be closely related to the epilepsy incidence.[30] GAD 65, an isoform of GAD, can continuously suppress the sensitivity of epilepsy and improve the neuropathologic changes.
Epilepsy commonly results in hippocampal neuron apoptosis, which is a main pathological manifestation.[30,31,32] Bcl-2 protein can inhibit neuronal apoptosis, related to many stimulus and damage, and significantly inhibit the increase of free radical and peroxide, and then prolong the cell survival.[33] Caspase-3 protein was a key enzyme, which can induce neuronal apoptosis.[34] Therefore, neuron apoptosis can be suppressed by up-regulating the Bcl-2 proteins and down-regulating the caspase-3 proteins.
In conclusion, valepotriate can significantly protect epileptic mice induced by MES and PTZ, and prolong pentobarbital sodium-induced sleeping time. Furthermore, valepotriate significantly up-regulated the expressions of GABAA, GAD 65, and Bcl-2 proteins, whereas down-regulated the expression of caspase-3 protein. These results indicated that valepotriate possess the evident of anti-epileptic activity and its mechanisms may be associated with up-regulation of inhibitory receptors and inhibition of neuronal apoptosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kale R. Global campaign against epilepsy: The treatment gap. Epilepsia. 2002;43(Suppl 6):31–3. doi: 10.1046/j.1528-1157.43.s.6.13.x. [DOI] [PubMed] [Google Scholar]
- 2.Wheless JW, Clarke DF, Arzimanoglou A, Carpenter D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. 2007;9:353–412. doi: 10.1684/epd.2007.0144. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Song Z, Liao DG, Zhang TY, Liu F, Zhuang K, et al. Anticonvulsant and sedative effects of Eudesmin isolated from Acorus tatarinowii on mice and rats. Phytother Res. 2015;29:996–1003. doi: 10.1002/ptr.5337. [DOI] [PubMed] [Google Scholar]
- 4.Engel J., Jr Surgery for seizures. N Engl J Med. 1996;334:647–52. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- 5.Schwabe U, Paffrath D. Arzneiverordnungsreport C (1990-2010) Berlin, Heidelberg, New York: Springer-Verlag; 2010. [Google Scholar]
- 6.Ekstein D, Schachter SC. Natural products in epilepsy – The present situation and prespectives for the future. Pharmaceuticals. 2010;3:1426–45. doi: 10.3390/ph3051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai CW, Lai YH. History of epilepsy in Chinese traditional medicine. Epilepsia. 1991;32:299–302. doi: 10.1111/j.1528-1157.1991.tb04655.x. [DOI] [PubMed] [Google Scholar]
- 8.Peng W, Ming QL, Han P, Zhang QY, Jiang YP, Zheng CJ, et al. Anti-allergic rhinitis effect of caffeoylxanthiazonoside isolated from fruits of Xanthium strumarium L. in rodent animals. Phytomedicine. 2014;21:824–9. doi: 10.1016/j.phymed.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Li Y. Traditional Chinese medicine. In: Devinsky O, Schachter SC, Pacia S, editors. Complementary and Alter Therapies for Epilepsy. New York, USA: Demos Medical Publishing; 2005. pp. 177–82. [Google Scholar]
- 10.Huang C, Li WG, Zhang XB, Wang L, Xu TL, Wu D, et al. a-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology. 2013;65:1–11. doi: 10.1016/j.neuropharm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh CL, Lin JJ, Chiang SY, Su SY, Tang NY, Lin GG, et al. Gastrodia elata modulated activator protein 1 via c-Jun N-terminal kinase signaling pathway in kainic acid-induced epilepsy in rats. J Ethnopharmacol. 2007;109:241–7. doi: 10.1016/j.jep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Liu CH, Lin YW, Tang NY, Liu HJ, Hsieh CL. Neuroprotective effect of Uncaria rhynchophylla in kainic acid-induced epileptic seizures by modulating hippocampal mossy fiber sprouting, neuron survival, astrocyte proliferation, and S100B expression. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/194790. 194790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury B, Bhattamisra SK, Das MC. Anti-convulsant action and amelioration of oxidative stress by Glycyrrhiza glabra root extract in pentylenetetrazole- induced seizure in albino rats. Indian J Pharmacol. 2013;45:40–3. doi: 10.4103/0253-7613.106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suleymanova E, Gulyaev M, Chepurnova N. Ginseng extract attenuates early MRI changes after status epilepticus and decreases subsequent reduction of hippocampal volume in the rat brain. Epilepsy Res. 2014;108:223–31. doi: 10.1016/j.eplepsyres.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Orellana-Paucar AM, Serruys AS, Afrikanova T, Maes J, De Borggraeve W, Alen J, et al. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models. Epilepsy Behav. 2012;24:14–22. doi: 10.1016/j.yebeh.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Borowicz KK, Jaszczyk B, Luszczki JJ, Czuczwar SJ. Interactions between two enantiomers of losigamone and conventional antiepileptic drugs in the mouse maximal electroshock model – An isobolographic analysis. Eur J Pharmacol. 2007;567:110–6. doi: 10.1016/j.ejphar.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Amabeoku GJ, Green I, Kabatende J. Anticonvulsant activity of Cotyledon orbiculata L. (Crassulaceae) leaf extract in mice. J Ethnopharmacol. 2007;112:101–7. doi: 10.1016/j.jep.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Ayoka AO, Akomolafe RO, Iwalewa EO, Akanmu MA, Ukponmwan OE. Sedative, anti-epileptic and anti-psychotic effects of Spondias mombin L. (Anacardiaceae) in mice and rats. J Ethnopharmacol. 2006;103:166–75. doi: 10.1016/j.jep.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Hirose S. Mutant GABA (A) receptor subunits in genetic (idiopathic) epilepsy. Prog Brain Res. 2014;213:55–85. doi: 10.1016/B978-0-444-63326-2.00003-X. [DOI] [PubMed] [Google Scholar]
- 20.Gong YH, Cheng GY, Cheng WP. Effect of Bunaozhixian power on neuronal apoptosis genes Bax and Bcl-2 expression in the hippocampus area of epileptic rats. Glob Tradit Chin Med. 2012;5:105–7. [Google Scholar]
- 21.You JS, Peng M, Shi JL, Zheng HZ, Liu Y, Zhao BS, et al. Evaluation of anxiolytic activity of compound Valeriana jatamansi Jones in mice. BMC Complement Altern Med. 2012;12:223. doi: 10.1186/1472-6882-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YL, Shi JL, Guo JY, Liu Y, Zheng HZ, Zhao BS. Anxiolytic activity of valepotriate. Chin J Exp Tradit Med Form. 2011;17:126–8. [Google Scholar]
- 23.Wang YL, Liu Y, Shi JL, Guo JY, Zheng HZ, Zhai YJ. Effects of valtrate on anxiety models in rats and its possible mechanisms. Chin Pharmcol Bull. 2011;27:501–4. [Google Scholar]
- 24.Sayyah M, Mandgary A, Kamalinejad M. Evaluation of the anti-convulsant activity of the seed acetone extracts of Ferula gummosa Boiss. against seizures induced by pentylenetetrazole and electroconvulsive shock in mice. J Ethnopharmacol. 2002;82:105–9. doi: 10.1016/s0378-8741(02)00166-6. [DOI] [PubMed] [Google Scholar]
- 25.Swinyard EA. Laboratory evaluation of antiepileptic drugs. Review of laboratory methods. Epilepsia. 1969;10:107–19. doi: 10.1111/j.1528-1157.1969.tb03838.x. [DOI] [PubMed] [Google Scholar]
- 26.Litchfield JT, Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 27.Gupta G, Kazim I, Afzal M, Rahman M, Saleem S, Ashraf MS. Sedative, anti-epileptic and anti-psychotic effects of Viscum album L. (Loranthaceae) in mice and rats. J Ethnopharmacol. 2012;141:810–6. doi: 10.1016/j.jep.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Ha JH, Lee DU, Lee JT, Kim JS, Yong CS, Kim JA, et al. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain. J Ethnopharmacol. 2000;73:329–33. doi: 10.1016/s0378-8741(00)00313-5. [DOI] [PubMed] [Google Scholar]
- 29.Huang BK, Qin LP, Liu MY, Zhang QY, Zheng HC. Sesquiterpene and iridoids from Valeriana pseudofficinalis roots. Chem Nat Compd. 2009;45:363–6. [Google Scholar]
- 30.Yang T, Kong B, Gu JW, Kuang YQ, Cheng L, Yang WT, et al. Anticonvulsant and sedative effects of paederosidic acid isolated from Paederia scandens (Lour.) Merrill. in mice and rats. Pharmacol Biochem Behav. 2013;111:97–101. doi: 10.1016/j.pbb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Pretel S, Applegate CD, Piekut D. Apoptotic and necrotic cell death following kindling induced seizures. Acta Histochem. 1997;99:71–9. doi: 10.1016/S0065-1281(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 32.Pollard H, Charriaut-Marlangue C, Cantagrel S, Represa A, Robain O, Moreau J, et al. Kainate-induced apoptotic cell death in hippocampal neurons. Neuroscience. 1994;63:7–18. doi: 10.1016/0306-4522(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 33.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100:2432–7. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao RJ, Wang H, Wang GB, Na RS, Jin DP, Guan WJ. Relationship of Caspase family and apoptosis. Chin J Anim Sci. 2010;46:73–8. [Google Scholar]


