Abstract
Background
Withaferin-A (WF-A) is a well-known dietary compound isolated from Withania sominifera. It has tremendous pharmacological potential and has been shown to exhibit antiproliferative activity against several types of cancerous cells. Currently, the main focus of anti-cancer therapeutic development is to identify apoptosis inducing drug-like molecules. Osteosarcoma is a rare type of osteocancer, affecting human. The present study therefore focused on the evaluation of antitumor potential of WF-A against several osteosarcoma cell lines.
Materials and Methods:
MTT assay was used to evaluate WF-A against osteosarcoma cell lines and to calculate the IC50. DAPI staining was used to confirm the apoptosis inducing potential of WF-A. Mitochondrial membrane potential, reactive oxygen species (ROS) assay, and Western blotting were used to confirm the basis of apoptosis.
Results:
The results revealed that that WF-A exhibited strong antiproliferative activity against all the cells lines, with IC50 ranging from 0.32 to 7.6 μM. The lowest IC50 (0.32 μM) was observed against U2OS cell line and therefore it was selected for further analysis. DAPI staining indicated that WF-A exhibited antiproliferative activity via induction of apoptosis. Moreover, WF-A induced ROS-mediated reduction in mitochondrial membrane potential ΔΨm) in a dose-dependent manner and activation of caspase-3 in osteosarcoma cells.
Conclusion
We propose that WF-A may prove a potent therapeutic agent for inducing apoptosis in osteosarcoma cell lines via generation of ROS and disruption of mitochondrial membrane potential.
SUMMARY
WF-A exhibits strong anticancer activity against osteosarcoma cell lines
Antiproliferative activity of WF-A is via induction of apoptosis
WF-A induced ROS-mediated reduction in mitochondrial membrane potential
WF-A induced expression of caspase-3 in osteosarcoma cells.
Abbreviations used: WA: Withaferin A; ROS: Reactive oxygen species; OS: Osteosarcoma; MMP: Mitochondrial membrane potential.
Keywords: Osteosarcoma, WF-A, caspase-3, apoptosis, U2OS
INTRODUCTION
Osteosarcoma (OS) is one of the most common primary osteotumor affecting young adults with an incidence of approximately 0.4 per 100,000 population per year.[1] OS is an aggressive malignant cancer of mesenchymal origin that[2] shows high variability in therapy response between patients. Therefore, a single therapeutic agent cannot be used for curing all OS patients. OS is currently being treated using the pre- and postoperative chemotherapy after surgical removal of tumor.[3] The survival rate of conventional treated OS patients is only 50-60%, while that for recurrent OS patients is 20-30%.[4] OS is generally characterized by several genetic alterations at varying frequencies. However, OS-specific mutation or pathway is still a big challenge and is yet to be fully explored. Therefore, it is imperative to identify the effective and novel drug-like molecules for improving the survival rate of patients and control the metastasis of OS. Although, in the last few decades, many novel molecules have been developed as the therapeutic agent to cure OS patients. These molecules are tested in multimodal therapies with dose variations of conventional drugs, but the vast majority of molecules failed to enter in phase III clinical trials due lack efficacy and selectivity. The rarity of OS disease, resources, and a limited number of patients are the major factors that limit the OS-related studies.[5,6] Currently, most of the research has focused to design the drug-like molecules for targeting the single mutation of kinases deregulated in OS such as insulin-like growth factor 1 receptor (IGF-1R). IGF-1 and -2 involved in cell growth as well as in cell survival pathways.[7,8] Phytomolecules are the vast variety of medically active compounds that are being frequently used as drugs against several types of diseases, which include, but are limited to, cancer, diabetes, and malaria.[9,10,11,12,13] Withania somnifera L. Dunal (Solanaceae) is an ancient Indian medicinal plant known as Ashwagandha. It is well known for its therapeutic properties and widely used in traditional systems of Indian medicine including, Siddha, Unani, and Ayurveda.[14] Withaferin-A (WF-A) is one of the dietary compounds [Figure 1A] isolated from Withania plant and medicinally used as antioxidant, antitumor, Cox-2 inhibitor, apoptosis inducer, and cell cycle modulator.[14,15] WF-A has been reported to arrest the cell cycle in G2/M in breast tumor through modulating the phosphorylation of CDK-1 and histone 3 as well as downregulating the expression of CDC25C and securin.[15] However, the antitumor potential of WA is demonstrated against various cancer types in vivo and in vitro such as the prostate (induction of Par-4 and inhibition of HSP-90),[16,17] breast (induction of G2/M arrest, FOXO3a and Bim regulation, decrease in the level of estrogen receptor-α, inhibition of STAT3),[14,18,19,20,21,22,23] colon (inhibition of Notch-1),[16] and cervical (downregulation of HPV E6 and E7 proteins, induction of p53 and apoptotic genes, and downregulation of STAT3 expression).[22] Moreover, WF-A activates the p38MAPK, leading to apoptosis through the marked reduction in mitochondrial membrane potential (ΔΨm) and caspase-3 activation in lymphoblastic and myeloid leukemia.[23,24] Likewise, WF-A inhibited the NF-κB nuclear transcriptional factor in a cellular model of cystic fibrosis inflammation, induced by Pseudomonas aeruginosa.[24] However, the antitumor potential of WF-A against OS has not been evaluated as yet. The present study was designed to determine the antiproliferative potential of WF-A against the OS and investigate the possible mechanism of action. In this study, we examined the antiproliferative potential of WF-A against low- and high-proliferative OS cell lines. We report that WF-A triggered apoptosis through induction of caspase-3 and reduced the level of mitochondrial membrane potential ΔΨm) in osteosarcoma cell lines.
Figure 1.
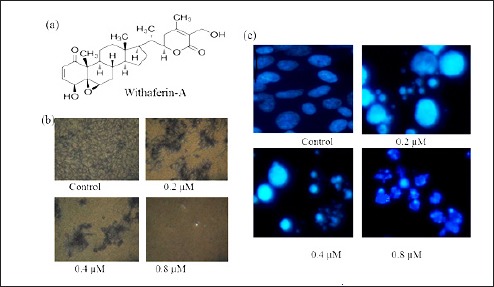
Antiproliferative effect of WF-A on U2OS cells. (a) Structure of WF-A phytomolecule (b) Formazen crystal assay. WF-A inhibits the formazen crystal formation and showed cell death in a dose-dependent manner. (C) DAPI staining showing WF-A induces apoptosis in a dose-dependent manner
MATERIALS AND METHODS
Cell lines and culture condition
HOS (ATCC CRL-1543, human), Saos-2 (ATCC HTB-85, human), 143B (ATCC CRL-8303, human), MG-63(ATCC CRL-1427, human), MNNG (ATCC CRL-1547, human), U2OS (ATCC 40432, human), and Vero cell line (VERO C1008; ATCC CRL-1586, valvet monkey kidney epithelial cells) cell cultures were obtained from the American Type Culture Collection (ATCC, Rockville, MD). All cells were grown in 75-cm2 flasks in RPMI-1640 (Cat-23400-62, Invitrogen, Germany) with 10% FBS (Gibco BRL), penicillin (10,000 U), and streptomycin (10,000 μg/mL) of stock (Gibco BRL) at 37°C in an atmosphere of 95% humidity and 5% CO2 atmosphere.
Reagents
WF-A was purchased from natural products company ChromoDex, Irvine, CA (ABS-00023249-010), MTT (Sigma-Aldrich, M-2128), DMSO (Merck-61857125001730, 80291225001730), caspase-3 Assay Kit (Sigma-Aldrich, CASP3F), active caspase-3 antibody (Sigma-Aldrich, C8487), cyt-c antibody (SC-13561, Santa Cruz, USA), B-actin antibody (Sigma-Aldrich, A2228), H2DCFDA (Sigma-Aldrich, D6883), and JC-1 (Sigma-Aldrich, T4069). Active PARP antibody (Abcam, ab32064), doxorubicin (Sigma-Aldrich, D-1515), and all other secondary antibodies were purchased from Santa Cruz, USA.
Cytotoxicity assay
In vitro anticancer activity of WF-A was carried out by MTT assay as described previously.[20] In total, 1–2 × 104 cells/well were cultured with 5% CO2 for 24 h in 96-well microplate (Grenier, Germany). WF-A was dissolved in 100% DMSO Gr. (Merck, Germany) at different concentrations. Cells were then treated with WF-A for 48 h at 37°C. Afterward, 10 μL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added and the plates were incubated for more 4 h at 37°C. One hundred microliters of DMSO was added to all the wells and mixed thoroughly to dissolve blue formazen crystals for 5 min at room temperature. The plates were read on a Fluostar Omega microplate reader (BMG LABTECH, Australia) at 570 nm. The experiment was done in triplicates and the inhibitory concentration (IC50) was calculated as follows:
Inhibition (%) = [1 - OD (570 nm) of sample well/OD (570 nm) of control well] × 100.
DAPI staining
U2OS cells were seeded at the density 1-2 × 104 cells into the six-well plates and treated with varied concentrations of WF-A for 48 h. In brief, cells were harvested and fixed in methanol and acetic acid in the ratio of 3:1 and kept overnight at 4°C. After incubation for overnight, the cells were centrifuged, resuspended in methanol and acetic acid, and then plated on chilled glass slides. After drying the slides, cells were stained with DAPI at the concentration of 1 μg/mL in dark for 30 min and then slides were washed. Finally, images were taken using fluorescence microscope.
Reactive oxygen species activation assay
Intracellular reactive oxygen species (ROS) activation was measured by a Fluostar Omega microplate reader (BMG LABTECH, Australia) following staining with H2DCFDA as described elsewhere.[26] A total of 2 × 104 cells were added in each well of 96-well microplate and grown overnight at 37°C and treated with WF-A at different concentrations (0.2, 0.4, and 0.8 μM). The cells were then stained with 5 μM H2DCFDA for 30 min at 37°C. The cells were collected and fluorescence was measured using a fluorescence plate reader.
Mitochondrial membrane disruption and potential assay
Mitochondrial membrane disruption assay was performed using JC-1 dye in accordance to the manufacture's instructions. U2OS cells were treated with or without WF-A for different time periods. Treated and untreated cells were collected and washed with PBS and then centrifuged at 300g at 4°C for 5 min. Treated and untreated cells were incubated with JC-1 dye for 30 min at 37°C. The cells were then visualized under fluorescence microscope. Mitochondrial membrane potential (ΔΨm) assay was performed with WF-A-treated and untreated cell lysate as per manufacturer's instructions using JC-1 and fluorescence was read at 540 and 490 nm using plate reader.
Caspase-3 activation
A total of 2 × 104 cells/well were incubated with or without WF-A in a time-dependent manner. Cells were collected and lysed in cell lysis buffer. Caspase-3 activation was measured fluorometrically using caspase-3 fluorescent assay kits (Sigma). Active caspase-3 cleaved substrate peptides Ac-DEVD-AMC and released of AMC (7-amido-4-methyl coumarin). Fluorescence was analyzed at 354 nm.
Western blot analysis
Cell lysate is prepared in lysis buffer 1 M Tris (7.4), 150 mM NaCl, 1% NP-40, 10% Triton-X, 0.5 M EDTA, 50% glycerol, 50 mM NaF, 1 mM Na3VO4, 15 mM glycerophosphate, and 200 X phosphatase inhibitors PMSF (Sigma, cat-7626), pepstatin A (Sigma, cat-P4265), leupeptin (Sigma, cat-L0649), and benzamidine (Sigma, cat-B6506) and were separated onto SDS-PAGE. The separated proteins were electrotransferred to PVDF membranes and blocked with 10% nonfat skim milk in 1 × TBST for 1 h. The blot membrane incubated with primary antibodies for overnight at 4°C. Blots were washed three times in 1 × TBST and incubated with HRP-conjugated secondary antibody for 2 h. Protein bands were spotted using enhanced chemiluminescence's reagent (ECL kit, Amersham Biosciences).
Statistical analysis
All experiments were carried out in triplicates and expressed as plus minus standard deviation (SD). Differences were considered significant at P less than 0.05.
RESULTS
Antiproliferative potential of WF-A on OS cell lines
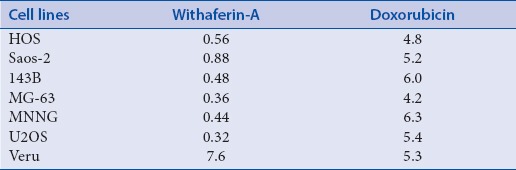
To identify the antiproliferative role of WF-A on OS cell lines. Cells were treated with WF-A concentration range 0.01–10 μM for 48 h. The WF-A showed a dose-dependent inhibition of OS cell lines with IC50 ranging from 0.32 to 7.6 μM [Table 1]. WF-A displayed the potent antiproliferative effect against U2OS with an IC50 0.32 μM. In the formazen crystal assay, we observed that WF-A-treated OS cells reduced the number of formazen crystals in a dose-dependent manner [Figure 1B].
Table 1.
IC50 (μM) value of WF-A against a panel of osteosarcoma cancer cell lines
WF-A trigger the apoptosis in U2OS cells
In order to examine whether the antiproliferative potential of WF-A is due to apoptosis induction, U2OS cells were incubated with different concentrations (0.2, 0.4, and 0.8 μM) of WF-A and subjected to DAPI staining for 48 h. The treated cells showed condensed and prominent fragmented nuclei in a dose-dependent manner. At 0.8 μM, most of cells underwent apoptosis and apoptic corps were observed in treated cells as compared with untreated control [Figure 1c].
WF-A trigger the ROS activation in U2OS cells
The proapoptotic potential of WF-A observed through DAPI staining study suggested that WF-A might induce generation of intracellular ROS. Therefore, we calculated the ROS level at varied concentrations of WF-A for 48 h. The results showed that the intracellular ROS levels of treated cells increased 70, 140, and 260% as compared with untreated cells [Figure 2A]. Our result suggested that WF-A is a potent molecule for activating ROS in U2OS cells to trigger the apoptosis.
Figure 2.
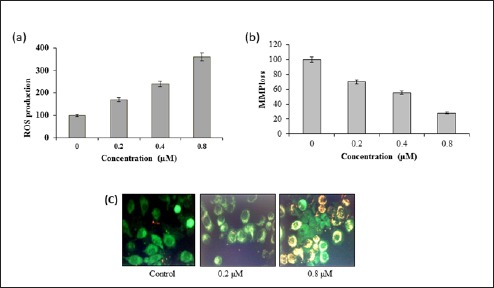
(A) Production of ROS by WF-A in a dose-dependent manner. (B) WF-A reduces the mitochondrial membrane potential (MMP) in U2OS cells. (C) WF-A-treated cells showed a significant reduction in MMP (yellowish-red fluorescence) as compared to untreated cells (green fluorescence)
AWF-A reduces the mitochondrial membrane potential (ΔΨm)
ROS generation is related to mitochondrial dysfunction. It disrupts the outer mitochondrial potential to release the death-promoting proteins like cytochrome C.[22] Therefore, we examined whether WF-A reduces the ΔΨm in U2OS cells treated with WF-A at varied concentrations (0.2, 0.4, and 0.8 μM) and measured the ΔΨm fluorometrically using JC-1 dye. WF-A-treated U2OS cells showed a significant reduction in ΔΨm in a dose-dependent manner. The ΔΨm reduced by 30, 45, and 72% at 0.2, 0.4, and 0.8 μM of WF-A, respectively, as compared to untreated control [Figure 2B]. The fluorescence microscopic analysis demonstrated that WF-A-treated cells displayed fluorescence (yellowish-red) indicating apoptotic cells as compared to control [Figure 2C]. Taken together, these results suggested that reduction in ΔΨm by ROS generation may be one of the reasons for induction of apoptosis in U2OS cells.
WF-A trigger apoptotic proteins caspase-3, cytosolic cyt-c, and PARP
To observe whether WF-A activates apoptosis-related proteins. We treated U2OS cells with WF-A at IC50 (0.32 μM) at different time points. Results indicated that WF-A treatment triggered the caspase-3 activation in U2OS cells in a time-dependent manner as compared to control (solvent), fluorometrically [Figure 3A]. Western blot analysis revealed that WF-A induced caspase-3, cytosolic cyt-c, and PARP protein expression at IC50 in a time-dependent manner [Figure 3B].
Figure 3.
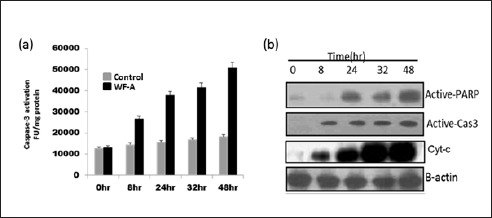
WF-A trigger the apoptosis through caspase-3, cyt-c, and PARP activation. (A) WF-A activates the caspase-3 in a dose-dependent manner leading to apoptosis at IC50. (B) In western blot analysis, WF-A-treated cells showed the activation of caspase-3, cytosolic cyt-c, and PARP in a time-dependent manner
DISCUSSION
Osteosarcoma is one of the rare type of bone tumor affecting children. The limited availability of biopsy samples is a one of the obstacles for studying the OS on the genetic and molecular level and to identify the OS-specific druggable target. In this study, we demonstrate that WF-A is a potent candidate for treating OS. WF-A showed potential growth inhibition activity against U2OS cells, as is evident from the proliferation assay. As reported previously, several drugs exhibit antiproliferative effects via induction of apoptosis. For instance, several chemotherapeutic drugs, such as cisplatin, 5-fluorouracil, and taxol[25,26,27,28,29,30,31] have been reported to induce specific apoptotic pathways. Additionally, resistance to drug is partly explained by the ability of cancer cells to flee apoptosis.[33] To asses weather WF-A induces apoptosis in U2OS, we carried out the DAPI staining of the treated cells. It was observed that WAF-A induces apoptosis in a concentration-dependent manner. Further, it was observed that WF-A-treated cells displayed ROS-mediated ΔΨm reduction and caspase-3 induction. Our results are in agreement with studies carried out previously.[22] Therefore, the results suggest that WF-A may induce apoptosis through increasing intracellular ROS and reduction in ΔΨm. Many anticancer drugs target cancer cells partly by generating high levels of intracellular ROS.[32] Moreover, mitochondria play a key role in ROS and apoptosis.[33] For example, capsaicin disrupts mitochondrial membrane potential ΔΨm and mediates oxidative stress resulting in apoptosis in pancreatic cancer cells.[34] Moreover, ΔΨm reduction is required for triggering the release of cyt-c. Released cyt-c is well-known protein that binds to apoptotic protease activating factor 1 (Apaf-1) in the cytosol and make a heterodimeric complex, namely, apoptosome. Apoptosomes activate the procaspase-9 for activating its downstream target caspase-3.[35,36] WF-A-treated cells indicated an increase in the level of cytosolic cyt-c in U2OS, suggesting that WF-A-treated cells undergo apoptotic cell death via activation of cyt-c.
CONCLUSION
We conclude that WF-A is a potential candidate for treating osteosarcoma. It induces apoptosis in U2OS cells via ROS-mediated ΔΨm reduction and activation of caspase-3. Further, mechanistic studies are required to establish WF-A as a lead molecule.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment-where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–32. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Hattinger CM, Fanelli M, Tavanti E, Vella S, Ferrari S, Picci P, et al. Advances in emerging drugs for osteosarcoma. Exp Opin Emerg Drugs. 2015;20:1–20. doi: 10.1517/14728214.2015.1051965. [DOI] [PubMed] [Google Scholar]
- 4.Xue Z, Zhao J, Niu L, An G, Guo Y, Ni L. Up-regulation of MiR-300 promotes proliferation and invasion of osteosarcoma by targeting BRD7. PLoS ONE. 2015;10:e0127682. doi: 10.1371/journal.pone.0127682. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Botter SM, Neri D, Fuchs B. Recent advances in osteosarcoma. Curr Opin Pharmacol. 2014;16:15–23. doi: 10.1016/j.coph.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 6.van Maldegem AM, Bhosale A, Gelderblom HJ, Hogendoorn PC, Hassan AB. Comprehensive analysis of published phase I/II clinical trials between 1990-2010 in osteosarcoma and Ewing sarcoma confirms limited outcomes and need for translational investment. Clin Sarc Res. 2012;2:5. doi: 10.1186/2045-3329-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maugg D, Rothenaigner I, Schorpp K, Potukuchi HK, Korsching E, Baumhoer D, et al. New small molecules targeting apoptosis and cell viability in osteosarcoma. PLoS ONE. 2015;10:e0129058. doi: 10.1371/journal.pone.0129058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Roth M, Piperdi S, Montoya K, Sowers R, Rao P, et al. Insulin-like growth factor 1 receptor and response to anti-IGF1R antibody therapy in osteosarcoma. PLoS ONE. 2014;9:e106249. doi: 10.1371/journal.pone.0106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Xu J, Li F, Xu L, Li C. A new antifungal isocoumarin from the endophytic fungus Trichoderma Sp 09 of Myoporum bontioides A gray. Pcog Mag. 2016;12:259. doi: 10.4103/0973-1296.192204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamboli FA, More HN. Evaluation of antiulcer and antioxidant activity of Barleria gibsoni Dalz. leaves. Phcog Res. 2016;8:226. doi: 10.4103/0974-8490.188879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uto T, Tung NH, Thongjankaew P, Lhieochaiphant S, Shoyama Y. Kayeassamin is isolated from the flower of Mammea siamensis triggers apoptosis by activating caspase-3/-8 in hl-60 human leukemia cells. Phcog Res. 2016;8:244–8. doi: 10.4103/0974-8490.188884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shankara BR, Ramachandra YL, Rajan SS, Ganapathy PS, Yarla NS, Richard SA, Dhananjaya BL. Evaluating the anticancer potential of ethanolic gall extract of Terminalia chebula (Gaertn.) Retz (combretaceae) Phcog Res. 2016;8:209. doi: 10.4103/0974-8490.182919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99:113. doi: 10.1016/s0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 14.Joshi P, Misra L, Siddique AA, Srivastava M, Kumar S, Darokar MP. Epoxide group relationship with cytotoxicity in with anolide derivatives from Withania somnifera. Steroids. 2014;79:19–27. doi: 10.1016/j.steroids.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nut Cancer. 2008;60:Suppl 151–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 2010;79:542–51. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–53. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 18.Hahm ER, Lee J, Huang Y, Singh SV. Withaferin a suppresses estrogen receptor-alpha expression in human breast cancer cells. Mol Carcinogenesis. 2011;50:614–24. doi: 10.1002/mc.20760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991–8. doi: 10.1093/carcin/bgq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–9. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 inhibition by Withaferin-A: a therapeutic target against colon carcinogenesis. Mol Cancer Ther. 2010;9:202–10. doi: 10.1158/1535-7163.MCT-09-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–705. doi: 10.1093/carcin/bgr192. [DOI] [PubMed] [Google Scholar]
- 23.Malik F, Kumar A, Bhushan S, Khan S, Bhatia A, Suri KA, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis Int J Prog Cell Death. 2007;12:2115–33. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 24.Mandal C, Dutta A, Mallick A, Chandra S, Misra L, Sangwan RS. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis. 13:1450–64. doi: 10.1007/s10495-008-0271-0. [DOI] [PubMed] [Google Scholar]
- 25.Maitra R, Porter MA, Huang S, Gilmour BP. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of cystic fibrosis inflammation. J Inflamm (Lond) 2009;6:15. doi: 10.1186/1476-9255-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74:214–26. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- 27.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Diff. 2006;13:1396–402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 28.Azuma M, Tamatani T, Ashida Y, Takashima R, Harada K, Sato M. Cisplatin induces apoptosis in oral squamous carcinoma cells by the mitochondria-mediated but not the NF-kappaB-suppressed pathway. Oral Oncol. 2003;39:282–89. doi: 10.1016/s1368-8375(02)00116-1. [DOI] [PubMed] [Google Scholar]
- 29.Yoneda K, Yamamoto T, Osaki T. p53- and p21-independent apoptosis of squamous cell carcinoma cells induced by 5-fluorouracil and radiation. Oral Oncol. 1998;34:529–37. doi: 10.1016/s1368-8375(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 30.Abal M, Andreu JM, Barasoain I. Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr Canc Drug Targs. 2003;3:193–203. doi: 10.2174/1568009033481967. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira CG, Epping M, Kruyt FA, Giaccone G. Apoptosis target of cancer therapy. Clin Cancer Res. 2002;8:2024–34. [PubMed] [Google Scholar]
- 32.Malaguarnera L. Implications of apoptosis regulators in tumorigenesis. Cancer Met Rev. 2004;23:367–87. doi: 10.1023/B:CANC.0000031774.32572.df. [DOI] [PubMed] [Google Scholar]
- 33.Ding H, Han C, Guo D, Chin YW, Ding Y, Kinghorn AD, et al. Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS mediated mechanism. Nutr Cancer. 2009;61:348–56. doi: 10.1080/01635580802567158. [DOI] [PubMed] [Google Scholar]
- 34.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med. 2009;47:333–43. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Pramanik KC, Boreddy SR, Srivastava SK. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE. 2011;6:e20151. doi: 10.1371/journal.pone.0020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S. Caspase function in programmed cell death. Cell Death Diff. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]


