Abstract
The lymphatic vasculature is not considered a formal part of the immune system, but it is critical to immunity. One of its major roles is in the coordination of the trafficking of antigen and immune cells. However, other roles in immunity are emerging. Lymphatic endothelial cells, for example, directly present antigen or express factors that greatly influence the local environment. We cover these topics herein and discuss how other properties of the lymphatic vasculature, such as mechanisms of lymphatic contraction (which immunologists traditionally do not take into account), are nonetheless integral in the immune system. Much is yet unknown, and this nascent subject is ripe for exploration. We argue that to consider the impact of lymphatic biology in any given immunological interaction is a key step toward integrating immunology with organ physiology and ultimately many complex pathologies.
Keywords: lymph, endothelium, lymph node, adhesion, migration
INTRODUCTION
Even though memory CD8+ T lymphocytes outside of secondary lymphoid organs outnumber their counterparts in secondary lymphoid organs (1–4), secondary lymphoid organs remain the critical meeting point for the initiation of immune responses to antigens previously not encountered. Accordingly, few naive T cells exist in peripheral tissues (4). The elegant organization of the spleen and lymph nodes that fosters rare, productive encounters to kick off immunity has long been appreciated (5–7). Lymph nodes are especially fascinating organs that provide a specialized microenvironment for the meeting of migratory immune cells, especially lymphocytes and antigen-presenting cells like dendritic cells (DCs). The function and assembly of this specialized lymphoid organ uniquely coordinate cell trafficking from two sources: the blood, where the majority of lymphocytes and DC precursors enter lymph nodes through distinct postcapillary venules called high endothelial venules; and the lymphatic vessels, which transport not blood but interstitial fluid derived exclusively from adjacent tissues.
This review focuses on the lymphatic vasculature. Our aim is to bring its physiology to life for the immunology-oriented reader, so as to reveal how this very specialized vasculature coordinates multiple physiological roles, only some of which are expressly directed toward the regulation of immunity. The coordination of these roles sometimes brings together unusual processes, such as the interface between lipid transport and immunity when the mesenteric lymphatic vasculature faces the need to transport fats through the mesenteric lymphatic vessels and mesenteric lymph nodes (8). The inevitable convergence of immunology with other disciplines through their common reliance on the lymphatic vasculature may have important implications for various inflammatory or other diseases.
INTERSTITIAL FLUID AND LYMPH FLOW GOVERN THE AVAILABILITY OF PROTEINS, PEPTIDES, AND MACROMOLECULES TO TISSUE CELLS
Often, the first issue that comes to mind when lymphatic vessels are discussed is their role in fluid clearance from tissues. Very often, the focus is on water, as accumulated water in tissues produces edema. However, one should not overlook the role of lymphatic transport in controlling the availability of proteins, peptides, and other macromolecules to cells within all tissues. A slow flow of fluid through all tissues facilitates efficient supply of nutrients and signaling molecules, including hormones and cytokines, to cells within these tissues. This fluid, present between cells in extravascular compartments, is called interstitial fluid (9).
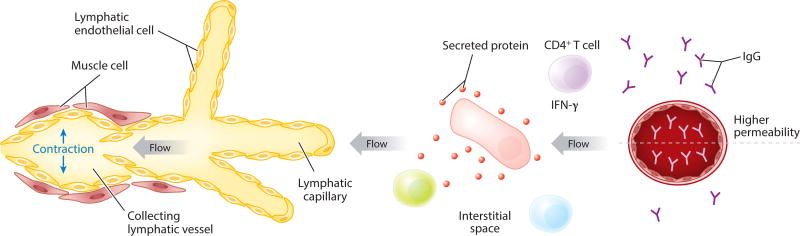
Although the composition and rate of flow of interstitial fluid are only sometimes discussed in the field of immunology, they have a broad impact on immunity. The basic tenet of Starling’s law of fluid filtration is that hydrostatic pressure is the driving force for fluid loss from plasma, and the osmotic gradient established by macromolecules, most notably albumin, counteracts fluid loss from the vasculature. Starling’s law, even as it has been modestly revised over time (10, 11), states that arterioles, capillaries, and venules all contribute to fluid filtration and that plasma proteins are pulled into the interstitium during such filtration. Venules, therefore, are unable to absorb the capillary filtrate, so the lymphatic vasculature is required to absorb and return the bulk of this capillary filtrate to the bloodstream. This fluid also passes across the endothelial glycocalyx, a layer of glycoproteins secreted by the endothelium, such that larger macromolecules present in plasma are most restricted in passage owing to their large size and negative charge (12). Proteins initially present in plasma, from albumin to complement and immunoglobulins, are found within interstitial fluid at lower concentrations than in plasma because of this filtration barrier (Figure 1) (12). Adjustments to the filtration barrier are made by vesicular transport (11) or existence and integrity of fenestrae (13), or by any of numerous signals that have been identified to increase intercellular endothelial permeability (14). Indeed, a long-standing tenet in the regulation of inflammation is that proinflammatory mediators increase vascular permeability, and the main purpose of this heightened permeability is to promote increased passage of important effector molecules like complement and immunoglobulin into the interstitium. We are still gaining insight into how this critical process is regulated. A recent study revealed how CD4+ T cells help regulate humoral immunity to herpes simplex virus by controlling vascular permeability in an IFN-γ-dependent manner that, in turn, allowed for sufficient antibody availability within the tissue (Figure 1) (15).
Figure 1.
Schema depicting the two major parts of the lymphatic vasculature: lymphatic capillaries and lymphatic collecting vessels. With contraction of lymphatic collecting vessels, flow through the lymphatic vessel occurs, and this dictates the direction of interstitial fluid flow within the adjacent tissue. The larger cell in the tissue (pink) is depicted as secreting a protein. Because of the distribution of the protein in the flow environment, the green cell would be subjected to more interaction with the protein than the nearby cell in light blue. The image also shows the distribution of IgG in the bloodstream and in the vasculature, with the concentration of IgG being lower in the interstitial space than in plasma under all conditions except when the permeability of the vessel has been increased, for instance, because of secretion of IFN-γ by T helper cells.
Along with regulation of vascular permeability, the molecular composition of the extracellular matrix between tissue cells and lymphatic transport are critical for the maintenance of any given tissue microenvironment (16). In the face of unfavorable oncotic pressure, molecules with a Stokes radius equal to or greater than that of the cytokine TNF are not cleared from the interstitium by returning to the venous vasculature (16, 17). Instead, for molecules that gained access to or were produced in the interstitium, eventual appearance in the blood requires that these molecules be further transported through the lymphatic vasculature, which, in the absence of mechanically stimulated interstitial flow (18), uses an active pump–based mechanism to export interstitial fluid and its contents directly out of tissue (Figure 1). If lymphatic transport is impaired, the flow of interstitial fluid will decrease. Edema is thus linked to the balance of fluid arriving from the plasma and removal of interstitial fluid via lymphatic action.
Interstitial flow, even though typical flow rates are a modest approximately 0.2 µm/s (19), allows the establishment of autologous gradients that can guide cell migration through its ability to polarize secreted proteases (20) or chemokines (21, 22), strongly affecting cell behavior. These gradients are oriented in the direction of flow, that is, in the direction of the lymphatic vasculature (Figure 1). However, very small, highly diffusible nutrients like glucose would be expected to depend little on interstitial flow for gaining access to the appropriate cell targets. In insulin-dependent cell types, like skeletal muscle cells and adipocytes, the interstitial transport of insulin is relevant to glucose metabolism (23). An argument has been put forward that hyperinsulinemia is driven by mechanisms that keep insulin concentrations constant in the interstitium even when filtration of insulin from the vasculature is decreased in diabetes scenarios. Although glucose concentrations measured in vivo show rapid equilibration between plasma and all tissue compartments (24, 25), it has been argued that glucose availability in insulin-independent tissues like tumors can become limiting and thereby pivotally affect antitumor immunity (26). The method used to support such a conclusion involved collection of interstitial fluid from tumor tissue disconnected from the circulation, making it impossible to be certain that areas of limited glucose develop in vivo, rather than from rapid consumption during preparation of the fluid. If glucose is limiting in microenvironments like tumors, the issue can be raised as to whether improved interstitial flow to the tumor might be part of a multipronged approach to tip antitumor immunity in a favorable direction. Interstitial pressure in tumors is high, and this may favor the tumor in multiple ways (27). Improved methods to interrogate the composition of the extracellular space with fine-tuned temporal and spatial dynamics would advance the growing interest in the effect of the microenvironment on immunity and interest in the many diseases where the innate or adaptive immune system is involved.
STRUCTURE AND PROPERTIES OF THE LYMPHATIC SYSTEM
Lymphatic Capillaries
As indicated above, lymphatic flow is a major driver of interstitial flow. Lymphatic flow is thought to be regulated by the contractile properties of the larger collecting lymphatic vessels (28), which will be discussed in detail below. These form through the convergence of many lymphatic capillaries, also called initial lymphatics, which exist in nearly all tissues as blind-ended vessels lined by a single endothelial layer (Figure 1). It is thought that the lymphatic capillaries are capable of absorbing interstitial contents rather nonselectively. Nonselective uptake is conceptually critical to the issue of allowing foreign antigens access to the draining lymph nodes and is supported by the long-standing use of a variety of dyes and compounds that seem to have unrestricted access to draining lymph nodes when introduced into tissue parenchyma. However, it is possible that additional selective mechanisms exist for certain molecules to gain entry into lymphatic capillaries (29).
Interendothelial junctions that are assembled in a punctate, or button-like, fashion with flaps or gaps between them would account for the rather nonselective uptake into lymphatic capillaries (Figure 2). They consist of adherens junction and tight junction proteins, including VE-cadherin, claudin 5, ZO-1, ESAM, and occludin (30). The orientation of these junctions as punctate or as more continuous and thus less accessible to nonselective entry of molecules is regulated developmentally (31). In chronic inflammation, the junctions also take on a continuous, more closed nature (31), which may be detrimental for resolution or removal of inflammatory components from the tissues. On the other hand, a regulated immune response may require a shift in the accessibility of tissue-derived mediators to the lymph node.
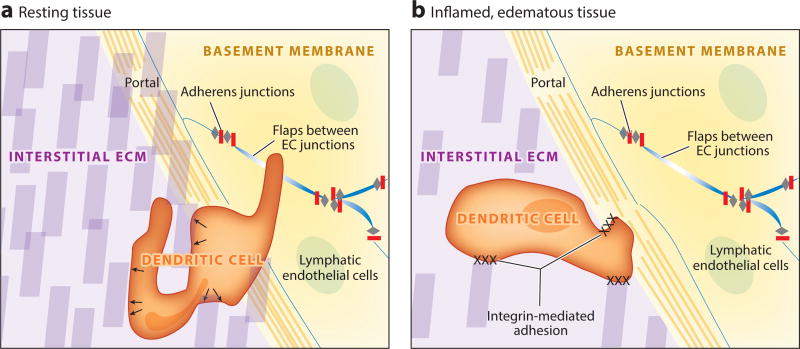
Figure 2.
Entry of immune cells into lymphatic capillaries. (a) Immune cells (dendritic cells are the most thoroughly studied) migrate through the extracellular matrix of a tissue (purple) with amoeboid movement by using matrix fibers (purple blocks) as structural supports, pushing against them with force generated in the cytoskeleton (arrows) to make their way to the lymphatic vessel. No specific adhesion is necessary between the cell and the extracellular matrix. Immune cells continue to utilize adhesion molecule–independent amoeboid motion to enter the lymphatic vessel, finding areas around the vessels with the least dense basement membrane (yellow) and flap-like areas (blue) between lymphatic endothelial cells that are not sealed by adherens junctions (gray and red). (b) In the context of an inflamed and edematous tissue, extracellular matrix fibers may become less dense as fluid accumulates in the tissue and increases the space between such fibers, making conditions unfavorable for amoeboid movement without use of adhesion molecules. Under these circumstances, immune cells employ integrins to anchor themselves to extracellular matrix fibers and lymphatic endothelial cells to make their way into the lymphatic lumen. Abbreviations: EC, endothelial cell; ECM, extracellular matrix.
In the presence of porous junctions, if interstitial fluid is flowing, it will enter through the substantial gaps between these junctions and form lymph. The lymph proteome is enriched in polypeptides that reflect the life cycle and turnover of cells and matrix in the extravascular tissue that the corresponding lymphatic vessels drain, thus providing a rich source of potential peptides for antigen presentation in draining lymph nodes on an ongoing basis (32).
Like the entry of interstitial fluid, the entry of DCs into lymph, then, appears to involve few barriers. Integrins are not essential for DC trafficking under basal circumstances (33). Here, amoeboid movement through matrix occurs without the need for formal adhesion between migrating cells and matrix components (34), and lymphatics with flap-like openings fit into this scheme of minimal molecular requirements for lymphatic uptake of cells or molecules. However, the situation in inflammation is different. Inflammation induces a loosening of the collagen structure within the interstitium (36), whereas efficient amoeboid migration is predicated on a dense matrix network (34). Thus, DC migration during inflammation relies on ICAM-1 and VCAM-1 (37–39). T lymphocytes migrate through the inflamed interstitium, relying on the αv integrin subunit to do so. The basement membrane around the lymphatic capillary is rather sparse and poses little in the way of a filtration barrier (35). Even the entry of immune cells into lymphatic capillaries is aided by the rather sparse basement membrane, as evidence suggests that DCs predominately gain access to the lymphatic lumen where the basement membrane is sparse. These areas are referred to as portals (35) (Figure 2). Like the entry of interstitial fluid, the entry of DCs into lymph, then, appears to involve few barriers.
Whatever the circumstances, it is essential that migrating cells find their way to the lymphatic entry point and be sufficiently motile to enter it. Random interaction would favor very little productive migration. Thus, chemotaxis to the lymphatic vessel is essential. DCs are the best-studied cell type in this regard, and it is well established that a particularly relevant chemokine-receptor pairing is CCL21, expressed by the lymphatic vessels (40) (Table 1), attracting DCs that express the CCL21 receptor CCR7 (41–43). CCR7 was initially reported to be necessary for T cell entry into lymphatics from skin (44), but further studies found that it was not required (45).
Table 1.
Key functional molecules expressed on lymphatic endothelium
| Molecule | Function | Reference(s) |
|---|---|---|
|
| ||
| Prox-1 | Transcription factor necessary for lymphatic development and maintenance. | 138 |
|
| ||
| LYVE-1 | Binds high molecular weight hyaluronic acids when presented as aggregates. May sense microbes. Also expressed by a subset of macrophages. | 58, 66, 139 |
|
| ||
| Podoplanin | Ligand for CLEC-2 that when expressed on platelets mediates lymphatic vessel stabilization during development. Also expressed by many stromal cells and podocytes. | 123, 140, 141 |
|
| ||
| CCL21 | Chemokine expressed on lymphatic endothelial cells to attract CCR7+ cells into the lymph system. Unique isoforms of CCL21 distinguish peripheral and lymph node lymphatic endothelia. | 40, 41, 46, 47, 73 |
|
| ||
| D6 | Atypical chemokine receptor that scavenges inflammatory chemokines to facilitate resolution of inflammation. | 49, 51, 54 |
When one thinks about the issue of chemotaxis to a lymphatic capillary in the face of interstitial fluid flow, a problem becomes apparent: Radiation of a chemotactic gradient is opposed by the direction of flow itself. That is, interstitial flow around the lymphatic capillary is, by definition, in the direction of lymph flow. Establishing a gradient to the vessel requires countermovement of chemokines against the direction of such flow. This problem is combatted by the establishment of a CCL21 gradient that is immobilized in the interstitium via binding to heparin sulfates.
So, although the establishment of a haptotactic, or immobilized, gradient helps to solve the issue of chemokine counterflow, it may nonetheless be the case that the necessary counterdiffusion needed to set up even a haptotactic CCL21 gradient poses an obstacle to DC migration into lymphatics. In the normal mouse, the haptotactic gradient established is steep and thus confined near the lymphatic vessel (46). It is interesting to consider the haptotactic gradient in light of the surprising finding that a 90% reduction in lymphatic capillary density did not impede either total DC migration through lymphatics or the pace of their migration to lymph nodes (47). Meanwhile, these more scarce lymphatics do impede the rate of interstitial flow, as measured by an assay that quantifies the loss of fluorescent tracer injected into the interstitium within a field of view (47). A possible contributor to the preservation of normal DC migration is the greater efficiency of a CCL21 gradient from the lymphatic capillary that is expected when overall interstitial flow is reduced, owing to a reduction in counterflow against the chemokine gradient. Indeed, CCL21 secretion by lymphatics increases in response to increased flow (48), which quite logically would facilitate the maintenance of aCCL21molecular broadcast in the face of a stronger countercurrent.
In brief, then, the literature appears most consistent with the following model. The lymphatic capillary system in the parenchyma of organs like skin is responsive to changes in interstitial flow. Since chemotaxis toward the vessel has to overcome the challenges of flow to establish a functional gradient, reductions in lymph transport have a rather minimal impact on immune cell migration, because the reduction in flow is offset by a more favorable chemotactic environment. By contrast, reductions in molecular transport, such as antigen or cytokines, are far more sensitive to changes in interstitial flow. Thus, one might anticipate that the greater impact of partial lymph stasis in immunity or inflammation would be on molecular transport rather than cellular.
The job of lymphatic capillaries as mediators of molecular clearance is critical to the regulation of inflammation. Indeed, it is so critical that the lymphatic vasculature possesses mechanisms to promote clearance of inflammatory mediators beyond acting as conduits for flow-mediated clearance. Namely, lymphatic capillaries particularly prominently express the nonsignaling G protein–coupled receptorD6, which serves as a chemokine scavenger (Table 1) (49). This scavenging function involves receptor-mediated internalization and degradation of several CC-type chemokines associated with inflammation, including CCR2 ligand CCL2 and CCR5 ligands CCL3, CCL4, and CCL5 (50, 51). Consequently, D6 participates pivotally in controlling inflammation (52, 53), preventing lymphatic congestion (54), and reducing sequelae in pathological scenarios like psoriasis (55) and autoantibody-triggered fetal loss (56).
In 2001, it became clear that one of the most selective receptors expressed on lymphatic capillary endothelium useful for distinguishing them from other cells is a receptor called LYVE-1 (Table 1), which, at least in cell lines, internalizes hyaluronic acid (HA) (57). HA is soluble within the extracellular matrix and is found in lymph (16). The lymph node participates in its turnover, which is quite high, with up to one-third of the total HA pool turning over each day (58). Fragments of HA are also known to trigger DC migration from the skin (59) and are associated with inflammation and tissue injury (60). Such fragments are implicated in the excess accumulation of neutrophils and other immune cells in various clinical contexts, such as transplant rejection (61). Expansion of lymphatic capillaries, through lymphangiogenesis, has been observed at sites of inflammation in many models and inflamed tissues, including transplants (62). While debate continues on the impact that lymphangiogenesis has in the progression of inflammation and clearance of inflammatory mediators through the lymphatic vasculature, one recent study showed that lymphangiogenesis in the transplanted lung protects against rejection by promoting clearance of HA from the grafted organ (63).
So, a role for the HA receptor LYVE-1 on lymphatic endothelium might seem obvious. However, native lymphatic endothelial cells expressing LYVE-1, either in vivo or in vitro, do not show binding to high molecular weight HA (57), even though cell lines expressing LYVE-1 do. Moreover, from analysis in knockout mice, a functional role for LYVE-1 on the endothelium remained elusive (64). Recently, the HA component of the cell wall of group A streptococci was observed to bind efficiently to LYVE-1 in lymphatic endothelial cells, and such binding mediated dissemination of the microorganism to lymph nodes (65). A follow-up study then revealed that clustering, or multimerization, of HA on the surface of microorganisms or cells allows for LYVE-1 binding on lymphatic endothelium (66). Thus, rather than the initial concept that LYVE-1 may fulfill a function on lymphatic vessels by interacting with interstitium-derived HA during its degradation in lymph and lymph nodes, it appears that LYVE-1 serves as a microbial recognition receptor. Another possibility is that LYVE-1 on lymphatic endothelial cells is involved in adhesion to cells that assemble multimeric HA on their surfaces, such as DCs (67) and macrophages (66). This possible role in host defense may extend to the subpopulation of macrophages that express LYVE-1 as well and exhibit an alternatively activated phenotype that is proangiogenic (68–71). Much remains to be clarified in this intriguing area of research.
Lymphatic Collecting Vessels
Lymphatic capillaries, specialized for uptake of lymph as described above, coalesce into contractile vessels that are called collecting vessels. As their name suggests, these vessels collect the interstitial fluid to transport it downstream to lymph nodes and ultimately to the bloodstream; the largest collecting vessel in the body, the thoracic duct, converges on the subclavian vein via the so-called lymphovenous valve. A key feature of collecting vessels is that they are not prone to absorb fluid or cells. They have continuous adherens junctions, in contrast to the lymphatic capillaries that drain into them. At the interface where lymphatic collecting vessels transition to collecting vessels, an interface sometimes called precollecting vessels, some specialized cell entry may occur, as selective expression of the chemokine CCL27 has been observed in this part of the vasculature (72). Furthermore, in the context of an inflamed lymph node, entry of immune cells may also occur (73). However, absorption is not a major function of the collecting vessels.
Collecting lymphatic vessels contain a single layer of endothelial cells, a basement membrane, intraluminal valves, and muscle cell coverage (Figure 3a) (74). The adjacent muscle cells are highly specialized to enable fluid transport from the low-pressure interstitium to the relatively high-pressure subclavian veins, as they express a mix of cardiac, skeletal, and vascular smooth muscle myosin isoforms that contribute to their ability to contract rapidly, forcefully, and spontaneously, very similar to the pumping of the mammalian heart (75, 76), to squeeze lymph along the length of the vessel. In addition to contractile machinery, lymphatic muscle also expresses various ion channels that regulate the timing of contractions, similar to cardiac pacemaker cells (77). Therefore, the main function of lymphatic muscle is to generate the pressure needed to pump lymph along the vessel length against gradually increasing pressure. A recent review discusses lymphatic contractile function in exquisite detail (78).
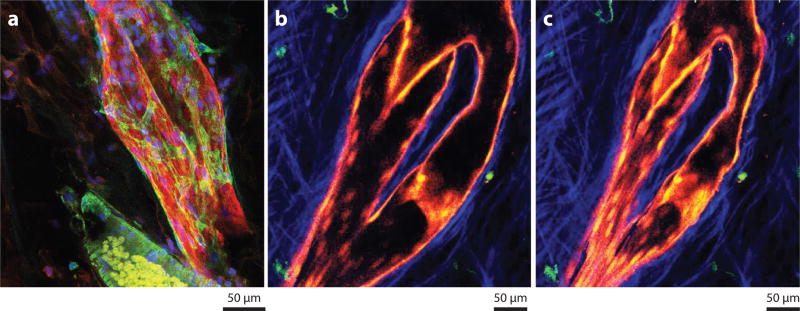
Figure 3.
Morphological features of lymphatic collecting vessels and lymph node lymphatic endothelium using lineage tracer mice and immunostaining. (a) Human mesenteric lymphatic collecting vessel stained for podoplanin (red) and smooth muscle actin (green) to reveal the veiled-like pattern of muscle around the collecting vessels and the bulge often seen around the valves. (b,c) Two single z-stacks of a branched collecting lymphatic vessel outside of the mouse popliteal lymph node, acquired from two-photon imaging in the Prox-1 ERCre × Tomatofl/fl mouse crossed with the CD11cYFP mouse. The images show the extremes at diastole and systole for the lymphangions in view. Lymphatics are red, dendritic cells are green, and collagen is blue.
To facilitate one-way lymph flow, intraluminal valves divide long collecting lymphatic vessels into a series of smaller chambers—called lymphangions—that act like the ventricles of the heart (Figure 3b,c). These valves prevent lymph from flowing back to the tissues after every contraction cycle, easily visualized in Prox-1 ER Cre × Tomatoflox/flox mice (79, 80) (Figure 3b,c). Valve formation and maintenance depend upon the transcription factors FOXC2 (81, 82) and GATA2 (83) in mice and humans, respectively, and the generation of a bridge formed by the integrin α9 and the extracellular matrix component EMILIN1 (84). Valves open and close in response to the pressure gradient on either side of the valve leaflets; i.e., when downstream pressure is lower than upstream pressure, the leaflets open to allow flow, whereas when downstream pressure is greater than upstream pressure, the leaflets close (85). More recent findings have shown that while correct, this view is oversimplified because certain aspects of valve function can lead to unexpected behavior. For example, when downstream pressure is increased sufficiently, the diameter of collecting lymphatic vessels increases to create pressure in the reverse direction, allowing fluid to move backward through a valve. When enough fluid has leaked through a valve in this manner, the valve leaflets may lock open and then allow reverse fluid flow toward the extremities (86).
Lymphatic pumping is negatively regulated by histamine (87, 88) and nitric oxide (NO), which potently inhibits lymphatic muscle contractions (89–91). In general, NO may be produced either by lymphatic endothelium through endothelial NO synthase (eNOS), or during inflammatory states by nearby immune cells through inducible NO synthase (iNOS) (90). During antigen-mediated immune responses, mouse myeloid-derived CD11b+Gr1+ cells and F4/80+ macrophages in the tissue produce copious amounts of NO through iNOS, which in turn inhibits lymphatic pumping (90). It has been further argued that immunosuppression is thus associated with inflammation via NO-mediated inhibition of lymphatic pumping. Indeed, associations between impaired lymphatic pumping and reduced flow to lymph nodes have been made (92). However, the transit of a variety of immune cells via lymph is often increased following acute and chronic inflammation (93–95). Thus, at present, it is unclear how the state of the lymphatic vasculature during immunosuppression associated with inflammation differs from the scenarios in which inflammatory stimuli support increased immune cell trafficking to lymph nodes. To bring clarity to this issue, we need a systematic and broad study examining the immune response and lymphatic function simultaneously and quantifying transport of both soluble and cellular components.
Lymphatics and the Lymph Node Microenvironment
A major factor in considering how inflammation affects immunity and antigen transport relates to the profound impact that inflammation can have on the arrangement of lymphatic vessels and sinuses in the lymph nodes, which directly drain the collecting lymphatic vessels. Under most conditions, steady state and inflammation alike, lymph passes into and through lymph nodes by flowing along the border of the lymph node, along the subcapsular sinus. LYVE-1+ lymphatic endothelial cells line the floor of the sinus, and these cells express CCL21 (96). A separate population of lymphatic endothelial cells lines the ceiling of the subcapsular sinus; these do not express CCL21 but display unique chemokines and chemokine receptors, like CCL1 (97, 98) and CCRL1 (96), another nonsignaling decoy receptor related to D6, discussed above. CCRL1 scavenges CCL21 and thus serves to maintain a robust CCL21 gradient that facilitates DC mobilization from lymph into the lymph nodes (96). The lymphatic endothelial cells that line the floor of the subcapsular sinus also express the protein called plasmalemma vesicle–associated protein (PLVAP), which forms endothelial fenestrae that act as a sieve to restrict access to the conduit network in the paracortex based on molecular size (99). Typically, these conduits have a cutoff of approximately 70 kDa, but this increases in mice lacking PLVAP. In addition to the control of cellular and molecular trafficking at the subcapsular sinus interface, lymphatic endothelial cells in the medullary sinuses control T cell egress from lymph nodes into efferent collecting lymphatic vessels. In the absence of lymphatic endothelial cell–expressed sphingosine kinase 1 and sphingosine kinase 2, sphingosine-1-phosphate is not detectable in lymph nodes, and T cells thus fail to egress (100). During inflammation, lymph node hypertrophy is accompanied by upregulation of CD69 that prevents T cell egress (101) and by an expansion of lymphatics initially near the subcapsular sinus (102, 103). As inflammation transitions to a phase of resolution, lymphangiogenesis shifts away from the subcapsular sinus toward the medulla and supports an expansion of the lymphatic vasculature interface that prompts T cell egress (103). Cells within the lymph node microenvironment, ranging from B cells to T cells to neutrophils, influence the extent and location of lymphatic remodeling that occurs therein (102, 104, 105).
Fitting with distinct functional roles in transport, lymphatic endothelial cells in different regions of the lymph node are phenotypically distinct (106, 107). One striking feature is the expression of self-antigen like tyrosinase, transgenes like α-galactosidase, and immune modulatory proteins like PD-L1in the lymphatic endothelial cells of the lymph node medulla (106, 108). AIRE-independent expression of tyrosinase in these lymph node lymphatic endothelial cells drives deletional tolerance of tyrosinase peptide-reactive MHC-I-restricted CD8+ T cells (106, 108). Thus, a subpopulation of lymphatic endothelial cells confined to the lymph node medulla mediates peripheral tolerance along with other lymph node stromal cells, fibroblastic reticular cells (109). Tolerance induced by lymphatic endothelium is not limited to peripheral antigens expressed by lymphatic endothelial cells, as avid endocytosis of soluble antigens in the environment by the PD-L1+ lymphatic endothelial cells also allows for presentation to CD8+ T cells to mediate deletional tolerance (Figure 4a) (110). Indeed, in yet another recent study, lymphatic endothelial cells took up and retained antigen for long periods of time—up to several weeks—particularly under conditions when lymphangiogenesis in the lymph node was induced and lymphatic endothelium was proliferating. This archiving of antigen was functionally pivotal in the immune response because during late phases after induction of a CD8+ T cell response, it provided antigen to DCs that would cross-present to these T cells to foster CD8+ T cell memory responses (111). The transfer of archived antigen occurred when the lymph node was contracting, and excess endothelial cells generated during lymphangiogenesis then gave rise to lymphatic endothelial cells undergoing apoptosis during contraction. The concept is that the engulfment of dying lymphatic endothelium by DCs would be a means to transfer antigen, with the presentation being carried out by professional antigen-presenting cells, that is, DCs (Figure 4b). These data are consistent with multiple ways in which lymphatic endothelial cells can affect CD8+ T cell immunity, by direct presentation to T cells to support deletion or transfer of antigen to DCs due to apoptosis during lymph node contraction to support sustenance of a productive memory response.
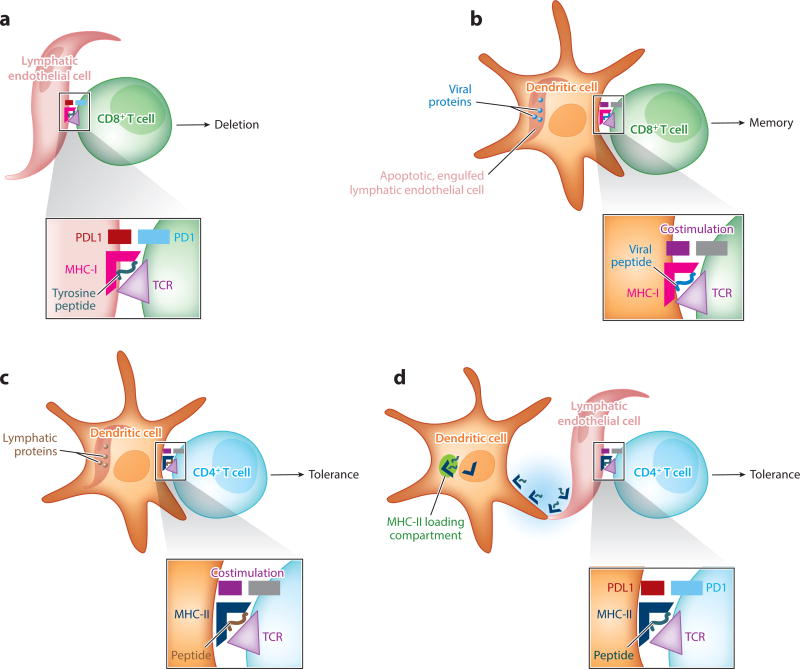
Figure 4.
Lymphatic endothelial cells in antigen presentation require cooperation with dendritic cells. The schema depict four scenarios described in the literature and covered in this review where lymphatic endothelium in the lymph node participates in presenting antigen, most generally to promote peripheral tolerance but also to serve as a long-term reservoir for antigen presentation late in a response for the promotion of CD8+ T cell memory. The scenario in panel a is the only one that does not involve dendritic cells as critical intermediates. (a) A subset of lymphatic endothelial cells express peripheral antigens, or acquire them through uptake of dying cells, for subsequent MHC-I-mediated presentation to CD8+ T cells, leading to immunological tolerance. (b) Lymphatic endothelial cells capable of long-term retention of antigens, such as viral proteins or particles, in lymph nodes undergo apoptosis during lymph node contraction in the late phases of an immune response. The dying lymphatic endothelial cells are engulfed by DCs that cross-present foreign antigens originally present in lymphatic endothelial cells. This mechanism fosters the generation of CD8+ T cell memory against viral antigens. (c) Lymphatic endothelial cells can express MHC-II but lack HLA-DM for appropriate peptide loading of the MHC. CD4+ T cell–associated immunological tolerance can be fostered when proteins from lymphatic endothelial cells are taken up by DCs, allowing for peptides derived from lymphatic endothelial cells to be loaded onto MHC-II molecules of the DCs. (d) Another mechanism that allows lymphatic endothelial cells to support CD4+ T cell immunity despite the lack of HLA-DM occurs when lymphatic endothelial cells acquire intact MHC class II–peptide complexes from DCs, resulting in their presentation of antigen to CD4+ T cells through a mechanism referred to as cross-dressing.
Lymphatic endothelial cells in anatomic sites outside of lymph nodes do not express PD-L1 or self-antigens like tyrosinase (106). This finding highlights the specialization of lymphatic endothelial cells in various anatomic sites. Yet, unfortunately, the diversity of lymphatic endothelial cells in different organs and locations remains largely unexplored. Defining this diversity will be an important endeavor in the field and will likely uncover additional immunological roles of lymphatic endothelium beyond the major function of mediating molecular and cellular transport.
Lymphatic endothelial cells express MHC-II, and upregulate it in response to IFN-γ stimulation (112). Thus, the question arises of whether lymphatic endothelial cells participate in antigen presentation to CD4+ T cells in an MHC-II-restricted manner. Because lymphatic endothelial cells are highly endocytic (110) and in contact with many polypeptides traversing lymph (32), they would encounter many relevant antigens. Two recent studies (113, 114) addressing the role of lymphatic endothelium in presenting antigen on MHC-II molecules came to different conclusions about the role of lymphatic endothelial cell antigen presentation that require further research to clarify. However, these studies converge on a common consensus—that antigens taken up or expressed within lymphatic endothelial cells are not primarily presented on MHC-II expressed by the endothelial cells themselves, but that DCs once again play key intermediary roles, as proposed earlier for CD8+ T cell responses (Figure 4b) (111, 114a). In one study, an antigen engineered to be restricted to lymphatic endothelial cells could not be presented by the endothelium, because the lymphatics did not express HLA-DM for peptide exchange onto MHC-II (113). Nonetheless, CD4+ T cell tolerance to the lymphatic endothelium–expressed antigen occurred because DCs acquired the antigen from lymphatics (Figure 4c) (113). In the other study, lymphatic endothelial cells served as antigen-presenting cells, but many of the surface peptide–MHC-II complexes displayed by lymphatic endothelial cells were derived from DCs that transferred complexes to the lymphatic endothelium (114) (Figure 4d). Thus, despite lacking HLA-DM (113), lymphatic endothelial cells were able to tolerize CD4+ T cells directly (114).
In summing up the role of lymphatic endothelial cells in lymph nodes, this section highlights their role in facilitating the routing of cells and molecules to different lymph node compartments and an additional role for lymphatic endothelium in presentation of antigen, in cooperation with DCs. Yet another key function of lymphatic endothelial cells in lymph nodes, and likely in nonlymphoid tissue, is the production of cytokines, which may be coordinated with the expression of molecules like LYVE-1 (66) and TLR-4, which is highly expressed by lymphatic endothelial cells and macrophages, but not DCs, as commonly thought (112, 115). With respect to cytokine production, lymphatic endothelial cells are the largest producer of IL-7 to maintain T cell homeostasis in lymph nodes (116–118) and to sustain inflammation-induced lymphoid follicles in the context of disease (119). They also produce many other cytokines. For example, recent studies suggest that macrophage-sustaining cytokines like CSF-1 in tumors depend substantially upon the presence of lymphatic vessels (120).
Lymph fluid and cells that leave the lymph node enter efferent collecting lymphatic vessels that, to the extent compared, possess similar properties as afferent lymphatic collecting vessels discussed above. However, lymph is of distinct composition in the efferent vessels versus afferent vessels; indeed, the lymph node typically substantially concentrates lymph so that the protein concentration of efferent lymph is similar to that of plasma, whereas afferent lymph typically has a protein concentration less than half that of plasma (121). Concentration of lymph occurs by the absorption of water into the high endothelial venules within the lymph node (121). Efferent collecting vessels ultimately converge to form the largest collecting vessel in the body, the thoracic duct. This duct returns lymph into the bloodstream at the lymphovenous valve, which separates the thoracic duct from the subclavian vein. The patency of the lymphovenous valve, in addition to a platelet thrombus at this junction, is crucial to preventing blood—which is at a higher pressure in veins than the pressure in the thoracic duct—from backing up into the thoracic duct and reaching upstream lymph nodes and even peripheral tissues like the intestine (122). Remarkably, the patency of the lymphovenous valve is dependent upon platelet-expressed CLEC-2 (122), a ligand for podoplanin that is rich in lymphatic endothelium; this interaction is also required during lymphatic development to maintain blood-lymph separation (123).
LYMPHATIC COLLECTING VESSEL PERMEABILITY AND INTEGRITY AS RELATED TO IMMUNITY—INFLAMMATORY BOWEL DISEASE
It stands to reason that immune and inflammatory responses would be dramatically altered if lymphatic vessel integrity were compromised (124). We have already discussed various scenarios when lymphangiogenesis appears to affect immunological and pathophysiological processes, and still other data on this topic exist. Yet, exactly what lymphangiogenesis achieves is still debated, as the capacity of the existing vessels for processes like cell trafficking seems more than sufficient to meet basal needs in transport (47). As discussed, lymphangiogenesis in the lymph node achieves multiple purposes, from expanding the capacity of immune cells to enter (102) and exit the lymph node (29) to, perhaps most important, serving as a reservoir for antigen as the contraction of expanded lymphatics proceeds (111). However, it is unclear whether in the periphery contraction of lymphatics, once they are expanded, ever occurs (31). With regard to fluid transport, it remains unclear whether newly formed lymphatic capillaries ever acquire a normal permeability after their formation.
With an intense focus on the remodeling of lymphatic capillaries that has been fostered by the attention to lymphangiogenesis in the literature, we turn to considering whether the functional drivers of lymphatic transport, the lymphatic collecting vessels, are linked to immunological regulation or disorders. In contrast to lymphatic capillaries, the lymphatic collecting vessel network does not appear amenable to the generation or maturation of new collecting vessels as a consequence of inflammation or perhaps even injurious insult. Indeed, one of the major causes of lymphedema that is too often associated with the removal of lymph nodes during treatment of breast cancer (125) is that collecting lymphatic vessels may have little capacity to regrow when lymph nodes and inevitably portions of collecting vessels that invest them are removed. So, the question arises: Are immune-mediated diseases associated with altered functionality or integrity of lymphatic collecting vessels? Recent developments in the study of inflammatory bowel disease (IBD) and experimental models of IBD have yielded insights that suggest the answer to this question may be yes.
Some of the earliest descriptions of Crohn’s disease, a major type of IBD, noted the disease was characterized by a prominent lymphangitis (126). Since then, it has become clear that immune dysregulation and changes in the intestinal microbiome are associated with Crohn’s disease (127). Can all of these various features be linked? If so, how?
The intestinal wall, particularly that of the ileum, contains three separate lymphatic capillary beds that originate with and drain distinct anatomic spaces: (a) Every villus contains a single blind-ended lymphatic capillary (lacteal), and (b) the underlying submucosa has its own lymphatic capillary network, as does (c) the muscularis (127). These all independently intersect with, and drain into, contractile lymphatic collecting vessels that originate at the mesenteric border and run antiparallel to the intestinal wall through mesenteric fat until they reach mesenteric lymph nodes (74). In humans, there is a fourth lymphatic capillary bed that is located in the mesenteric fat itself, mostly localized just beneath the serosal epithelial covering of the mesenteric adipose tissue (43). These four lymphatic capillary networks carry many critical antigens to draining lymph nodes, and the villus lacteals also transport dietary fat that is packaged into chylomicrons (8). Thus, the mesenteric lymph node is subjected to periodic high loads of fat that filter through this space, and the lymph node has had to evolve mechanisms to prevent fatty acid–driven inflammation, for instance (8). It is quite possible that microbial lipids, likely available to the host (128), are components of chylomicrons and regularly affect the mesenteric lymphatic corridor.
One of the several unexplained morphological manifestations of Crohn’s disease is the presence of creeping fat, the expansion of fat in the mesentery that overrides the usual mesenteric border and extends up onto the intestinal wall (129). When, during surgical resection of Crohn’s disease–affected ileum, dyes are injected into the mucosa to drain out through mesenteric collecting vessels, embedded in mesenteric adipose tissue, the dye front is often deviated and follows the nascent frontier of the expanding creeping fat (43). This relationship caused us to wonder if significant remodeling of the fat-localized mesenteric collecting vessels occurred. We developed a method to better identify human mesenteric collecting lymphatic vessels, which only weakly stain for many lymphatic markers. Remarkably, we find that the collecting vessels are interrupted by the development of B cell–rich tertiary lymphoid structures that obstruct the path to the usual draining lymph node (43). Tertiary lymphoid structures are common features in many inflammatory diseases and in cancers (130), but until we were able to view them in three-dimensional analyses, it was unclear that the structures were connected to existing collecting lymphatic vessels and thus in a position to affect both lymph transport and which cells and molecules arrived to the draining lymph node. It will be interesting to determine whether this is true in other inflammatory diseases.
It is not possible to know what the consequences of such obstruction would be in humans. However, various studies in mice may offer a clue. One illuminating study arose from an analysis of the consequence of Yersinia pseudotuberculosis infection in mice. Even after mice recover from oral infection with this bacterium, intestinal inflammation continues, along with evidence of suppressed immunity (131), features reminiscent of Crohn’s disease. The explanation for the development of chronic inflammation and impaired immunity is that migratory DCs arising from the lamina propria failed to arrive in the draining lymph node, apparently because collecting vessels became excessively leaky, allowing for a spilling out of immune cells and chylomicrons within lymph into the adjacent fat (131). As far as reported, the obstruction was not related to formation of tertiary lymphoid structures, thus differing morphologically from human Crohn’s disease specimens. However, the concept of leaking, or high permeability, of collecting vessels as the basis of disease deserves more attention. Collecting vessels are known to have a basal level of permeability to proteins like albumin (132). This permeability is sufficient to broadcast antigens to DCs and macrophages that closely associate with the muscular wall of the collecting vessel (73). Indeed, the associated DCs appear to support collecting vessel integrity and lower permeability (80), suggesting that high permeability might be associated with infection or inflammation-mediated loss or modification of these support DCs.
Although development of tertiary lymphoid structures was not reported in connection with the Y. pseudotuberculosis model, such structures have been reported in the context of the disodium sulfate (DSS) model of colitis. The structures form within ten days after cessation of DSS administration. In this experimental model, as in humans, they are highly enriched in B cells (133). The structures formed in the absence of lymphoid tissue inducer cells, and they functioned to contain bacteria and perhaps bacterial products transported from the DSS-damaged epithelial border (133). However, they were also proinflammatory and drove immune pathology (133). The study did not carry out three-dimensional imaging or look at the preexisting lymphatic network. This will be important to do in the future, to allow a fuller comparison to data from human Crohn’s disease.
FUTURE DIRECTIONS
A number of research questions related to the lymphatic vasculature are ripe to be addressed. The possibility that inflammatory diseases like Crohn’s disease might be closely connected to pathophysiological changes in lymphatic vessels, for example, is intriguing. However, too little is known about the mechanisms at play to maintain normal physiology of the vessels at present. Some of these mechanisms no doubt relate to the properties of the vessels themselves and the response to local mediators; others may relate to the status of neighboring immune cells (73, 80, 90). Still others may relate to mechanisms that operate at a distance—via neural communication, for instance. Why does the application of an inflammatory mediator like IL-1b in the vicinity of a collecting vessel cause markedly enhanced permeability in the analogous contralateral collecting vessel of the same experimental subject (134)? Is there a neural cue? Many an experimental design would assume that the contralateral tissue is the ideal control, yet even such basic assumptions require close examination. Clearly, much remains to be learned. Some of the more straightforward tasks may include comprehensive profiling of lymphatic endothelium from different organs and parts of the network. We have a hint, mostly derived from work in the lymph node, that lymphatic vessel diversity exists and peripheral lymphatic capillaries are distinct from those in the lymph node (http://www.immgen.org) (40, 106). Indeed, such work highlights a number of genes relatively selectively expressed in the lymph node by lymphatic endothelium. Some of these selected genes have already been connected to lymphatic valve formation and maintenance (Table 2), yet lymphatic valves have not been described in lymph nodes. Other genes suggest connections that might link lymph node lymphatics to osmolyte transport (135, 136) and pacemaker activity (137). Clearly, there are many new functional connections to be made as we explore lymphatic diversity in full. The tools to do so are at hand.
Table 2.
Survey of gene expression profiling in the Immunological Genome Project identifies novel genes selectively expressed by lymphatic endothelial cells in lymph nodes
| Gene | Functions | Reference(s) |
|---|---|---|
|
| ||
| Xlr5a | X-linked lymphocyte related; unknown function. | None |
|
| ||
| Msr1 | Encodes Scavenger Receptor A, traditionally associated with antigen uptake by macrophages. | 142 |
|
| ||
| Stab2 | Stabilin 2; hyaluron receptor that mediates endocytic clearance of heparin. Known to be expressed on subset of human lymph node lymphatic endothelium. | 107, 143 |
|
| ||
| Sema3a | Semaphorin 3a/neuropilin 1; role in lymphatic vessel maturation and valve formation. | 144 |
|
| ||
| Mmrn/Emilin 4 | Emilin family member; Emilin 1 is described as a crucial ligand for α9 integrin that maintains lymphatic valve structure in peripheral collecting lymphatic vessels. | 84 |
|
| ||
| Fhdc1 | Microtubule-binding member of the formin family that regulates Golgi morphology. | 145 |
|
| ||
| Nts | Neurotensin; neuropeptide that modulates dopamine signaling. In the Immgen database (www.immgen.org), MHC-II+ thymic epithelial cells are the only cells expressing Nts besides lymphatic endothelial cells. | 146 |
|
| ||
| Gpm6a | Glycoprotein M6A; transmembrane protein associated with filopodia formation. Previously associated with neurite growth and guidance. | 147 |
|
| ||
| Slc45a3 | Sucrose symporter that functions in osmoregulation in the kidney. | 135 |
|
| ||
| Popdc2 | Popeye domain–containing protein associated with the regulation of cardiac pacemaking in the sinus node. | 137 |
Acknowledgments
Grant support for the authors includes NIH grants AI049653, HL118206, DP1DK1109668, and HL124142.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–31. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, et al. Quantifying Memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–49. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–17. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 5.Roozendaal R, Mebius RE. Stromal cell–immune cell interactions. Annu. Rev. Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 6.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012;12:762–73. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 7.Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–18. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. J. Clin. Investig. 2014;124:929–35. doi: 10.1172/JCI71610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escolano A, Martinez-Martinez S, Alfranca A, Urso K, Izquierdo HM, et al. Specific calcineurin targeting in macrophages confers resistance to inflammation via MKP-1 and p38. EMBO J. 2014;33:1117–33. doi: 10.1002/embj.201386369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michel CC, Phillips ME. Steady-state fluid filtration at different capillary pressures in perfused frog mesenteric capillaries. J. Physiol. 1987;388:421–35. doi: 10.1113/jphysiol.1987.sp016622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- 12.Michel CC, Nanjee MN, Olszewski WL, Miller NE. LDL and HDL transfer rates across peripheral microvascular endothelium agree with those predicted for passive ultrafiltration in humans. J. Lipid Res. 2015;56:122–28. doi: 10.1194/jlr.M055053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev. Cell. 2012;23:1203–18. doi: 10.1016/j.devcel.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 15.Iijima N, Iwasaki A. Access of protective antiviral antibody to neuronal tissues requires CD4 T-cell help. Nature. 2016;533:552–56. doi: 10.1038/nature17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012;92:1005–60. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- 17.Miller NE, Michel CC, Nanjee MN, Olszewski WL, Miller IP, et al. Secretion of adipokines by human adipose tissue in vivo: partitioning between capillary and lymphatic transport. Am. J. Physiol. Endocrinol. Metab. 2011;301:E659–67. doi: 10.1152/ajpendo.00058.2011. [DOI] [PubMed] [Google Scholar]
- 18.Negrini D, Moriondo A. Lymphatic anatomy and biomechanics. J. Physiol. 2011;589:2927–34. doi: 10.1113/jphysiol.2011.206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chary SR, Jain RK. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. PNAS. 1989;86:5385–89. doi: 10.1073/pnas.86.14.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ. Res. 2003;92:801–8. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 21.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005;5:617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 22.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–38. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Kolka CM, Castro AV, Kirkman EL, Bergman RN. Modest hyperglycemia prevents interstitial dispersion of insulin in skeletal muscle. Metabolism. 2015;64:330–37. doi: 10.1016/j.metabol.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu A, Dube S, Veettil S, Slama M, Kudva YC, et al. Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J. Diabetes Sci. Technol. 2015;9:63–68. doi: 10.1177/1932296814554797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossetti P, Bondia J, Vehi J, Fanelli CG. Estimating plasma glucose from interstitial glucose: the issue of calibration algorithms in commercial continuous glucose monitoring devices. Sensors. 2010;10:10936–52. doi: 10.3390/s101210936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swartz MA, Lund AW. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer. 2012;12:210–19. doi: 10.1038/nrc3186. [DOI] [PubMed] [Google Scholar]
- 28.Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ. Res. 2004;95:204–9. doi: 10.1161/01.RES.0000135549.72828.24. [DOI] [PubMed] [Google Scholar]
- 29.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, et al. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17:671–84. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am. J. Pathol. 2012;180:2561–75. doi: 10.1016/j.ajpath.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clement CC, Santambrogio L. The lymph self-antigen repertoire. Front. Immunol. 2013;4:424. doi: 10.3389/fimmu.2013.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 34.Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 2009;21:636–44. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J. Exp. Med. 2009;206:2925–35. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun YM, et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat. Immunol. 2013;14:949–58. doi: 10.1038/ni.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J, Wang JH, Guo YJ, Sy MS, Bigby M. In vivo treatment with anti-ICAM-1 and anti-LFA-1 antibodies inhibits contact sensitization-induced migration of epidermal Langerhans cells to regional lymph nodes. Cell Immunol. 1994;158:389–99. doi: 10.1006/cimm.1994.1285. [DOI] [PubMed] [Google Scholar]
- 38.Xu H, Guan H, Zu G, Bullard D, Hanson J, et al. The role of ICAM-1 molecule in the migration of Langerhans cells in the skin and regional lymph node. Eur. J. Immunol. 2001;31:3085–93. doi: 10.1002/1521-4141(2001010)31:10<3085::AID-IMMU3085>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006;203:2763–77. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, et al. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 1999;190:1183–88. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Förster R, Schubel A, Breitfeld D, Kremmer E, Renner-Müller I, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 42.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–88. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Randolph GJ, Bala S, Rahier JF, Johnson MW, Wang PL, et al. Lymphoid aggregates remodel lymphatic collecting vessels that serve mesenteric lymph nodes in Crohn disease. Am. J. Pathol. 2016;186(12):3066–73. doi: 10.1016/j.ajpath.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Debes GF, Arnold CN, Young AJ, Krautwald S, Lipp M, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005;6:889–94. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vander Lugt B, Tubo NJ, Nizza ST, Boes M, Malissen B, et al. CCR7 plays no appreciable role in trafficking of central memory CD4 T cells to lymph nodes. J. Immunol. 2013;191:3119–27. doi: 10.4049/jimmunol.1200938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328–32. doi: 10.1126/science.1228456. [DOI] [PubMed] [Google Scholar]
- 47.Platt AM, Rutkowski JM, Martel C, Kuan EL, Ivanov S, et al. Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J. Immunol. 2013;190:4608–20. doi: 10.4049/jimmunol.1202600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 2009;183:4273–83. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- 49.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, et al. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am. J. Pathol. 2001;158:867–77. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locati M, Torre YM, Galliera E, Bonecchi R, Bodduluri H, et al. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 2005;16:679–86. doi: 10.1016/j.cytogfr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Weber M, Blair E, Simpson CV, O’Hara M, Blackburn PE, et al. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol. Biol. Cell. 2004;15:2492–508. doi: 10.1091/mbc.E03-09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, et al. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur. J. Immunol. 2005;35:1342–46. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- 53.Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, et al. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut. 2010;59:197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- 54.Lee KM, McKimmie CS, Gilchrist DS, Pallas KJ, Nibbs RJ, et al. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood. 2011;118:6220–29. doi: 10.1182/blood-2011-03-344044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh MD, King V, Baldwin H, Burden D, Thorrat A, et al. Elevated expression of the chemokine-scavenging receptor D6 is associated with impaired lesion development in psoriasis. Am. J. Pathol. 2012;181:1158–64. doi: 10.1016/j.ajpath.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R, et al. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. PNAS. 2007;104:2319–24. doi: 10.1073/pnas.0607514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 2001;276:19420–30. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 58.Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol. Rev. 2009;230:216–31. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 59.Muto J, Morioka Y, Yamasaki K, Kim M, Garcia A, et al. Hyaluronan digestion controls DC migration from the skin. J. Clin. Investig. 2014;124:1309–19. doi: 10.1172/JCI67947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 61.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, et al. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am. J. Respir. Crit. Care Med. 2014;189:556–66. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerjaschki D. Lymphatic neoangiogenesis in renal transplants: a driving force of chronic rejection? J. Nephrol. 2006;19:403–6. [PubMed] [Google Scholar]
- 63.Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, et al. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J. Clin. Investig. 2015;125:4255–68. doi: 10.1172/JCI79693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gale NW, Prevo R, Espinosa J, Ferguson DJ, Dominguez MG, et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol. Cell Biol. 2007;27:595–604. doi: 10.1128/MCB.01503-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynskey NN, Banerji S, Johnson LA, Holder KA, Reglinski M, et al. Rapid lymphatic dissemination of encapsulated group A streptococci via lymphatic vessel endothelial receptor-1 interaction. PLOS Pathog. 2015;11:e1005137. doi: 10.1371/journal.ppat.1005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrance W, Banerji S, Day AJ, Bhattacharjee S, Jackson DG. Binding of hyaluronan to the native lymphatic vessel endothelial receptor LYVE-1 is critically dependent on receptor clustering and hyaluronan organization. J. Biol. Chem. 2016;291:8014–30. doi: 10.1074/jbc.M115.708305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, Takashima A. Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J. Immunol. 2002;169:4322–31. doi: 10.4049/jimmunol.169.8.4322. [DOI] [PubMed] [Google Scholar]
- 68.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, et al. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+,CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J. Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 69.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ. Res. 2007;100:e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 70.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLOS ONE. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gautier EL, Ivanov S, Williams JW, Huang SC, Marcelin G, et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J. Exp. Med. 2014;211:1525–31. doi: 10.1084/jem.20140570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wick N, Haluza D, Gurnhofer E, Raab I, Kasimir MT, et al. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27-expressing lymphatic endothelial cells. Am. J. Pathol. 2008;173:1202–9. doi: 10.2353/ajpath.2008.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuan EL, Ivanov S, Bridenbaugh EA, Victora G, Wang W, et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J. Immunol. 2015;194:5200–10. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol. Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 75.Scallan JP, Wolpers JH, Muthuchamy M, Zawieja DC, Gashev AA, Davis MJ. Independent and interactive effects of preload and afterload on the pump function of the isolated lymphangion. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H809–24. doi: 10.1152/ajpheart.01098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H795–808. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathias R, von der Weid PY. Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:G623–34. doi: 10.1152/ajpgi.00392.2012. [DOI] [PubMed] [Google Scholar]
- 78.Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ. Lymphatic pumping: mechanics, mechanisms and malfunction. J. Physiol. 2016;594:5749–68. doi: 10.1113/JP272088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–32. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ivanov S, Scallan JP, Kim KW, Werth K, Johnson MW, et al. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. J. Clin. Investig. 2016;126:1581–91. doi: 10.1172/JCI84518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 2004;10:974–81. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 82.Mellor RH, Brice G, Stanton AW, French J, Smith A, et al. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation. 2007;115:1912–20. doi: 10.1161/CIRCULATIONAHA.106.675348. [DOI] [PubMed] [Google Scholar]
- 83.Kazenwadel J, Betterman KL, Chong CE, Stokes PH, Lee YK, et al. GATA2 is required for lymphatic vessel valve development and maintenance. J. Clin. Investig. 2015;125:2979–94. doi: 10.1172/JCI78888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danussi C, Del Bel Belluz L, Pivetta E, Modica TM, Muro A, et al. EMILIN1/α9β1 integrin interaction is crucial in lymphatic valve formation and maintenance. Mol. Cell Biol. 2013;33:4381–94. doi: 10.1128/MCB.00872-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE., Jr Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H48–60. doi: 10.1152/ajpheart.00133.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scallan JP, Wolpers JH, Davis MJ. Constriction of isolated collecting lymphatic vessels in response to acute increases in downstream pressure. J. Physiol. 2013;591:443–59. doi: 10.1113/jphysiol.2012.237909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, et al. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation. 2014;21:640–48. doi: 10.1111/micc.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurtz KH, Moor AN, Souza-Smith FM, Breslin JW. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation. 2014;21:593–605. doi: 10.1111/micc.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J. Physiol. 2002;540:1023–37. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liao S, Cheng G, Conner DA, Huang Y, Kucherlapati RS, et al. Impaired lymphatic contraction associated with immunosuppression. PNAS. 2011;108:18784–89. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scallan JP, Davis MJ. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J. Physiol. 2013;591:2139–56. doi: 10.1113/jphysiol.2012.250662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bouta EM, Wood RW, Brown EB, Rahimi H, Ritchlin CT, Schwarz EM. In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. J. Physiol. 2014;592:1213–23. doi: 10.1113/jphysiol.2013.266700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown MN, Fintushel SR, Lee MH, Jennrich S, Geherin SA, et al. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J. Immunol. 2010;185:4873–82. doi: 10.4049/jimmunol.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, et al. The skin, a novel niche for recirculating B cells. J. Immunol. 2012;188:6027–35. doi: 10.4049/jimmunol.1102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacPherson GG, Jenkins CD, Stein MJ, Edwards C. Endotoxin-mediated dendritic cell release from the intestine: characterization of released dendritic cells and TNF dependence. J. Immunol. 1995;154:1317–22. [PubMed] [Google Scholar]
- 96.Ulvmar MH, Werth K, Braun A, Kelay P, Hub E, et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014;15:623–30. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- 97.Qu C, Edwards EW, Tacke F, Angeli V, Llodra J, et al. Role of CCR8 and other chemokine pathways in the migration of monocyte-derived dendritic cells to lymph nodes. J. Exp. Med. 2004;200:1231–41. doi: 10.1084/jem.20032152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. J. Exp. Med. 2013;210:1509–28. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rantakari P, Auvinen K, Jappinen N, Kapraali M, Valtonen J, et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 2015;16:386–96. doi: 10.1038/ni.3101. [DOI] [PubMed] [Google Scholar]
- 100.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–44. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 102.Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Tan KW, Yeo KP, Wong FH, Lim HY, Khoo KL, et al. Expansion of cortical and medullary sinuses restrains lymph node hypertrophy during prolonged inflammation. J. Immunol. 2012;188:4065–80. doi: 10.4049/jimmunol.1101854. [DOI] [PubMed] [Google Scholar]
- 104.Tan KW, Chong SZ, Wong FH, Evrard M, Tan SM, et al. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D. Blood. 2013;122:3666–77. doi: 10.1182/blood-2012-11-466532. [DOI] [PubMed] [Google Scholar]
- 105.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, et al. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 106.Cohen JN, Tewalt EF, Rouhani SJ, Buonomo EL, Bruce AN, et al. Tolerogenic properties of lymphatic endothelial cells are controlled by the lymph node microenvironment. PLOS ONE. 2014;9:e87740. doi: 10.1371/journal.pone.0087740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park SM, Angel CE, McIntosh JD, Mansell C, Chen CJ, et al. Mapping the distinctive populations of lymphatic endothelial cells in different zones of human lymph nodes. PLOS ONE. 2014;9:e94781. doi: 10.1371/journal.pone.0094781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, et al. Lymph node–resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J. Exp. Med. 2010;207:681–88. doi: 10.1084/jem.20092465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chang JE, Turley SJ. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 2015;36:30–39. doi: 10.1016/j.it.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 110.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, et al. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J. Immunol. 2014;192:5002–11. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat. Commun. 2014;5:3989. doi: 10.1038/ncomms4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Malhotra D, Fletcher AL, Astarita J, Lukacs-Kornek V, Tayalia P, et al. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rouhani SJ, Eccles JD, Tewalt EF, Engelhard VH. Regulation of T-cell tolerance by lymphatic endothelial cells. J. Clin. Cell Immunol. 2014;5:1000242. doi: 10.4172/2155-9899.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J. Exp. Med. 2014;211:1153–66. doi: 10.1084/jem.20132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114a.Kedl R, Finlon J, Tamburini B. Contraction of the hypertrophic lymph node and death of lymphatic endothelial cells as a process for antigen exchange; Presented at Lymphat. Gordon Res. Conf.; Mar. 22; Ventura, CA. 2016. [Google Scholar]
- 115.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, et al. IL-7-producing stromal cells are critical for lymph node remodeling. Blood. 2012;120:4675–83. doi: 10.1182/blood-2012-03-416859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miller CN, Hartigan-O’Connor DJ, Lee MS, Laidlaw G, Cornelissen IP, et al. IL-7 production in murine lymphatic endothelial cells and induction in the setting of peripheral lymphopenia. Int. Immunol. 2013;25:471–83. doi: 10.1093/intimm/dxt012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hara T, Shitara S, Imai K, Miyachi H, Kitano S, et al. Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J. Immunol. 2012;189:1577–84. doi: 10.4049/jimmunol.1200586. [DOI] [PubMed] [Google Scholar]
- 119.Shinoda K, Hirahara K, Iinuma T, Ichikawa T, Suzuki AS, et al. Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. PNAS. 2016;113:E2842–51. doi: 10.1073/pnas.1512600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Steinskog ES, Sagstad SJ, Wagner M, Karlsen TV, Yang N, et al. Impaired lymphatic function accelerates cancer growth. Oncotarget. 2016;7:45789–802. doi: 10.18632/oncotarget.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adair TH, Moffatt DS, Paulsen AW, Guyton AC. Quantitation of changes in lymph protein concentration during lymph node transit. Am. J. Physiol. 1982;243:H351–59. doi: 10.1152/ajpheart.1982.243.3.H351. [DOI] [PubMed] [Google Scholar]
- 122.Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Investig. 2014;124:273–84. doi: 10.1172/JCI70422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bertozzi CC, Hess PR, Kahn ML. Platelets: covert regulators of lymphatic development. Arterioscler. Thromb. Vasc. Biol. 2010;30:2368–71. doi: 10.1161/ATVBAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomas SN, Rutkowski JM, Pasquier M, Kuan EL, Alitalo K, et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J. Immunol. 2012;189:2181–90. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patel KM, Manrique O, Sosin M, Hashmi MA, Poysophon P, Henderson R. Lymphatic mapping and lymphedema surgery in the breast cancer patient. Gland Surg. 2015;4:244–56. doi: 10.3978/j.issn.2227-684X.2015.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Warren S, Sommers SC. Pathology of regional ileitis and ulcerative colitis. J. Am. Med. Assoc. 1954;154:189–93. doi: 10.1001/jama.1954.02940370001001. [DOI] [PubMed] [Google Scholar]
- 127.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016;13:13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 128.Donia MS, Fischbach MA. Small molecules from the human microbiota. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Colombel JF, Watson AJ, Neurath MF. The 10 remaining mysteries of inflammatory bowel disease. Gut. 2008;57:429–33. doi: 10.1136/gut.2007.122192. [DOI] [PubMed] [Google Scholar]
- 130.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautes-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–80. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 131.Fonseca DM, Hand TW, Han SJ, Gerner MY, Glatman Zaretsky A, et al. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell. 2015;163:354–66. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Scallan JP, Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J. Physiol. 2010;588:243–54. doi: 10.1113/jphysiol.2009.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J. Exp. Med. 2011;208:125–34. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine. 2013;64:362–69. doi: 10.1016/j.cyto.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vitavska O, Edemir B, Wieczorek H. Putative role of the H+/sucrose symporter SLC45A3 as an osmolyte transporter in the kidney. Pflugers Arch. 2016;468:1353–62. doi: 10.1007/s00424-016-1841-6. [DOI] [PubMed] [Google Scholar]
- 136.Hofmeister LH, Perisic S, Titze J. Tissue sodium storage: evidence for kidney-like extrarenal countercurrent systems? Pflugers Arch. 2015;467:551–58. doi: 10.1007/s00424-014-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boukens BJ, Christoffels VM. Popeye proteins: muscle for the aging sinus node. J. Clin. Investig. 2012;122:810–13. doi: 10.1172/JCI62588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang Y, Oliver G. Transcriptional control of lymphatic endothelial cell type specification. Adv. Anat. Embryol. Cell Biol. 2014;214:5–22. doi: 10.1007/978-3-7091-1646-3_2. [DOI] [PubMed] [Google Scholar]
- 139.Jackson DG. The lymphatics revisited: new perspectives from the hyaluronan receptor LYVE-1. Trends Cardiovasc. Med. 2003;13:1–7. doi: 10.1016/s1050-1738(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 140.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999;154:385–94. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 142.Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? The mouse’s tale. J. Clin. Investig. 2001;108:649–54. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harris EN, Weigel JA, Weigel PH. The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J. Biol. Chem. 2008;283:17341–50. doi: 10.1074/jbc.M710360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, et al. An unexpected role of semaphorin3A–neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ. Res. 2012;111:426–36. doi: 10.1161/CIRCRESAHA.112.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Copeland SJ, Thurston SF, Copeland JW. Actin- and microtubule-dependent regulation of Golgi morphology by FHDC1. Mol. Biol. Cell. 2016;27:260–76. doi: 10.1091/mbc.E15-02-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moore TC. Modification of lymphocyte traffic by vasoactive neurotransmitter substances. Immunology. 1984;52:511–18. [PMC free article] [PubMed] [Google Scholar]
- 147.Formoso K, Garcia MD, Frasch AC, Scorticati C. Filopodia formation driven by membrane glycoprotein M6a depends on the interaction of its transmembrane domains. J. Neurochem. 2015;134:499–512. doi: 10.1111/jnc.13153. [DOI] [PubMed] [Google Scholar]