Abstract
The inbred Fischer (F344) and Lewis (LEW) rats, while originally developed as animal models for cancer and tissue transplantation research, have since been used to study genetic differences in a variety of physiological and behavioral endpoints. In this context, LEW rats show greater sensitivity to the aversive effects of cocaine as compared to F344 rats in a conditioned taste avoidance procedure. Like cocaine, 3,4-methylenedioxypyrovalerone (MDPV; “Bath Salts”) acts as a dopamine transport blocker and possesses aversive properties, making it a good candidate for assessing whether the aforementioned strain differences with cocaine would generalize to drugs with similar biochemical action. Accordingly, male F344 and LEW rats were exposed to a novel saccharin solution followed by injections of one of four doses of MDPV in a taste avoidance procedure. Over the four saccharin/MDPV pairings during conditioning, core body temperatures were also assessed. Similar to previous research, MDPV induced robust dose-dependent taste avoidance, although no effect of strain was observed. MDPV also produced hyperthermia that was independent of strain and unrelated to the conditioned taste avoidance. These findings argue for a complex influence of multiple (and likely interacting) monoaminergic systems mediating MDPV-induced taste avoidance in the two strains and suggest different mechanisms of avoidance learning for cocaine and MDPV.
Keywords: F344 and LEW rats, strain differences, MDPV, conditioned taste avoidance, hyperthermia
1. Introduction
The inbred Fischer (F344) and Lewis (LEW) rat strains, while originally developed as animal models for cancer and tissue transplantation research (Billingham et al., 1962; see also Riley et al., 2009), have since been used to study genetic differences in a wide variety of physiological and behavioral endpoints. For example, these strains have been shown to differ in terms of stress reactivity and HPA activation, with F344 rats more reactive to stressors (Dhabhar et al., 1993; Sternberg et al., 1992; Stöhr et al., 2000).
These strains also differ in regards to exploratory tendencies and drug reactivity (see Kosten and Ambrosio, 2002), including their relative sensitivity to the rewarding and aversive effects of various drugs. Although initially characterized for their differences to the rewarding effects of drugs (Davis et al., 2007; Picetti et al., 2010; Picetti et al., 2012; Stöhr et al., 1998), the F344 and LEW strains have recently been assessed for their differential sensitivity to the drugs’ aversive effects, those which might limit drug intake. In relation to these aversive effects, Lancellotti et al. (2001) reported that while F344 rats developed robust morphine-induced conditioned taste avoidance (CTAs), LEW rats failed to acquire such avoidance at any dose tested and even after repeated conditioning trials (see also Davis et al., 2012), indicating a relative insensitivity of LEW rats to the aversive motivational properties of morphine. F344 rats also show greater CTAs induced by nicotine and ethanol (see Pescatore et al., 2005; Roma et al., 2006). Interestingly, and in contrast to these findings, LEW rats show a greater sensitivity to the aversive effects of cocaine as compared to F344 rats (Glowa et al., 1994; Grigson and Freet, 2000). These differences suggest that there is a genetic component in the relative sensitivity to these affective properties, with the direction of the difference being drug-dependent (for similar analyses of strain differences with the rewarding effects of drugs, see Cunningham et al., 1992a; Cunningham, 2014; Davis et al., 2007).
Although the basis for the differences reported between the F344 and LEW strains in relation to taste avoidance learning is not known, it might be predicted that drugs acting via similar biochemical mechanisms would induce similar strain differences. In this context, examining drugs with similar biological actions might provide insight into the basis for strain differences with these drugs, as well as the mechanisms mediating avoidance induced by these compounds. As noted above, cocaine-induced taste avoidance differs significantly for the F344 and LEW strains (LEW > F344). In outbred rats, this effect appears to be mediated primarily by cocaine’s actions on dopamine (DA), as DA antagonists such as pimozide and haloperidol have been shown to block cocaine-induced CTA (see Hunt et al., 1985; Serafine et al., 2012b). Consistent with these results, animals exposed to the selective dopamine transporter (DAT) inhibitor GBR 12909 prior to taste avoidance conditioning with cocaine display weaker cocaine-induced CTAs (Serafine et al., 2012a), suggesting an adaptation to DA-mediated effects as a consequence of the preexposure and a role of DA in cocaine’s aversive effects (for a review of drug preexposure, see Riley & Simpson, 2001). Cocaine-induced taste avoidance is also weaker in knockout mice with a DAT deletion (although it should be noted that NET- and SERT-knockout mice showed stronger attenuation in this preparation; see Jones et al., 2010; for a complete discussion of monoamine regulation of cocaine-induced taste avoidance, see Serafine and Riley, 2013). It is clear from this evidence that DA plays some role in mediating taste avoidance induced by cocaine.
Recently, the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV; one of many potential constituents of “Bath Salts”) has received attention due to its increased use and associated anecdotal reports of paranoid psychotic behavior, agitation, hallucinations and delirium (see Bronstein et al., 2011; Penders, 2012). Like cocaine, MDPV acts as a DAT blocker that inhibits clearance of endogenous DA and, thereby, increases extracellular concentrations of DA in the nucleus accumbens and elsewhere. MDPV appears much more efficient and potent than cocaine at inhibiting DA clearance (as well as in producing locomotor activation, tachycardia, and hypertension; see Baumann et al., 2013a). Like cocaine, MDPV possesses multiple stimulus properties. For example, Watterson et al. (2014) found that MDPV maintained self-administration in rats across a range of doses, progressively escalated intake over long-access conditions and significantly lowered ICSS thresholds, results which demonstrate reinforcing or rewarding properties and suggest possible abuse liability. On the other hand, Merluzzi et al. (2014) reported that MDPV produced dose-dependent taste avoidance (at 1, 1.8 and 3.2 mg/kg) in both adolescent and adult male Sprague-Dawley rats, indicating the presence of aversive properties of the drug.
Given that both cocaine and MDPV induce robust conditioned taste avoidance and have a similar mechanism of action, i.e., DA reuptake inhibition, it might be predicted that the aforementioned strain differences with cocaine-induced taste avoidance (LEW > F344) would also be seen using MDPV as the avoidance-inducing agent. Such a finding would support the role of DA in cocaine- and MDPV-induced taste avoidance and provide some insight into the basis for any reported differences between the two strains in avoidance induced by these compounds, e.g., differential sensitivities to the aversive effects mediated by DA. Accordingly, F344 and LEW rats in the present study underwent taste avoidance conditioning with one of four doses of MDPV (0, 1, 1.8, 3.2 mg/kg).
MDPV, like many stimulants, has also been reported to induce hyperthermia (see Fantegrossi et al., 2013; Merluzzi et al., 2014). Although we have recently noted that MDPV-induced hyperthermia was unrelated to aversion learning in outbred Sprague-Dawley rats (see Merluzzi et al., 2014), it is not known if and to what extent core body temperature is affected by MDPV in these strains and if any reported differences are associated with MDPV-induced taste avoidance. Work with other stimulants has demonstrated that hyperthermia-induced neurotoxicity is mediated at least in part by excess DA activity at several DA receptor subtypes (D1/D3; see Ares-Santos et al., 2012; Granado et al., 2011; Granado et al., 2014). Given that the F344 and LEW strains differ significantly in DA reactivity (and the molecular pathways involved in DA synthesis and release; see Beitner-Johnson et al., 1991; Flores et al., 1998; Guitart et al., 1992; Strecker et al., 1994), it might be expected that core temperatures would be differentially affected by MDPV. If these changes are involved in MDPV-induced avoidance learning, there should be some relationship between the two in these strains. Accordingly, in addition to the behavioral assessments, core body temperatures for both strains were assessed prior to and following each drug injection during taste avoidance conditioning.
2. Materials and Methods
Experimentally-naïve male F344 (n = 32) and LEW (n = 32) rats were obtained from Harlan Sprague-Dawley (Indianapolis, IN) on postnatal day (PND) 21. Procedures recommended by the National Research Council (1996), the Committee on Guidelines for the Care and Use of Animals in Neuroscience and Behavioral Research (2003) and the Institutional Animal Care and Use Committee at American University were followed at all times.
Upon arrival to the animal facility on PND 21, subjects were group housed (three rats per polycarbonate bins; 23 cm × 4 cm × 21cm) and maintained on ad-libitum food and water until PND 89. On PND 77, subjects were handled and weighed and temperature probes were implanted. Specifically, the injection site was aseptically cleaned with alcohol and a temperature transponder (Bio Medic Data Systems, Seaford, DE; Model #IPTT-300) was rapidly inserted subcutaneously into each animal’s left flank with a hypodermic needle. For the next 7 days (PND 77–83), the temperature transponders were checked daily to assess placement by palpating the injection site and for proper function by attempting to record the temperatures. From PND 83-PND 88, animals were weighed, handled and scanned for body temperature daily and each subject’s daily water consumption was recorded to the nearest tenth of a milliliter (ml).
2.1. Drugs and Solutions
3,4-methylenedioxypyrovalerone hydrochloride (synthesized at the Chemical Biology Research Branch of the National Institute on Drug Abuse) was dissolved in sterile isotonic saline (0.9%) at a concentration of 1 mg/ml and was subsequently filtered through a 0.2 mm filter to remove any contaminants before being administered intraperitoneally (IP) at a dose of 1, 1.8 or 3.2 mg/kg (Merluzzi et al., 2014). Sterile isotonic saline was also filtered before being administered to vehicle controls equivolume to the highest dose of MDPV administered (3.2 mg/kg). Volume of the injection was manipulated in favor of concentration given the influence concentration has on the absorption/distribution of the drug. Sodium saccharin (0.1%; Sigma-Aldrich, St. Louis, MO) was prepared daily as a 1 g/L solution in tap water.
2.2 Habituation
On PND 89, water was removed from all subjects for the next 24 h to encourage consumption during training and testing. On the following day (PND 90), subjects were removed from their group-housed bins, scanned for body temperature, weighed and placed in individual hanging wire-mesh test cages (24.3 cm × 19 cm × 18 cm) where they received 45-min access to water in graduated 50-ml Nalgene tubes. Following removal of the water tubes, the animals spent an additional 20 min in the test cages before they were returned to their group-housed bins; water was then made freely available for 23 h. This procedure (24-h water deprivation followed by 45-min water access in test cages followed by 23-h ad-libitum water access) was repeated three additional times to ensure adaptation to test cage fluid consumption.
2.3 Conditioning
On the day following the final water-adaptation cycle, water was again removed for 24 h. On the next day, all subjects were weighed, handled, scanned for body temperature, placed in the test cages and given 45-min access to a novel saccharin solution. The initial scan during handling was to ensure that the probe was functioning, and these data were not considered in any statistical analyses. Immediately following saccharin access, subjects were assigned to one of four groups such that saccharin consumption was comparable among groups. Subjects were then scanned for body temperature and given an IP injection of either 0 (vehicle) or 1, 1.8 or 3.2 mg/kg MDPV. Animals were then returned to their group-housed home cages where water was available ad libitum for the next 23 h. In addition to the scan immediately prior to drug administration, animals were scanned 30-, 60-, 90- and 120-min post injection. For each temperature recording, the probe was scanned three times and the three measurements averaged, with the three measurements never differing by more than 0.9°C. The temperature data were uploaded to a spreadsheet from the Bio Medic Data Systems scanner. This procedure (24-hour restriction; test cage saccharin access, injection and ad-libitum access water access) was repeated an additional three times.
2.4 Statistical Analyses
On the initial conditioning trial (before drug administration), consumption by the F344 strain was significantly less than that of the LEW strain (means of 4.40 and 8.62, respectively; F (1, 63) = 160.334; for similar differences between the F344 and LEW strains, see Davis and Riley, 2007; Hurwitz et al., 2013). Consequently, saccharin consumption was analyzed separately for each strain with a 4 × 4 repeated measures ANOVA with between-subjects factors of Dose (0, 1, 1.8 and 3.2) and a within-subjects factor of Trial (1 – 4). In the case of two-way interactions, simple effects of Dose at each Trial (univariate analysis) and of Trial at each Dose (multivariate analysis) were assessed, with Bonferroni-corrected multiple comparisons as warranted. To enable direct comparison between strains (given strain differences in consumption on Trial 1), consumption data on the final conditioning trial (Trial 4) were standardized as percent of Group 0 for each strain and analyzed with a 2 × 4 factorial ANOVA with factors of Strain (F344 and LEW) and Dose (0, 1, 1.8 and 3.2). In the event of an interaction, simple effects were assessed with Bonferroni-corrected multiple comparisons as warranted.
Statistical analyses of body temperature were based on the mean of three serial scans per animal per interval. Temperature differences were analyzed with a 2 × 4 × 4 × 5 mixed model ANOVA with between-subjects factors of strain (F344 and LEW) and Dose (0, 1, 1.8 and 3.2) and within-subjects factors of Trial (1 – 4) and Interval (1 – 5). Any interactions were followed by tests of simple effects, with Bonferroni-corrected multiple comparisons as warranted. All statistical analyses were based on a significance level of 0.05.
3. Results
3.1 CTA
Saccharin consumption (ml) for F344 and LEW rats over the four repeated conditioning trials are represented in Figures 1A and B, respectively. As illustrated, MDPV produced comparable dose-dependent taste avoidance in both strains.
Figure 1.
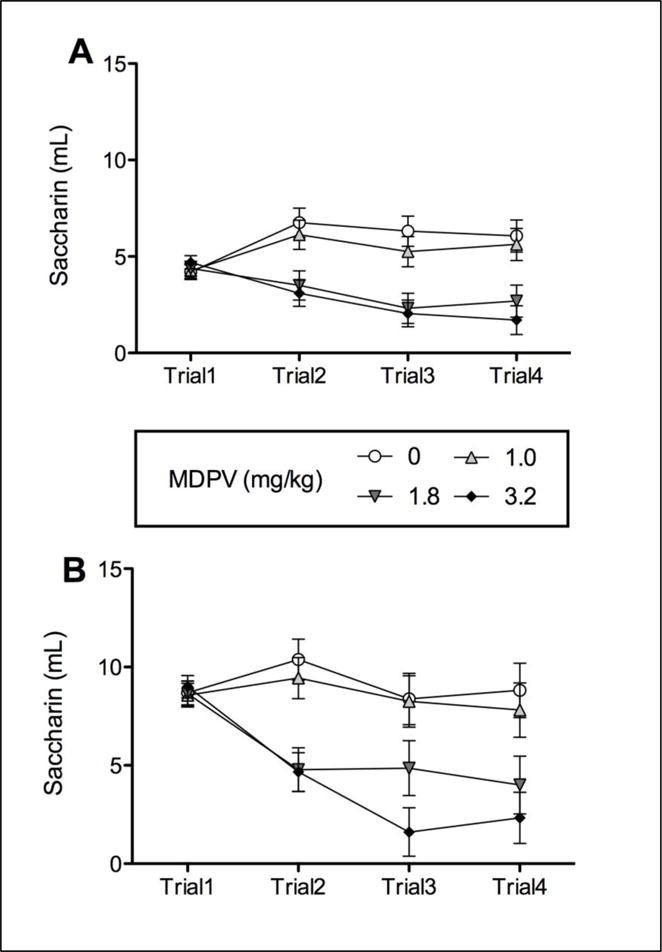
Saccharin consumption (ml) over repeated trials at each dose (0, 1, 1.8 and 3.2 mg/kg) of MDPV for F344 (Panel A) and LEW (Panel B) rats
F344
The 4 × 4 mixed model ANOVA for saccharin consumption revealed significant effects of Dose [F (3, 30) = 6.524] and Trial [F (3, 90) = 3.624], as well as a significant Dose × Trial [F (9, 90) = 7.113] interaction. To further explore the Dose × Trial interaction, simple effects of Dose at each Trial were assessed with a univariate analysis, which revealed significant differences on Trials 2–4 [Trial 2: F (3, 30) = 6.326; Trial 3: F (3, 30) = 7.956; Trial 4: F (3, 30) = 7.239]. Corrected multiple comparisons indicated that Groups 1.8 and 3.2 drank significantly less than Group 0 on Trials 2–4. Additionally, Group 3.2 drank significantly less than Group 1 on Trials 2–4. A multivariate analysis examining changes in consumption over trials for each dose indicated that Groups 0, 1 and 3.2 showed a significant change in consumption across trials [Vehicle: F (3, 28) = 5.831; 1: F (3, 28) = 3.057; 13.2: F (3, 28) = 6.394]. Multiple comparisons indicated that Group 0 significantly increased consumption from Trial 1 to Trials 2 and 3, but consumption on Trial 4 did not significantly differ from Trial 1. Group 1 significantly increased consumption from Trial 1 to Trial 2, but consumption on Trials 3 and 4 did not differ from Trial 1. Conversely, Group 1.8 drank significantly less saccharin on Trial 3 than on Trial 1, but showed no difference between Trials 2 and 4 and Trial 1. Finally, Group 3.2 drank significantly less saccharin on Trials 2–4 than on Trial 1.
LEW
The 4 × 4 mixed model ANOVA for saccharin consumption from Trial 1 revealed significant effects of Dose [F (3, 28) = 7.48] and Trial [F (3, 84) = 10.939], as well as a significant Dose × Trial [F (9, 84) = 4.004] interaction. To further explore the Dose × Trial interaction, simple effects of Dose at each Trial were assessed with a univariate analysis, which revealed significant differences on Trials 2–4 [Trial 2: F (3, 28) = 8.44; Trial 3: F (3, 28) = 6.482; Trial 4: F (3, 28) = 5.165]. Corrected multiple comparisons indicated that on Trial 2, Groups 1.8 and 3.2 drank significantly less than Groups 0 and 1. Additionally, on Trials 3–4, Group 3.2 drank significantly less than Groups 0 and 1. A multivariate analysis examining changes in consumption over trials for each dose indicated that Groups 1.8 and 3.2 showed a significant change in consumption across trials [1.8: F (3, 26) = 4.675; 3.2: F (3, 26) = 11.695]. Multiple comparisons indicated that Group 1.8 significantly decreased consumption from Trial 1 to Trials 2 and 4, but consumption on Trial 3 did not significantly differ from Trial 1. Group 3.2 significantly decreased consumption from Trial 1 to Trials 2–4. No differences were seen across Trials for Groups 0 and 1.
Final Aversion Test (Trial 4)
To directly compare subjects in the two strains, consumption for each dose group in each strain was calculated as a percentage of its respective control group (Group 0) on the final conditioning trial (Trial 4). These transformations are presented in Figure 2. As illustrated, although the percent of control consumption was dose-dependent, there were no strain differences in these percentage shifts at any dose. Specifically, the 4 × 4 factorial ANOVA revealed a main effect of Dose [F (3, 58) = 12.17], but no effect of Strain nor an interaction between Strain and Dose. In relation to the main effect of Dose, a one-way ANOVA (collapsed across Strain) with Tukey’s post-hoc revealed that Groups 1.8 and 3.2 showed significantly greater percentage differences from control values compared to Groups 0 and 1.
Figure 2.
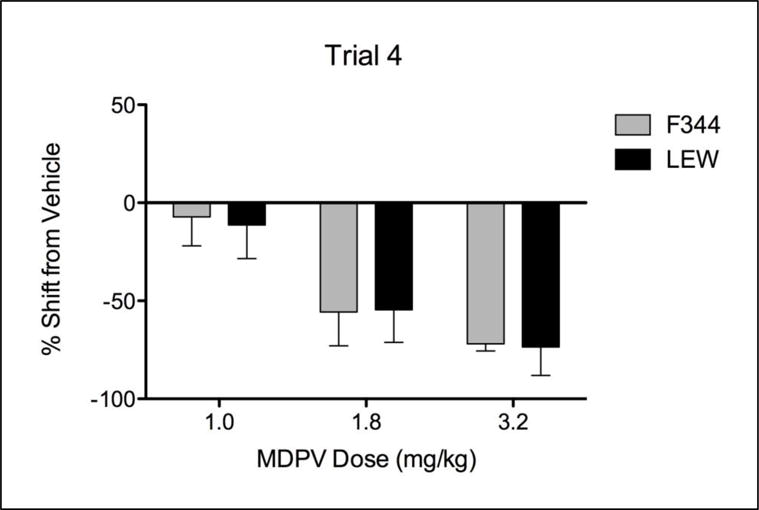
Percentage shift in saccharin consumption from their respective vehicle control subjects for F344 and LEW rats at each dose of MDPV (1, 1.8 and 3.2 mg/kg) on the final conditioning trial (Trial 4)
3.2 Temperature Assessment
The temperature probes of one subject in Group LEW 1.8 and one subject in Group LEW 3.2 failed to function midway through conditioning. All data from these subjects were removed from temperature assessments, leaving n=7 and n=9, respectively. The initial 2 × 4 × 4 × 5 mixed model ANOVA on body temperature yielded significant effects of Strain [F (1, 56) =6.263], Dose [F (3, 56)=3.355] and Interval [F (4, 672) = 27.315], as well as significant Strain × Interval [F (4, 672) =3.926] and Dose × Interval [F (12, 672) =2.566] interactions. Given that no significant effect of Trial was found, data were collapsed across Trials and analyzed with a 2 × 4 × 5 mixed model ANOVA. This analysis found significant main effects of Interval [F (4, 224) =27.315], Strain [F (1, 56) = 6.263] and Dose [F (3, 56) = 3.355], as well as significant Interval × Strain [F (4, 224)= 3.926] and Interval × Dose [F (12, 224) = 2.566] interactions. No three-way interaction was found. Body temperature over the five intervals (collapsed across Trials) is illustrated in Figures 3A (F344) and 3B (LEW).
Figure 3.
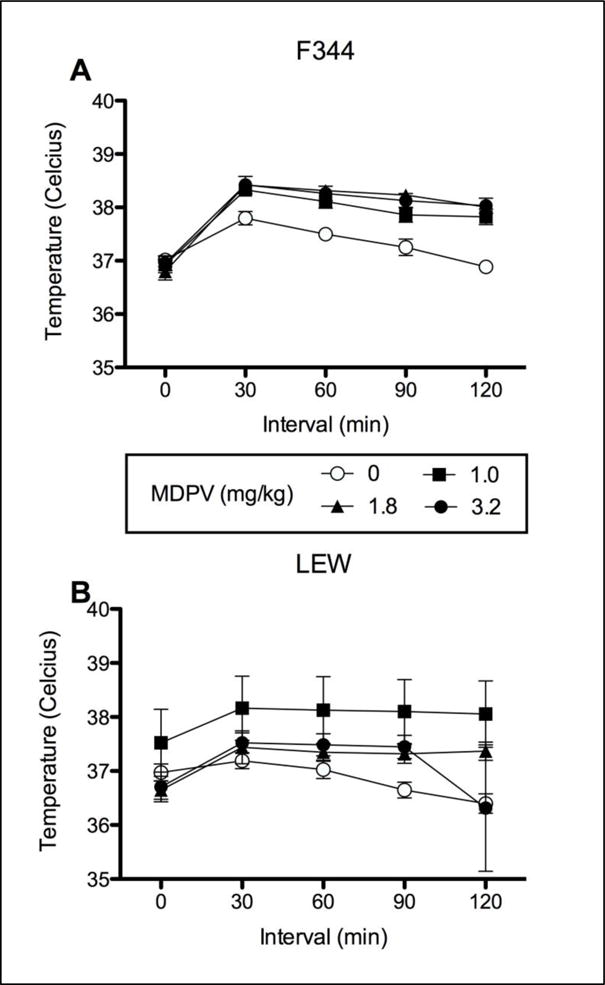
Body temperature (collapsed across trials) immediately prior to and 30, 60, 90 and 120 min following an injection of 0, 1, 1.8 and 3.2 mg/kg MDPV for F344 (A) and LEW (B) rats
In relation to the Dose × Interval interaction (collapsed across Strain), multivariate test of simple effects indicated significant differences across intervals for all dose groups [VEH: F (4, 53) = 17.852; 1: F (4, 53) = 24.114; 1.8: F (4, 53) = 30.326; 3.2: F (4, 53)= 34.716]. Pairwise comparisons indicated that at 30, 60 and 90 min post-injection, all groups injected with MDPV displayed increases in temperature from the initial sampling period (i.e., 0 min). This increase was also seen in the vehicle-treated animals at 30 min, which likely reflected a stress response in the F344 strain (see Discussion below). By 120 min post injection, no group differed from the initial temperature sample.
4. Discussion
Given that MDPV and cocaine both impact DA (Baumann et al., 2013b; Eshleman et al., 2013), which is thought to mediate (at least in part) cocaine-induced taste avoidance (Serafine et al., 2012a; Serafine et al., 2012b), it might be expected that the strain differences seen with cocaine-induced taste avoidance (LEW > F344) would also be evident with MDPV. As described, MDPV induced dose-dependent taste avoidance in both strains. However, there was no evidence of any consistent strain differences in this avoidance. That is, the rate of acquisition of the taste avoidance, as well as the degree of suppression was comparable between the two strains. For example, following the initial conditioning trial, subjects in the two high dose groups (i.e., Groups 1.8 and 3.2) in both strains displayed significantly less consumption than control subjects (Group 0). Further, subjects in Groups 3.2 in both strains drank less than those in Groups 1 (displaying typical dose-related avoidance). Group 1 never differed from controls at any point in conditioning. Over trials, subjects in Groups 1.8 and 3.2 decreased consumption from their own Trial 1 baselines (although the specific trials on which this occurred differed for the two strains at the 1.8 mg/kg dose, e.g., it was evident at Trial 3 for the F344 strain and Trials 2 and 4 for the LEW subjects). Neither the control nor low dose MDPV group (Group 1) drank less than baseline over repeated trials. These parallels were also evident when direct comparisons between the two strains were made on the final conditioning trial. Specifically, when the percentage shifts from their respective controls were compared there were no differences between the two strains at any dose. Since no drug group showed complete suppression of consumption during conditioning nor complete suppression relative to controls on the final test, the lack of strain differences was not likely due to a floor effect. Avoidance was dose-dependent and graded, allowing for differences to be evident, should they exist.
Two issues are relevant to these findings. First, why don’t the F344 and LEW strains display differences with MDPV given that MDPV and cocaine share a similar mechanism of action? Secondly, what accounts for MDPV-induced taste avoidance and why don’t the F344 and LEW strains differ in these effects? In relation to the first issue, the basis for the current lack of differences between the two strains (like those seen with cocaine) is unknown, but the differential monoamine binding profiles between cocaine and MDPV may provide a possible answer. While MDPV is significantly more potent than cocaine as an uptake blocker at DAT (MDPV: IC50 4.1 ± 0.6 nM, COC: IC50 211 ±19 nM) and NET (MDPV: IC50 25.9 ± 5.6 nM, COC: IC50 292 ±34 nM), it is significantly weaker at SERT (MDPV: IC50 3305 ± 485 nM, COC: IC50 313 ±17 nM), with DAT/SERT ratios of 806:1 (MDPV) and 1.5:1 (COC; see Marusich et al., 2014). MDPV’s limited effects on serotonin (5-HT) could explain the lack of differential strain effects seen in the present assessment, a possibility substantiated by prior research on cocaine-induced taste avoidance. As noted above, 5-HT has also been implicated in cocaine-induced taste avoidance. For example, transgenic mice with SERT deletions display attenuated acquisition of cocaine-induced taste avoidance compared to wild-type and DAT knockout mice (Jones et al., 2010), although it should be noted that NET knockouts also produced significant attenuation of avoidance, precluding a solitary role of 5-HT in this behavioral effect. Additionally, Serafine et al. (2010) reported that animals exposed to cocaine prior to taste avoidance conditioning with fluoxetine (a selective 5-HT reuptake inhibitor) displayed attenuated fluoxetine-induced taste avoidance, suggesting some adaptation to their common aversive effects (see Braveman, 1975; Cappell et al., 1975; for a review see Simpson and Riley, 2001). It should be noted that fluoxetine preexposure had no impact on cocaine-induced taste avoidance, although as noted by the authors, it is possible this was a dose-dependent effect. Given that the doses of fluoxetine used for preexposure were relatively low (3.2, 5.6, 10 and 18 mg/kg), it is possible that larger doses would have produced a stronger attenuation. This is supported by prior work by Jones et.al. (2009) showing attenuated cocaine-induced taste avoidance in mice when the animals were preexposed to a higher dose of fluoxetine (50 mg/kg). Additionally, Serafine et al. (2012a) exposed animals to two doses of GBR 12909, a selective DAT inhibitor, prior to cocaine-induced taste avoidance conditioning and found that only the high dose (50 mg/kg) blocked subsequent avoidance, again suggesting the dose-dependent nature of the attenuating effects of drug preexposure (for more on parameters of the preexposure effect, see Riley and Simpson, 2001). Together, these studies suggest a possible role for 5-HT in cocaine-induced taste avoidance and the failure of MDPV to induce strain-dependent differences in avoidance learning given its limited effects on 5-HT.
In relation to what mediates taste avoidance with MDPV and why no differences are evident between the two strains, it is important to note that only a single study to date has examined such avoidance with this drug (see Merluzzi et al., 2014), so the mechanism mediating MDPV’s aversive effects simply is not known. Consequently, it is difficult to determine why the strains do not differ with this compound. It is interesting in this context that differences in taste avoidance between the F344 and LEW strains have been reported for a wide variety of compounds (see above) with the direction of the differences dependent upon the specific drug tested. Although such differences have been widely reported, there are a handful of drugs (and manipulations) for which they are not evident. For example, Wakeford and Riley (2014) found that both the F344 and LEW strains acquired dose-dependent THC-induced taste avoidance, but no significant strain effect was seen. Related preparations outside of drugs of abuse have also produced comparable avoidance between the two strains, including those induced by wheel-running (Nakajima, 2014), LiCl (Foynes and Riley, 2004), spontaneous withdrawal (in opiate dependent animals; Cobuzzi and Riley, 2011) and the peripherally-acting opiate agonist, loperamide (Davis et al., 2012).
Wakeford and Riley (2014) proposed a possible explanation for the lack of strain differences in taste avoidance induced by these manipulations. Specifically, drugs such as LiCl and THC (at high doses) that do not produce strain differences in taste avoidance do produce disgust reactions, such as gaping, chin rubbing and paw pushing to solutions with which they are paired (Berridge et al., 1981; Parker and Gillies, 1995). That these behaviors are generally thought to be associated with sickness supports the position that illness may mediate the avoidance response induced by these drugs (see Parker, 2003). It is interesting to note that Dwyer et al. (2008) reported that wheel running also induced palatability changes consistent with those induced by emetics such as LiCl. Alternatively, drugs that do produce differential avoidance between the F344 and LEW strains do not produce these disgust reactions (see Parker, 2003 for a review). In relation to these findings, Wakeford and Riley suggested the possibility that these strains do not differ in their sensitivity to emetic or sickness-inducing agents. Interestingly, clinical reports have shown that MDPV use has been associated with symptoms of nausea and vomiting (Coppola and Mondola, 2012; Wright et al., 2013). Although these findings suggest that the failure to see strain differences in MDPV-induced avoidance may be related to its sickness-inducing effects, it will be important to validate such effects in preclinical models of emesis and palatability shifts (see Parker, 2014).
Although the focus of the current study was to determine whether strain differences seen with cocaine would be evident with a drug with similar biochemical action, the secondary issue was whether hyperthermia may be involved in any differential MDPV-induced avoidance learning in these strains. As previously mentioned, the DA system has been implicated in stimulant-induced hyperthermia, and given that the F344 and LEW animals differ in DA reactivity (Cadoni and Chiara, 2007; Flores et al., 1998), body temperature at multiple intervals following MDPV injections was assessed. As noted, MDPV induced significant hyperthermia in both strains (an effect consistent with previous work assessing MDPV-induce changes in temperature in outbred rats; see Merluzzi et al., 2014; Ross et al., 2012). Although the two strains differed in core temperature, with LEW subjects displaying significantly lower temperature than the F344 strain, there was no significant Strain by Dose interaction, suggesting that these differences were independent of MDPV. Similar effects have been reported with THC, suggesting that the differential changes in core temperature may be a function of differential stress reactivity in the two strains (for a discussion, see Wakeford and Riley, 2014).
The question initially posed was how taste avoidance induced by MDPV was related to any changes in MDPV-induced changes in temperature. While MDPV did induce a hyperthermic effect, this effect was not dose-dependent (in contrast to the effects seen with CTA). Subsequent Pearson’s correlations confirmed that there were no significant relationships between saccharin consumption and body temperature for either F344 or LEW animals on any injection day at any time point post drug injection (Bonferroni corrected, all ps>.0125, data not shown). Although there seemed to be a trend towards a dose effect in the Lewis animals, with the low dose producing the highest temperatures, this effect was not statistically significant, likely due to high variability in those animals. This absence of a relationship between avoidance and temperature is again consistent with Merluzzi et.al. (2013), which showed a similar hyperthermic effect that was independent of MDPV-induced avoidance learning in outbred rats. Although some prior work with ethanol has suggested a contributory influence of ethanol’s thermic effects on strength of ethanol-induced taste avoidance (see Cunningham et al., 1988; Cunningham et al., 1992b), this effect has not consistently been found in these strains, or in outbred rats, with other drugs of abuse (see Roma et al., 2006; Wakeford and Riley, 2014).
The findings presented here further demonstrate that while the subjective effects and mechanisms of action of cocaine and MDPV have been linked (see Baumann et al., 2013b; Coppola and Mondola, 2012), F344 and LEW animals do not differ in MDPV-induced taste avoidance in a manner seen with cocaine. The drug and dose specificity of differential avoidance between F344 and LEW animals suggests a complex genetic influence of multiple (and likely interacting) monoaminergic systems and argues for independent mechanisms of taste avoidance learning for cocaine and MDPV.
Highlights.
-
-
MDPV induced dose-dependent conditioned taste avoidance (CTA).
-
-
There were no strain differences in MDPV-induced taste avoidance.
-
-
MDPV induced hyperthermia independent of strain.
-
-
There was no relationship between MDPV-induced CTA and hyperthermia.
-
-
MDPV-induced taste avoidance may be a function of its emetic effects.
Acknowledgments
This research was supported by a grant from the Mellon Foundation to ALR. A portion of this research was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism, NIH, US Department of Health and Human Services.
Footnotes
The authors have no conflicts of interest to declare.
References
- Ares-Santos S, Granado N, Oliva I, O’Shea E, Martin E, Colado M, et al. Dopamine D1 receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiology of Disease. 2012;45:810–20. doi: 10.1016/j.nbd.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. European Journal of Pharmacology. 2013a;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3, 4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013b;38:552–62. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X, Nestler EJ. Dopaminergic brain reward regions of Lewis and Fischer rats display different levels of tyrosine hydroxylase and other morphine-and cocaine-regulated phosphoproteins. Brain Research. 1991;561:147–50. doi: 10.1016/0006-8993(91)90759-o. [DOI] [PubMed] [Google Scholar]
- Berridge K, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. Journal of Comparative and Physiological Psychology. 1981;95:363. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- Billingham R, Hodge BA, Silvers WK. An estimate of the number of histocompatibility loci in the rat. Proceedings of the National Academy of Sciences of the United States of America. 1962;48:138. doi: 10.1073/pnas.48.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR, Green JL, Rumack BH, Dart RC. 2010 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th Annual Report. Clinical Toxicology. 2011;49:910–41. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Chiara GD. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. Journal of Neurochemistry. 2007;103:487–99. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Cobuzzi JL, Riley AL. Spontaneous withdrawal in opiate-dependent Fischer 344, Lewis and Sprague–Dawley rats. Pharmacology Biochemistry and Behavior. 2011;98:28–34. doi: 10.1016/j.pbb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Coppola M, Mondola R. 3,4-methylenedioxypyrovalerone (MDPV): chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicology Letters. 2012;208:12–5. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Hawks DM, Niehus DR. Role of hypothermia in ethanol-induced conditioned taste aversion. Psychopharmacology. 1988;95:318–22. doi: 10.1007/BF00181940. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992a;107:385–93. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Bachtold JF. Ambient Temperature Effects on Taste Aversion Conditioned by Ethanol: Contribution of Ethanol‐Induced Hypothermia. Alcoholism: Clinical and Experimental Research. 1992b;16:1117–24. doi: 10.1111/j.1530-0277.1992.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Cunningham CL. Genetic Relationship Between Ethanol-Induced Conditioned Place Preference and Other Ethanol Phenotypes in 15 Inbred Mouse Strains. 2014 doi: 10.1037/a0036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CM, Riley AL. The effects of cocaine preexposure on cocaine-induced taste aversion learning in Fischer and Lewis rat strains. Pharmacology Biochemistry and Behavior. 2007;87:198–202. doi: 10.1016/j.pbb.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davis CM, Roma PG, Dominguez JM, Riley AL. Morphine-induced place conditioning in Fischer and Lewis rats: Acquisition and dose-response in a fully biased procedure. Pharmacology Biochemistry and Behavior. 2007;86:516–23. doi: 10.1016/j.pbb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Davis CM, Cobuzzi JL, Riley AL. Assessment of the aversive effects of peripheral mu opioid receptor agonism in Fischer 344 and Lewis rats. Pharmacology Biochemistry and Behavior. 2012;101:181–6. doi: 10.1016/j.pbb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS, Spencer RL. Stress response, adrenal steroid receptor levels and corticosteroid-binding globulin levels—a comparison between Sprague-Dawley, Fischer 344 and Lewis rats. Brain Research. 1993;616:89–98. doi: 10.1016/0006-8993(93)90196-t. [DOI] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochemical Pharmacology. 2013;85:1803–15. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’constituent 3, 4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–73. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Wood GK, Barbeau D, Quirion R, Srivastava LK. Lewis and Fischer rats: a comparison of dopamine transporter and receptors levels. Brain Research. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Foynes MM, Riley AL. Lithium-chloride-induced conditioned taste aversions in the Lewis and Fischer 344 rat strains. Pharmacology Biochemistry and Behavior. 2004;79:303–8. doi: 10.1016/j.pbb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology. 1994;114:229–32. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva I, Martin ED, Colado MI, Moratalla R. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiology of Disease. 2011;42:391–403. doi: 10.1016/j.nbd.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Moratalla R. D1 but not D4 dopamine receptors are critical for MDMA-induced neurotoxicity in mice. Neurotoxicity Research. 2014;25:100–9. doi: 10.1007/s12640-013-9438-8. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride, are greater in Lewis than in Fischer rats: Evidence for the reward comparison hypothesis. Behavioral Neuroscience. 2000;114:353. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Guitart X, Beitner‐Johnson D, Marby DW, Kosten TA, Nestler EJ. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse. 1992;12:242–53. doi: 10.1002/syn.890120310. [DOI] [PubMed] [Google Scholar]
- Hunt T, Switzman L, Amit Z. Involvement of dopamine in the aversive stimulus properties of cocaine in rats. Pharmacology Biochemistry and Behavior. 1985;22:945–8. doi: 10.1016/0091-3057(85)90300-4. [DOI] [PubMed] [Google Scholar]
- Hurwitz ZE, Cobuzzi JL, Merluzzi AP, Wetzell B, Riley AL. Prepubertal fischer 344 rats display stronger morphine-induced taste avoidance than prepubertal lewis rats. Developmental psychobiology. 2013 doi: 10.1002/dev.21176. [DOI] [PubMed] [Google Scholar]
- Jones JD, Hall FS, Uhl GR, Rice K, Riley AL. Differential involvement of the norepinephrine, serotonin and dopamine reuptake transporter proteins in cocaine-induced taste aversion. Pharmacology Biochemistry and Behavior. 2009;93:75–81. doi: 10.1016/j.pbb.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Hall FS, Uhl GR, Riley AL. Dopamine, norepinephrine and serotonin transporter gene deletions differentially alter cocaine-induced taste aversion. Pharmacology Biochemistry and Behavior. 2010;94:580–7. doi: 10.1016/j.pbb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL. Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacology Biochemistry and Behavior. 2001;68:603–10. doi: 10.1016/s0091-3057(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, et al. Age-dependent MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Developmental Psychobiology. 2014 doi: 10.1002/dev.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S. Running-based taste aversion learning in five strains of rats. Physiology & Behavior. 2014;123:200–13. [PubMed] [Google Scholar]
- Parker LA, Gillies T. THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behavioral Neuroscience. 1995;109:71–8. doi: 10.1037//0735-7044.109.1.71. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Animal Learning & Behavior. 2003;31:165–72. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Parker LA. Conditioned flavor avoidance and conditioned gaping: Rat models of conditioned nausea. European Journal of Pharmacology. 2014;722:122–33. doi: 10.1016/j.ejphar.2013.09.070. [DOI] [PubMed] [Google Scholar]
- Penders TM. How to recognize a patient who’s high on “bath salts”. The Journal of Family Practice. 2012;61:210–2. [PubMed] [Google Scholar]
- Pescatore KA, Glowa JR, Riley AL. Strain differences in the acquisition of nicotine-induced conditioned taste aversion. Pharmacology Biochemistry and Behavior. 2005;82:751–7. doi: 10.1016/j.pbb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Picetti R, Ho A, Butelman ER, Kreek MJ. Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology. 2010;211:313–23. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology. 2012;220:163–72. doi: 10.1007/s00213-011-2464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL, Simpson GR. The attenuating effects of drug preexposure on taste aversion conditioning: Generality, experimental parameters, underlying mechanisms, and implications for drug use and abuse. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2001. pp. 505–59. [Google Scholar]
- Riley AL, Davis CM, Roma PG. Strain Differences in Taste Aversion Learning: Implications for Animal Models of Drug Abuse. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Neural and Behavioral Processes. New York, NY: Oxford University Press; 2009. pp. 226–61. [Google Scholar]
- Roma PG, Flint WW, Higley JD, Riley AL. Assessment of the aversive and rewarding effects of alcohol in Fischer and Lewis rats. Psychopharmacology. 2006;189:187–99. doi: 10.1007/s00213-006-0553-6. [DOI] [PubMed] [Google Scholar]
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. The American Journal of Medicine. 2012;125:854–8. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Riley AL. Preexposure to cocaine attenuates aversions induced by both cocaine and fluoxetine: Implications for the basis of cocaine-induced conditioned taste aversions. Pharmacology Biochemistry and Behavior. 2010;95:230–4. doi: 10.1016/j.pbb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Briscione MA, Rice KC, Riley AL. Dopamine mediates cocaine-induced conditioned taste aversions as demonstrated with cross-drug preexposure to GBR 12909. Pharmacology Biochemistry and Behavior. 2012a;102:269–74. doi: 10.1016/j.pbb.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Briscione MA, Riley AL. The effects of haloperidol on cocaine-induced conditioned taste aversions. Physiology & Behavior. 2012b;105:1161–7. doi: 10.1016/j.physbeh.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Riley AL. Cocaine-Induced Conditioned Taste Aversions: Role of Monoamine Reuptake Inhibition. In: Hall FS, editor. Serotonin: Biosynthesis, Regulation, and Health Implications. New York, NY: Nova Science Publishers; 2013. pp. 257–91. [Google Scholar]
- Sternberg EM, Glowa JR, Smith MA, Cologero AE, Listwak SJ, Aksentijevich S, et al. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Research. 1992;570:54–60. doi: 10.1016/0006-8993(92)90563-o. [DOI] [PubMed] [Google Scholar]
- Stöhr T, Wermeling DS, Weiner I, Feldon J. Rat strain differences in open-field behavior and the locomotor stimulating and rewarding effects of amphetamine. Pharmacology Biochemistry and Behavior. 1998;59:813–8. doi: 10.1016/s0091-3057(97)00542-x. [DOI] [PubMed] [Google Scholar]
- Stöhr T, Szuran T, Welzl H, Pliska V, Feldon J, Pryce CR. Lewis/Fischer rat strain differences in endocrine and behavioural responses to environmental challenge. Pharmacology Biochemistry and Behavior. 2000;67:809–19. doi: 10.1016/s0091-3057(00)00426-3. [DOI] [PubMed] [Google Scholar]
- Strecker RE, Eberle WF, Ashby CR., Jr Extracellular dopamine and its metabolites in the nucleus accumbens of Fischer and Lewis rats: basal levels and cocaine-induced changes. Life Sciences. 1994;56:PL135–PL41. doi: 10.1016/0024-3205(94)00913-9. [DOI] [PubMed] [Google Scholar]
- Wakeford AG, Riley AL. Conditioned taste avoidance induced by Δ9-tetrahydrocannabinol in the Fischer (F344) and Lewis (LEW) rat strains. Pharmacology Biochemistry and Behavior. 2014;116:39–44. doi: 10.1016/j.pbb.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV) Addiction Biology. 2014;19:165–74. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TH, Cline-Parhamovich K, Lajoie D, Parsons L, Dunn M, Ferslew KE. Deaths involving methylenedioxypyrovalerone (MDPV) in upper east Tennessee. Journal of Forensic Sciences. 2013;58:1558–62. doi: 10.1111/1556-4029.12260. [DOI] [PubMed] [Google Scholar]


