Abstract
OBJECTIVE
Most drugs of abuse have both aversive and rewarding effects, and the use and abuse potential of such drugs is thought to be a function of a balance of these affective properties. Characterizing these effects and their relative balance may provide insight into abuse vulnerability. One drug that has received recent attention is methylenedioxyparavalerone (MDPV), a monoamine transport inhibitor similar to, but significantly more potent than, cocaine. MDPV is self-administered and has been shown to produce aversive and rewarding effects in adult rats. The present study extended this characterization of the affective properties of MDPV by examining its ability to support place conditioning at a range of doses known to produce taste avoidance.
METHODS
Male Sprague-Dawley rats were injected with MDPV (1, 1.8 or 3.2 mg/kg) or saline and placed on the non-preferred side of a place conditioning apparatus for 30 min. On the next day, they were given an injection of saline and placed on the preferred side. This was repeated three times for a total of four conditioning cycles, and side preference was assessed on a final test.
RESULTS
All doses of MDPV produced significant increases in time spent in the drug-paired chamber, an effect not seen in vehicle-treated animals.
CONCLUSIONS
That the same doses of MDPV induced both taste avoidance and place preference allows assessments of how other factors might impact these effects and how they may, in turn, contribute to its abuse liability.
Keywords: MDPV, place preference, reward
1. Introduction
Since synthetic cathinones emerged in the United States in 2010, the subsequent rise in popularity and abuse poses a significant threat for public health (Bronstein et al., 2011; Rosenbaum et al., 2012; Spiller et al., 2011). These drugs have been marketed as “legal highs” and have been commonly sold in gas stations and “head shops”, as well as over the internet. Law enforcement in various countries has attempted to circumvent the use and distribution of the most common of these, namely 3, 4-methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxymethcathinone (methylone) and 4-methylmethcathinone (mephedrone). For example, these compounds were given emergency Schedule I classification in the US in 2011 and now have permanent classification as such (Drug Enforcement Administration, 2011; Fass et al., 2012). These compounds, as well as several behaviorally active derivatives, however, are still being produced and distributed, thus their continued characterization is crucial (Araújo et al., 2014; Baumann, 2014; for a review, see German et al., 2014; Marusich et al., 2014).
One of the aforementioned parent compounds, MDPV, is the main substance found in patients presenting with bath salts overdose in the United States (see Baumann, 2014); it is a monoamine transport inhibitor similar to, but significantly more potent than, cocaine, with primary actions at dopamine and norepinephrine transporters (with minimal effects on serotonin) (Baumann et al., 2013; Baumann et al., 2012). Like cocaine, this compound has also been shown to maintain self-administration and promote escalation of intake across a range of doses in adult rats (Aarde et al., 2013; Watterson et al., 2014). Drug self-administration, in general, is thought to be a function of the balance of the drug’s aversive and rewarding effects, thus understanding each of these affective properties of MDPV may provide some insight into its use and abuse vulnerability (for a review, see Riley, 2011; Verendeev and Riley, 2012; Verendeev and Riley, 2013). Independent assessments of MDPV’s aversive and rewarding effects (and their relative contributions to drug self-administration), however, are limited.
In relation to its aversive effects, MDPV has recently been assessed for its ability to induce a conditioned taste avoidance in both adolescent and adult male Sprague Dawley rats (see Merluzzi et al., 2014), wherein MDPV (1.0, 1.8 or 3.2 mg/kg) produced dose-dependent avoidance of saccharin over trials. Further, Marusich et al. (2012) reported that MDPV produced significant increases in exploration, circling, hyperactivity and stereotypy (see also Aarde et al., 2013). In relation to its rewarding effects, MDPV has been shown to significantly lower ICSS thresholds across a range of doses in adult rats (comparable to those producing avoidance; see Watterson et al., 2014), an effect generally assumed to reflect the rewarding effects of the drug (Kornetsky and Bain, 1992; Wise, 1996). Additionally, Karlsson et. al. (2014) reported that in male C57BL/6J mice MDPV induced dose-dependent (0.5 – 20 mg/kg) conditioned place preference (CPP, a commonly used measure of a drug’s rewarding effects; see Tzschentke, 1998; Tzschentke, 2007). Although this latter study illustrates that MDPV induces CPP (and at a range of doses), it utilized an inbred mouse strain that is particularly susceptible to the reinforcing effects of ethanol and other drugs (see Cunningham et al., 2009; Orsini et al., 2005; Risinger and Brown, 1996). Given that both strain (e.g., inbred versus outbred) and species (e.g., mice versus rats) have been shown to influence a host of effects induced by drugs other than MDPV, for example, D1 and D2 dopamine receptor activation (Ralph and Caine, 2005), alcohol-induced taste avoidance (Broadbent et al., 2002) and psychomotor stimulant effects of cocaine (Thomsen and Caine, 2011), it may be difficult to generalize results between strains and across species. Accordingly, the present study attempted to evaluate the ability of MDPV to induce CPP in adult male Sprague-Dawley rats, in which both MDPV’s aversive effects and self-administration of MDPV have been previously demonstrated. Characterizing the rewarding effects of MDPV in these rats allows for a baseline to assess how a range of factors might impact them and, in turn, the drug’s abuse liability.
2. Materials and Methods
Forty-eight experimentally-naïve male Sprague-Dawley rats were obtained from Harlan Sprague-Dawley (Indianapolis, IN) on postnatal day (PND) 21. Procedures recommended by the National Research Council (1996), the Committee on Guidelines for the Care and Use of Animals in Neuroscience and Behavioral Research (2003) and the Institutional Animal Care and Use Committee at American University were followed at all times. Upon arrival to the animal facility on PND 21, subjects were group housed (three rats per OptiRat Plus polycarbonate bins; 100 cm × 99 cm × 201 cm) and maintained on ad-libitum food and water until PND 90, when experimental procedures began. Animals remained drug- and experimentally-naïve until this time.
2.1 Apparatus
The place conditioning apparatus (San Diego Instruments Place Preference System, San Diego, CA) consisted of two main conditioning chambers (28 × 21 × 34.5 cm) joined by a smaller middle chamber (14 × 21 × 34.5 cm). One of the conditioning chambers featured a white aluminum diamond plate floor with white walls; the other conditioning chamber featured a haircell-textured black plastic floor with black walls; the smaller middle chamber was outfitted with a steel rod floor and gray walls. Each individual chamber in each apparatus had its own white LED lights, and the lights were set on maximum, producing a biased apparatus (for a discussion on the impact of light in place conditioning, see Roma and Riley, 2005). A total of eight identical apparatuses were used; each apparatus featured a 16 × 4 photobeam array for recording time (in seconds) spent in each chamber. The CPP room was illuminated by a 25-W red light mounted to the ceiling, and a white noise generator was used to mask background noise.
2.2 Drugs and Solutions
3,4-methylenedioxypyrovalerone hydrochloride (synthesized at the Chemical Biology Research Branch of the National Institute on Drug Abuse) was dissolved in sterile isotonic saline (0.9%) at a concentration of 1 mg/ml and was subsequently filtered through a 0.2 mm filter to remove any contaminants before being administered intraperitoneally (IP) at a dose of 1.0, 1.8 or 3.2 mg/kg. Sterile isotonic saline was also filtered before being administered to saline controls, as well as to MDPV-treated subjects on non-drug paired days (see below). Injections for vehicle controls were equivolume to the highest dose of MDPV (3.2 mg/kg). Volume of the injection was manipulated in favor of concentration, given the influence that concentration has on the absorption/distribution of the drug.
2.3 CPP Pre-test
On PND 90, each animal was allowed 15-min access to freely explore the entire place conditioning apparatus to obtain individual baseline times spent in each chamber. Immediately following the pre-test, animals were returned to their home cages. No injections were given on this day. A biased conditioning procedure was used during CPP conditioning, i.e., each animal was subsequently given MDPV prior to placement on its own initially non-preferred side (see Cunningham et al., 2003; Davis et al., 2007; Schenk et al., 1986).
2.4 CPP Conditioning and Test
On PND 91 (Conditioning Day 1), animals were randomly assigned to receive an injection of either MDPV (1.0, 1.8 or 3.2 mg/kg; n = 12/dose) or saline (n = 12) and immediately confined to their respective non-preferred side (drug-paired side; DPS) of the CPP apparatus for 30 min. On the next day, all animals were given an injection of saline equivolume to the conditioning injections and then confined to the opposite chamber (non-drug paired side; NDPS). This two-day cycle of the drug given prior to animal’s placement on the non-preferred side followed by vehicle given prior to its placement on the preferred side was repeated three additional times (for a 8 total conditioning days). Order of conditioning sessions (i.e., drug vs. saline) was not counterbalanced (see King and Riley, 2013; Lepore et al., 1995; Mayer and Parker, 1993). Following the final cycle, all of the animals were given a test for CPP during which they were placed in the middle gray compartment and allowed to freely explore the apparatus for 15 min, after which animals were returned to their home cages. No injections were given on this day.
2.5 Statistical Analyses
Percent time spent on the DPS on the pretest and final test was compared with a 2 × 4 repeated measures ANOVA, with a within-subjects variable of Trial (Pretest and Test) and a between-subjects variable of Drug (0, 1.0, 1.8 and 3.2). In the case of a significant interaction, simple effects of Trial for each Drug condition were assessed with multivariate analyses. All statistical analyses were based on a significance level of 0.05.
3. Results
Percent time spent on the DPS for all groups (n = 12 for each group) before (Pre) and following (Post) conditioning is presented in Figure 1. As illustrated, all doses of MDPV produced a significant increase in time spent on the DPS, with no such increase seen with vehicle-treated animals. The 2 × 4 repeated measures ANOVA revealed a main effect of Trial [F (1, 44) = 64.517], as well as a significant Trial × Drug interaction [F (3, 44) = 4.906]. There was no main effect of Drug. To further explore the Trial × Drug interaction, simple effects of Trial for each Drug condition were explored with multivariate analyses, which revealed significant increases for all MDPV-injected groups [1.0: F (1, 44) =34.737; 1.8: F (1, 44) = 17.213; 3.2: F (1, 44) = 26.526], but no significant increase for vehicle-treated animals.
Figure 1.
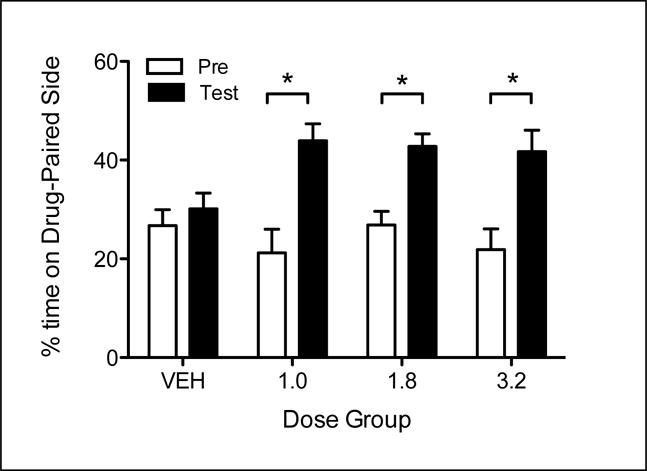
Percent time spent on the DPS for all groups (n = 12 for each group) before (Pre) and following (Post) conditioning. Significant within-group changes from Pre-Test to Post-Test are denoted by asterisk (p<.05).
4. Discussion
The present study assessed the ability of MDPV to induce CPP in adult male Sprague-Dawley rats. As described, all doses of MDPV were shown to produce significant increases in time spent on the drug paired side (from pretest to test), suggesting that MDPV does produce rewarding effects (Brielmaier et al., 2008; Cunningham et al., 2003). It should be noted, however, that actual preference was not produced, i.e., animals still spent a majority of time on the non-drug paired side. These relatively weak conditioning effects were likely a function of initial strong preference for the non-drug paired side (possibly a function of the chamber lights being set to maximum; see above). Additionally, these shifts in preference were not dose-dependent, contradictory to results seen with other stimulants (Costello et al., 1989; Durazzo et al., 1994). A meta-analysis of place conditioning studies with amphetamine and cocaine reported a significant effect of amphetamine dose on the magnitude of place conditioning effect, and a trend toward this same relationship with cocaine (Bardo et al., 1995). It is possible, however, that the doses used produced a ceiling effect, and that dose-dependent differences may have emerged at lower doses.
The fact that MDPV produces a positive shift in place conditioning is not surprising, given that other dopaminergic compounds have been shown to produce conditioned place preference (Bilsky et al., 1990; Calcagnetti and Schechter, 1993; Spyraki et al., 1982). Further, the present findings would be expected given that MDPV has been shown to lower ICSS thresholds (see introduction; Watterson et al., 2014), another common indicator of a drug’s rewarding effects. The current findings are unique in that they demonstrate MDPV’s rewarding effects in outbred rats, utilizing the same doses that have been shown to produce taste avoidance. That the same doses of MDPV are capable of producing both aversion and reward may seem paradoxical (Hunt and Amit, 1987; Riley, 2011), but it is consistent with data from a host of other drugs, including morphine (King and Riley, 2013; Simpson and Riley, 2005), amphetamine (Wang et al., 2010), and caffeine (Brockwell et al., 1991), that induce both taste avoidance and place preference at the same dose in the same animals in concurrent CTA/CPP assessments.
Further studies will be needed to determine how the balance of MDPV’s subjective effects might impact self-administration. Additionally, given that both the aversive and rewarding effects likely contribute to drug taking, it will also be necessary to determine which factors may influence the strength of each of these and, thus, their overall balance. For example, age has already been shown to influence MDPV-induced aversions (Merluzzi et al., 2014), but a number of other experiential and subject factors such as sex, strain, dose and drug history should also be examined given their impact on the aversive and rewarding effects of other drugs of abuse (Freeman and Riley, 2009; Klosterhalfen and Klosterhalfen, 1985; Tzschentke, 1998). Determining how the balance between aversion and reward influences MDPV-induced self-administration, and the factors that can alter this balance, may provide valuable insight into possible risk factors for use and overall abuse potential.
Acknowledgments
This research was supported by a grant from the Mellon Foundation to ALR. The authors have no conflicts of interest to declare. A portion of this research was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism, NIH, US Department of Health and Human Services. Requests for reprints should be sent to: Heather King, Psychopharmacology Laboratory, Department of Psychology, American University, Washington, DC 20016.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo AM, Valente MJ, Carvalho M, da Silva DD, Gaspar H, Carvalho F, de Lourdes Bastos M, de Pinho PG. Raising awareness of new psychoactive substances: chemical analysis and in vitro toxicity screening of ‘legal high’packages containing synthetic cathinones. Arch Toxicol. 2014 doi: 10.1007/s00204-014-1278-7. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bardo M, Rowlett J, Harris M. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Baumann MH. Awash in a sea of ‘bath salts’: implications for biomedical research and public health. Addiction. 2014;109:1577–1579. doi: 10.1111/add.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol. 2013;698:1–5. doi: 10.1016/j.ejphar.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH. Powerful cocaine-like actions of 3, 4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2012;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky EJ, Hui Y, Hubbell CL, Reid LD. Methylenedioxymethamphetamine’s capacity to establish place preferences and modify intake of an alcoholic beverage. Pharmacol Biochem Behav. 1990;37:633–638. doi: 10.1016/0091-3057(90)90538-s. [DOI] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Nicotine place preference in a biased conditioned place preference design. Pharmacol Biochem Behav. 2008;89:94–100. doi: 10.1016/j.pbb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Brockwell NT, Eikelboom R, Beninger RJ. Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacol Biochem Behav. 1991;38:513–517. doi: 10.1016/0091-3057(91)90006-n. [DOI] [PubMed] [Google Scholar]
- Bronstein AC, Spyker DA, Cantilena LR, Green JL, Rumack BH, Dart RC. 2010 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 28th annual report. Clin Toxicol. 2011;49:910–941. doi: 10.3109/15563650.2011.635149. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place preference for the psychostimulant cathinone is blocked by pretreatment with a dopamine release inhibitor. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:637–649. doi: 10.1016/0278-5846(93)90011-g. [DOI] [PubMed] [Google Scholar]
- Costello N, Carlson J, Glick S, Bryda M. Dose-dependent and baseline-dependent conditioning with d-amphetamine in the place conditioning paradigm. Psychopharmacology. 1989;99:244–247. doi: 10.1007/BF00442816. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology. 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic Influences on Conditioned Taste Aversion. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York, NY: 2009. pp. 387–421. [Google Scholar]
- Davis CM, Roma PG, Dominguez JM, Riley AL. Morphine-induced place conditioning in Fischer and Lewis rats: Acquisition and dose-response in a fully biased procedure. Pharmacol Biochem Behav. 2007;86:516–523. doi: 10.1016/j.pbb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Administration, D.o.J. chedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order Fed Regist. 2011:65371–65375. [PubMed] [Google Scholar]
- Durazzo TC, Gauvin DV, Goulden KL, Briscoe RJ, Holloway FA. Cocaine-induced conditioned place approach in rats: the role of dose and route of administration. Pharmacol Biochem Behav. 1994;49:1001–1005. doi: 10.1016/0091-3057(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Fass JA, Fass AD, Garcia AS. Synthetic cathinones (bath salts): legal status and patterns of abuse. Ann Pharmacother. 2012;46:436–441. doi: 10.1345/aph.1Q628. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Riley AL. The Origins of Conditioned Taste Aversion Learning: A Historical Analysis. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York, NY: 2009. pp. 9–33. [Google Scholar]
- German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci. 2014;97:2–8. doi: 10.1016/j.lfs.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T, Amit Z. Conditioned taste aversion induced by self-administered drugs: paradox revisited. Neurosci Biobehav Rev. 1987;11:107–130. doi: 10.1016/s0149-7634(87)80005-2. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, Methylone and 3, 4 - Methylenedioxypyrovalerone (MDPV) Induce Conditioned Place Preference in Mice. Basic Clin Pharmacol Toxicol. 2014 doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- King HE, Riley AL. A history of morphine-induced taste aversion learning fails to affect morphine-induced place preference conditioning in rats. Learn Behav. 2013;41:433–442. doi: 10.3758/s13420-013-0118-6. [DOI] [PubMed] [Google Scholar]
- Klosterhalfen S, Klosterhalfen W. Conditioned taste aversion and traditional learning. Psychol Res. 1985;47:71–94. doi: 10.1007/BF00309122. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Bain G. Brain-stimulation reward: a model for the study of the rewarding effects of abused drugs. NIDA Res Monogr. 1992;124:73–93. [PubMed] [Google Scholar]
- Lepore M, Vorel SR, Lowinson J, Gardner EL. Conditioned place preference induced by Δ 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56:2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3, 4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neurotoxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer LA, Parker LA. Rewarding and aversive properties of IP and SC cocaine: assessment by place and taste conditioning. Psychopharmacology. 1993;112:189–194. doi: 10.1007/BF02244909. [DOI] [PubMed] [Google Scholar]
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, Riley AL. Differential expression of MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Dev Psychobiol. 2014;56:943–954. doi: 10.1002/dev.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology. 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129×1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther. 2005;312:733–741. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM. Genetic differences in nicotine-induced conditioned taste aversion. Life Sci. 1996;58:PL223–PL229. doi: 10.1016/0024-3205(96)00051-3. [DOI] [PubMed] [Google Scholar]
- Roma PG, Riley AL. Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav. 2005;82:163–169. doi: 10.1016/j.pbb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Rosenbaum CD, Carreiro SP, Babu KM. Here today, gone tomorrow… and back again? A review of herbal marijuana alternatives (K2, Spice), synthetic cathinones (bath salts), kratom, Salvia divinorum, methoxetamine, and piperazines. J Med Toxicol. 2012;8:15–32. doi: 10.1007/s13181-011-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Hunt T, Malovechko R, Robertson A, Klukowski G, Amit Z. Differential effects of isolation housing on the conditioned place preference produced by cocaine and amphetamine. Pharmacol Biochem Behav. 1986;24:1793–1796. doi: 10.1016/0091-3057(86)90523-x. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Riley AL. Morphine preexposure facilitates morphine place preference and attenuates morphine taste aversion. Pharmacol Biochem Behav. 2005;80:471–479. doi: 10.1016/j.pbb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Dopaminergic substrates of amphetamine-induced place preference conditioning. Brain Res. 1982;253:185–193. doi: 10.1016/0006-8993(82)90685-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp Clin Psychopharmacol. 2011;19:321–341. doi: 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Review on CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: History and interpretation. Neuroscience & Biobehavioral Reviews. 2012;36:2193–2205. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav Pharmacol. 2013;24:363–374. doi: 10.1097/FBP.0b013e32836413d5. [DOI] [PubMed] [Google Scholar]
- Wang YC, Huang ACW, Hsiao S. Paradoxical simultaneous occurrence of amphetamine-induced conditioned taste aversion and conditioned place preference with the same single drug injection: a new “pre-and post-association” experimental paradigm. Pharmacol Biochem Behav. 2010;95:80–87. doi: 10.1016/j.pbb.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]


