Abstract
Replication and transmission of avian influenza virus in humans poses a pandemic threat. The molecular determinants that facilitate this process are not well understood. We used DBA/2 mice to identify viral factors that mediate the difference in pathogenesis between a virulent (H7N3) and a non-virulent (H7N9) avian influenza virus from North America. In vitro and in vivo characterization of reassortant viruses identified the PB2 and PA polymerase genes as major determinants of H7N3 pathogenesis. Analysis of individual residues in the PB2 and PA genes identified position 358 (E358V) in PB2 and positions 190 (P190S) and 400 (Q400P) in PA that reduced the virulence of H7N3 virus. The E358V and P190S substitutions also caused reduced inflammation after infection. Our results suggest that specific residues in the polymerase proteins PB2 and PA are important for replication and virulence of avian influenza viruses in a mammalian host.
Keywords: Avian Influenza virus, H7 subtype, DBA/2J, pathogenesis
Introduction
Avian influenza A viruses (IAV) continue to pose a significant threat to human health. H5 and H7 subtype IAV sporadically transmit from avian species to humans, sometimes causing fatal disease. The molecular mechanisms, associated with replication and pathogenesis of avian influenza viruses in a mammalian host, are not well understood. A better understanding of the molecular basis for mammalian tropism will improve influenza virus surveillance and epidemiology and provide insights into fundamental influenza biology.
Highly pathogenic avian H5N1 IAV emerged in Hong Kong in 1997 and has since spread to across Asia to other continents. The World Health Organization has identified over 650 cases and nearly 50% of these have died. In 2003, a highly pathogenic avian H7N7 IAV caused a large outbreak in the Netherlands with approximately 80 confirmed human cases (Fouchier et al., 2004). Besides these larger outbreaks, there have been several reports of human infections with avian H9N2, H7N2, H7N3, H6N1 or H10N8 virus (Chen et al., 2014; Reperant et al., 2012; Wei et al., 2013). In March of 2013 the first human cases of a novel H7N9 IAV were reported in China (Chen et al., 2013; Li et al., 2014; Watanabe et al., 2013). Since then nearly 700 cases have been reported and of those nearly 40% have died. The factors allow these viruses to transmit and replicate in a human host are only partially defined. The surface proteins HA and NA are very important for transmission of IAV from birds to mammals due to their role in attachment and release of the virus (Imai and Kawaoka, 2012). Avian HA proteins bind predominantly to α2-3 linked sialic acids, while HA proteins from human IAV bind α2-6 linked sialic acids. This differential binding provides a significant barrier for avian IAV replication in the human host, because human airway epithelial cells express predominantly α2-6 linked sialic acids. However, certain mammalian species, such as pigs, express both α2-3 and α2-6 linked sialic acids in the upper respiratory tract providing an environment for adaptation to human-like receptors. The polymerase proteins PB2, PB1 and PA of influenza virus are also major determinants of host range and transmission. A lysine at PB2 position 627 is found in most human influenza viruses and in avian influenza viruses isolated from humans (Jonges et al., 2014; Mok et al., 2014). In contrast, avian influenza viruses isolated from birds have a glutamic acid at this position. Genetic differences in the host factor ANP32A, account for the species specificity of this residue (Long et al., 2016). Additional residues at 701, 590 and 591 of PB2 have similar effects and allow avian IAV to replicate more efficiently in mammalian host cells (Liu et al., 2012). Similarly, several residues on the PA and PB1 protein have been associated with increased replication and virulence in mammalian hosts (Manz et al., 2013; To et al., 2014; Yamayoshi et al., 2014).
The continued transmission of avian influenza viruses to humans, specifically H7 influenza viruses, makes the identification of virus and host factors important for this process a priority. Despite this, H7 virus pathogenesis and transmission have not been extensively studied (Belser et al., 2013a; Belser et al., 2013b; Belser et al., 2007; Belser and Tumpey, 2014; Joseph et al., 2007). One of the reasons is that H7 viruses often do not cause severe disease in mice and ferrets, preventing us from identifying viral factors associated with pathogenesis and disease. Recently, we and others reported on the utility of the DBA/2 mouse strain to study in vivo replication and pathogenesis of natural isolates of avian and human influenza viruses (Boon et al., 2010; Pica et al., 2011), including avian H7 influenza viruses. We have now extended this model to identify molecular determinants of pathogenesis in North-American avian H7 viruses. A genetic analysis between a virulent H7N3 and a non-virulent H7N9 virus identified three residues on the PB2 and PA polymerase proteins that impact virulence and disease. The protein domains containing these residues are therefore potentially important for pathogenesis in a mammalian host.
Results
In vivo and in vitro characterization of H7N3 and H7N9 influenza A virus
Previously we had described a significant difference in virulence between an avian H7N3 virus (A/shorebird/Delaware/22/2006) and an avian H7N9 virus (A/mallard/Alberta/177/2004) (Boon et al., 2010). This H7N9 virus is very different from the avian H7N9 virus that is circulating in China and is closely related to other avian IAV circulating in North America (Supplemental Table 1). The H7N3 virus is nearly identical to other H7N3 viruses that were isolated near the Delaware Bay area in 2006 (Supplemental Table 1). To identify the molecular determinants that define this reported difference in virulence we first generated molecular clones of these two avian IAV and inoculated female DBA/2J mice intranasally with 103 TCID50 of either wild-type or reverse genetics derived H7N3 and H7N9 virus. Weight-loss and mortality were monitored over a 21 day period. Similar to our previous report (Boon et al., 2010), the wild-type H7N9 induced minimal weight loss (Table 1) and the majority of the animals survived the infection. Inoculation with 103 TCID50 of wild-type H7N3 induced significantly more weight-loss (P<0.001) and mortality (P<0.01) in these animals (Figure 1a and Table 1). Importantly, the molecular clones of both H7N3 and H7N9 demonstrated identical in vivo pathologies compared to the E2 passage of the respective virus isolates .
Table 1.
Morbidity and mortality in DBA/2 mice after inoculation with mutant or reassortant avian H7 influenza viruses.
| Virus | Dose1 | N | % Mortality | % Weight-loss on d8 (SEM) |
|---|---|---|---|---|
| H7N3-wt& | 103 | 4 | 100 | 26.8 (0.4) |
| H7N3-rg | 103 | 15 | 100 | 29.0 (0.7) |
| H7N3-5+3# | 103 | 8 | 63 | 20.8 (1.5) |
| H7N3-6+2 | 103 | 8 | 100 | 23.5 (1.1) |
| H7N3-7+1 | 103 | 9 | 100 | 27.7 (0.9) |
| H7N9-wt | 103 | 5 | 20 | 7.2 (3.5) |
| H7N9-rg | 103 | 13 | 15 | 7.0 (2.6) |
| H7N9-5+3 | 103 | 8 | 75 | 25.1 (1.4) |
| H7N9-6+2 | 103 | 8 | 62 | 16.6 (2.5) |
| H7N9-7+1 | 103 | 7 | 29 | 15.0 (2.4) |
| H7N9 PB2V358E | 103 | 15 | 33 | 15.9 (1.5) |
| H7N3-rg | 102 | 40 | 83 | 23.9 (1.1) |
| H7N3-5+3 | 102 | 8 | 0 | -0.6 (2.9) |
| H7N3 PB2N9 | 102 | 33 | 37 | 12.0 (2.5) |
| H7N3 PB2G62R | 102 | 10 | 90 | 23.0 (1.1) |
| H7N3 PB2E358V | 102 | 10 | 20 | 15.3 (2.4) |
| H7N3 PB2A471T | 102 | 10 | 100 | 29.9 (0.7) |
| H7N3 PB2E681G | 102 | 10 | 90 | 24.4 (1.7) |
| H7N3 PB1N9 | 102 | 7 | 86 | 15.8 (1.2) |
| H7N3 PAN9 | 102 | 17 | 12 | 5.8 (2.8) |
| H7N3 PAN184S | 102 | 10 | 90 | 23.3 (0.8) |
| H7N3 PAP190S | 102 | 9 | 44 | 13.6 (2.4) |
| H7N3 PAQ400P | 102 | 10 | 50 | 9.8 (3.7) |
| H7N9-rg | 102 | 4 | 0 | -7.0 (2.4) |
| H7N9-5+3 | 102 | 4 | 50 | 18.7 (2.4) |
Dose in TCID50/30μl inoculum volume.
wt = wild-type virus, rg = reverse genetics or plasmid-derived virus.
H7N3 5+3 denotes H7N3 influenza A virus genetic backbone with the PB2, PB1 and PA of H7N9 virus. Similarly 6+2, and 7+1 denotes a reassortment of the HA and NA, or NS gene-segment respectively.
Figure 1. Characterization of H7N3 and H7N9 Influenza A virus.
(A) Wild-type and plasmid-derived H7N3 and H7N9 influenza A virus were used to inoculate 6-8 week old female DBA/2J mice and mortality was observed for 21 days. (B) Virus titers in lungs homogenates of H7N3 and H7N9 infected DBA/2J mice. Male DBA/2J mice were inoculated with 103 TCID50 of H7N3 or H7N9 virus and lungs were harvested at 2, 3, 6, and 9 days post inoculation. Lung virus titers were quantified on MDCK cells. (C) MDCK cells were inoculated with an MOI of 0.0001 and the virus titer in the supernatant was quantified at 6, 24, 32 and 48 hours post infection on MDCK cells. The data (TCID50/ml) is the average of two wells per virus and is representative of two experiments. Dotted line is the limit of detection. ** = P<0.01; *** = P<0.001
To assess if the difference in pathogenesis was caused by a difference in virus replication, we quantified viral titers in lung homogenates of male DBA/2J mice at 2, 3, 6, and 9 days post inoculation with 103 TCID50. Significant differences in virus titer were observed at 2, 3 and 9 days post-infection (Figure 1b). The differences in titer at days 2 (100-fold, P<0.001) and 3 (100-fold, P<0.001) were particularly striking and provided additional evidence that early virus replication and increased viral load are major determinants of severe disease. At day 6, the viral load in the lungs of infected animals was similar (P>0.45) between H7N3 and H7N9, while at day 9, the viral load in H7N9 infected animals was lower (10-fold, P<0.05).
The in vitro growth properties of these two viruses in mammalian cell culture were also different. In MDCK cells, the H7N3 virus grew significantly faster compared to H7N9. Within 24 hours after inoculation with 50 TCID50 of H7N3 the culture supernatant contained 106 TCID50/ml. In contrast, it took 48 hours for the H7N9 virus to reach similar peak titers (Figure 1c).
The polymerase proteins of H7N3 virus determine the difference in disease severity
A genetic comparison between H7N3 and H7N9 identified a total of 53 amino-acid differences across 7 gene segments (Figure 2). The NA gene-segments were not included in this analysis as they belong to different serotypes (N3 versus N9). The majority of the differences were found in the HA (10 changes), PB1 (17 changes including PB1-F2) and PA (12 changes including PA-X) gene segments. In order to identify the specific residues associated with the increase in pathogenicity of H7N3, we first wanted to identify the gene segment(s) responsible for the difference in disease severity. The effects of PB2, PB1, and PA polymerase proteins (H7N35+3), the surface HA and NA glycoproteins (H7N36+2) or the non-structural proteins (NS) (H7N37+1) on pathogenesis were evaluated. The three polymerase genes had the largest impact on pathogenesis. H7N35+3 virus, containing the PB2, PB1 and PA gene segments of H7N9, caused significantly (P<0.001 at 103 TCID50 and P<0.0001 at 102 TCID50) reduced mortality compared to H7N3 (Figure 3a/c and Table 1). In the converse experiment, we observed a significant (P<0.005 at 103 TCID50) increase in mortality and morbidity when we exchanged the polymerase genes of H7N9 with those of H7N3 (H7N95+3). Exchange of the HA/NA gene-segments also reduced (P<0.01 at 103 TCID50 for H7N36+2) and increased (P<0.05 at 103 TCID50 for H7N96+2) mortality of H7N3 and H7N9 virus respectively. However, the effects on morbidity and mortality were smaller compared to the polymerase genes (Figure 3a/b and Table 1). Finally, we did not observe an effect of NS gene-segment on mortality of H7N9 and H7N3 virus (Figure 3a/b and Table 1).
Figure 2. Amino acid variation between H7N3 and H7N9 virus.
A total of 53 amino-acid differences were identified between H7N3 (in red) and H7N9 (in blue) influenza A virus. The consensus (Cons.) amino-acid for each position is indicated in grey and residues identified in >10% of all the sequences from North American avian influenza viruses are included. Neuraminidase was not evaluated because they belong to different subtypes and contains many amino-acid differences. 1 HA numbering based on the mature HA protein according to recommended numbering scheme (Burke and Smith, 2014) and the consensus is from all H7 HA proteins from North American avian influenza A viruses. 2 PA-X residues include only those found in the +61 amino-acids that represent the C-terminal tail of the protein.
Figure 3. The polymerase complexes of H7N3 and H7N9 IAV affect pathogenesis.
Reassortant H7N3 and H7N9 viruses were generated by reverse genetics containing the three polymerase genes (PB2, PB1, and PA), the two glycoproteins (HA and NA), or the non-structural proteins (NS) from the reciprocal virus. (A) Female DBA/2J mice were infected with 103 TCID50 of H7N3 virus containing the PB2, PB1, PA (H7N35+3), or HA and NA (H7N36+2) or NS (H7N37+1) of the H7N9 virus, and morbidity and mortality were monitored for 21 days. (B) Female DBA/2J mice were infected with 103 TCID50 of H7N9 containing the PB2, PB1, PA (H7N95+3), or HA and NA (H7N96+2) or NS (H7N97+1) of the H7N3 virus, and morbidity and mortality were monitored for 21 days. (C) Female DBA/2J mice were infected with 102 TCID50 of H7N3 virus containing all three (H7N35+3) or individual polymerase gene-segments (H7N3 PB2N9, H7N3 PB1N9, H7N3 PAN9) of the H7N9 IAV and morbidity and mortality was monitored for 21 days. Each virus strain was tested in two or more separate experiments and includes at least seven animals per strain of IAV. * = P<0.05; ** = P<0.01; *** = P<0.001.
In vitro characterization of the reassortant viruses also revealed that exchanging the polymerase genes (H7N35+3 or H7N95+3) had the largest impact on virus replication in MDCK cells. The presence of the PB2N9, PB1N9 and PAN9 genes in H7N3 (H7N35+3) significantly decreased (P<0.05) the viral titer in the supernatant of infected MDCK cells 24 hours post infection (Table 2). Introducing the PB2N3, PB1N3 and PAN3 genes of H7N3 on a H7N9 backbone (H7N95+3) increased (P<0.05) viral titers in the supernatant 24 hours post infection (Table 2). Substitution of the HA/NA or NS gene-segment had no significant effect on the replication of H7N3 virus in MDCK cells. Similarly, substitution of the NS segment in H7N9 virus also did not significantly change the growth properties of the reassortant virus.
Table 2.
The polymerase proteins PB2, PB1 and PA of H7N3 and H7N9 virus affect replication in MDCK cells.
| Virus | Substituted gene-segments | Virus Titer (log10/ml) |
|---|---|---|
| H7N3 | 5.9 (1.0) | |
| PB2, PB1, PA | 4.4 (1.1) * | |
| HA, NA | 6.4 (0.5) | |
| NS | 5.1 (0.6) | |
| H7N9 | 4.5 (0.8) | |
| PB2, PB1, PA | 6.4 (0.7) # | |
| HA, NA | - | |
| NS | 3.8 (0.4) |
Results are the average and standard deviation of the log10-transformed virus titers 24 hours after inoculation of MDCK cells derived from at least two independent experiments.
= P<0.05 compared to H7N3 virus.
= P<0.05 compared to H7N9 virus.
“-”, no data.
The PB2 and PA proteins of H7N3 virus cause the increase in pathology
Next we interrogated the effect of individual gene segments on H7N3 replication and pathogenesis. Because H7N3 virus is highly virulent at a dose of 103 TCID50 (100% mortality) we used a 10-fold lower inoculum (102 TCID50) to detect small effects of single gene-segments on viral pathogenesis. DBA/2J mice were inoculated with H7N3 virus containing the PB2 (H7N3-PB2N9), PB1 (H7N3-PB1N9) or PA (H7N3-PAN9) gene-segment from H7N9 and weight loss and mortality were monitored for 21 days. As shown in Figure 3c and Table 1, H7N3-PB2N9 and H7N3-PAN9 inoculated animals lost significantly less weight (12%, P<0.0001 and 5.8%, P<0.0001 respectively) compared to those inoculated with the H7N3 virus (23.9%). Also, fewer animals succumbed to infection with H7N3-PB2N9 (37%, (P<0.0001) and H7N3-PAN9 (12%, (P<0.0001)) compared to H7N3 virus infection (83%). The introduction of the PB1 gene segment of H7N9 (H7N3-PB1N9) had a significant effect on morbidity (15.8%, P<0.01), but no effect on mortality (86%, P>0.6).
Increased polymerase activity of the H7N3 virus polymerase complex
The polymerase activity of IAV can be quantified with a mini-genome assay using firefly luciferase activity as the readout for activity (Salomon et al., 2006). The polymerase activity of H7N3 was 3-fold higher (P<0.001) compared to that of H7N9 (Figure 4). Upon substitution of individual gene-segments between H7N9 and H7N3, we observed significant changes in polymerase activity. The PB2 and PA gene-segment of H7N9 reduced the polymerase activity of the H7N3 complex by 75% (P<0.001) and 49% (P<0.01) respectively (Figure 4a). Consistent with this result, the inclusion of the PB2 (P<0.01) or PA (P<0.05) gene from H7N3 increased the polymerase activity of the H7N9 complex (Figure 4b). Interestingly, the PB1 gene of H7N9 increased polymerase activity of the H7N3 polymerase complex 3-fold (Figure 4a, P<0.001), while the addition of H7N3 PB1 to the H7N9 complex reduced activity (P<0.01, Figure 4b).
Figure 4. H7N9 influenza A virus demonstrates reduced polymerase activity.
293T cells were transfected with expression plasmids (pcDNA3.1) encoding the PB2, PB1, PA, and NP protein of H7N3 (red squares), H7N9 (blue squares), or a mutated PB2 or PA protein of influenza A virus in addition to a vector producing an influenza virus like gene-segment containing the firefly luciferase protein in the negative orientation and a transfection control plasmid encoding for the Renilla luciferase protein. Forty-eight hours later the amounts of firefly and Renilla luciferase protein activity is quantified. The results are normalized based on Renilla luciferase activity and compared to the polymerase activity of H7N3 (A and C) or H7N9 (B) polymerase genes. The data represents the mean relative activity + SD from three or more independent experiments in duplicate. * = P<0.05; ** = P<0.01; *** = P<0.001 compared to H7N3 (A) or H7N9 activity (B).
Position 358 in the PB2 protein affects replication and pathogenicity of H7N3 virus
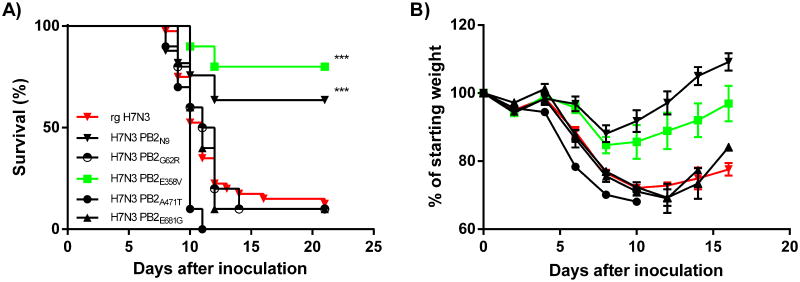
Six amino-acid differences were identified between the PB2 protein of H7N3 (PB2N3) and H7N9 (PB2N9) (Figure 2). Four positions were selected (G62R, E358V, A471T, and E681G) based on the difference in chemical properties of the amino-acids involved. These 4 residues were modified in the PB2 gene of H7N3 and the viruses were generated and tested in our DBA/2J mouse model. Compared to the wild type H7N3, position G62R, and E681G had no effect on morbidity and mortality (Figure 5 and Table 1). The A471T substitution significantly increased morbidity (P<0.01 on 6 dpi) and mortality (P<0.05) of the mutant virus compared to wild type H7N3. Finally, the E358V change in the PB2N3 gene resulted in significantly reduced weight loss and increased survival (P<0.001). Inoculation of DBA/2J mice with the H7N3-PB2E358V virus induced a maximum weight loss of 15.3% on day 8 and only 20% of the inoculated animals succumbed to infection. In contrast, 83% of the H7N3 virus infected animals succumbed to the infection and the average weight-loss on day 8 post inoculation is significantly higher (23.9%, P<0.001).
Figure 5. A Valine at Position 358 in the PB2 gene of H7N3 attenuates the virus.
H7N3 viruses were generated containing single point mutations at position 62 (G62R), 358 (E358V), 471 (A471T) and 681 (E681G) of the H7N3 PB2 polymerase gene. Female DBA/2J mice were infected with 102 TCID50 of H7N3 viruses containing each of the four point mutations and mortality (A) and morbidity (B) was compared to H7N3 and H7N3 PB2N9 virus. Each virus strain was tested in two or more separate experiments and includes at least eight animals per strain of IAV. * = P<0.05; ** = P<0.01; *** = P<0.001.
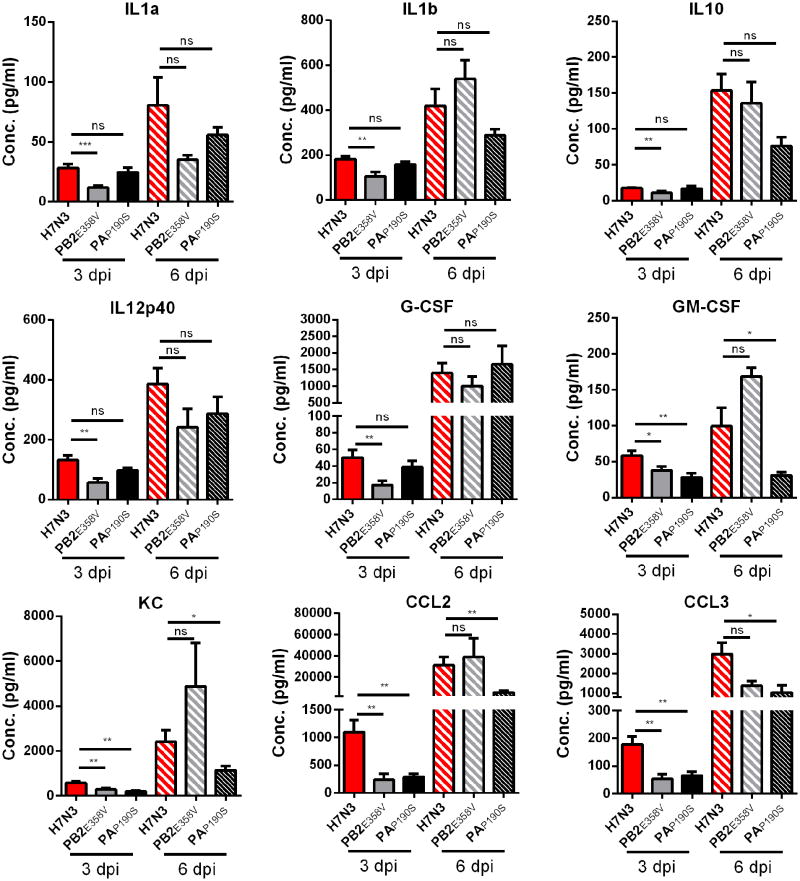
To determine how PB2358 affects pathogenesis in vivo, we inoculated animals with 100 TCID50 of H7N3 and H7N3-PB2E358V virus and determined lung viral titers on days 2, 3, 6 and 9 post inoculation. On day 2, the viral load was significantly lower (P<0.001) in the H7N3-PB2E358V inoculated animals compared to the H7N3 infected animals (Table 3). On days 3 and 6 the viral load between the two viruses was similar, while on day 9 the viral load in the H7N3-PB2E358V infected animals was again significantly reduced (P<0.01). Cytokines and chemokines play an important role in the pathogenesis of influenza virus (Boon et al., 2011; Brandes et al., 2013; Hatta et al., 2010). To assess inflammation, we quantified the production of pro-inflammatory cytokines in lung homogenates 3 and 6 days post inoculation with H7N3 and H7N3-PB2E358V. The concentration of several pro-inflammatory cytokine and chemokines (CCL2, CCL3, G-CSF, IL-1β and GM-CSF) was significantly lower 3 dpi in the H7N3-PB2E358V infected animals compared to the H7N3 infected mice (P<0.05, Figure 7). No differences were observed on 6 dpi. Finally, the glutamic acid to valine change at position 358 of the PB2 of H7N3 virus significantly reduced the polymerase activity (P<0.001, Figure 2C). Combined, these data suggest that a valine at position 358 in the PB2 of H7N3 attenuates virus replication resulting in reduced amounts of pro-inflammatory cytokines, reduced weight loss and increased survival after inoculation.
Table 3.
Effects of PB2E358V and PAP190S substitutions on virus replication in vivo.
| Virus | Lung virus titer (log10/ml) | |||
|---|---|---|---|---|
|
| ||||
| Day 2 | Day 3 | Day 6 | Day 9 | |
| H7N3 | 3.9 (0.6) | 5.4 (0.7) | 6.0 (0.8) | 5.9 (0.5) |
| H7N3 PB2E358V | 2.2 (0.5)*** | 4.9 (1.0) | 6.2 (0.5) | 4.4 (1.2)** |
| H7N3 PAP190S | 4.5 (1.0)* | 4.9 (1.1) | 5.6 (0.7) | ND |
Average lung virus titers in H7N3 or mutant virus infected DBA/2J animals. Results are the average and standard deviation of the log10-transformed virus titer obtained from at least two different experiments.
= P<0.001;
= P<0.01;
= P<0.05.
ND = not done.
Figure 7. Cytokine and chemokine concentration in lung homogenates after H7N3, H7N3-PB2E358V, and H7N3-PAP190S virus infection.
Male DBA/2J mice were inoculated with 100 TCID50 of the H7N3, H7N3 PB2E358V, or H7N3 PAP190S and lungs were collected at 3 and 6 days post infection (dpi). The tissue was homogenized in 1.0ml of PBS and used for cytokine and chemokine quantification. The results are the average plus standard error of the mean of at least seven mice from two different experiments. * = P<0.05; ** = P<0.01; *** = P<0.001; ns = not significant.
To evaluate the importance of PB2358 in the context of the H7N9 virus, we introduced a glutamic acid (V358E) at this position and evaluated pathogenesis. Compared to H7N9 virus, inoculation with H7N9-PB2V358E resulted in an increase in weight-loss on day 8 (7% versus 15%, P<0.01, Table 1) and mortality (15% versus 33%, P = 0.25, Table 1).
Positions 190 and 400 in the PA protein affect H7N3 pathogenesis
The PA gene-segment of H7N9 also attenuated the H7N3 virus in vivo (Figure 6 and Table 1). Sequence analysis identified seven amino-acid differences in the full-length PA protein and seven differences in the newly identified PA-X protein (Figure 2) (Jagger et al., 2012). Three positions in the PA gene were selected (N184S, P190S, and Q400P) based on the difference in chemical properties of the amino-acids involved. Interestingly position 190 is within the decanucleotide sequence that is involved in the +1 ribosomal frameshift required to produce the PA-X protein. Following intranasal infection, two of the three PA mutant viruses demonstrated an attenuated phenotype. Animals infected with the P190S mutant or the Q400P mutant lost significantly (P<0.01) less weight and fewer animals succumbed to infection (Figure 6 and Table 1). The N184S substitution had no effect on weight loss or survival after intranasal infection in DBA/2J mice. To determine if the P190S substitution reduced virus replication we analyzed viral loads at day 2, 3 and 6 post inoculation. A small but significant increase in virus titer was found on 2 dpi (P<0.05, Table 3), but not on 3 (P>0.4) or 6 dpi (P>0.1). Analysis of the cytokine and chemokine concentration in lung homogenates of H7N3 and H7N3-PAP190S inoculated mice, we found significantly less CCL2, CCL3, KC, and GM-CSF at 3 dpi (P<0.01) and 6 dpi (P<0.05, Figure 7). Finally, we measured the impact of N184S, P190S and Q400P substitutions on the polymerase activity of H7N3 virus (Figure 2C). A serine at position 184 increased (P<0.05) the polymerase activity, while a serine or proline at positions 190 and 400 respectively reduced (P<0.001) the polymerase activity.
Figure 6. Residue 190 and 400 of PA affect virulence of an avian H7N3 influenza virus.
H7N3 viruses were generated containing single point mutations at position 184 (N184S), 190 (P190S), and 400 (Q400P) of the H7N3 PA polymerase gene. Female DBA/2J mice were infected with 102 TCID50 of H7N3 viruses containing each of the four point mutations and mortality (A) and morbidity (B) was compared to H7N3 and H7N3 PAN9 virus. Each virus strain was tested in two or more separate experiments and includes at least eight animals per strain of IAV. ** = P<0.01; *** = P<0.001.
Discussion
Avian influenza viruses continue to pose a significant threat to human health as exemplified by the most recent outbreak of avian H7N9 influenza virus in the People's Republic of China. Therefore, there is a growing need to identify residues that impact replication and pathogenesis of avian-lineage influenza viruses in mammalian hosts. Our study shows that virulence and pathogenesis of avian H7 subtype influenza viruses are influenced by multiple residues on the polymerase genes. The identification of several residues on the PB2 and PA gene segment highlight the importance of the polymerase complex for virulence and pathogenesis. The identified determinants and implied functions may improve genotype screening of avian and human isolates, and support the generation of novel antiviral drugs.
PB2358 was found to have a significant effect on virus replication and pathogenesis. H7N3 virus harboring a glutamic acid (E) at this position was more virulent compared to a virus with a Valine at this position. This is in contrast to avian H1N1 viruses, in which a Valine at PB2358 was associated with increased virulence in DBA/2J mice (Kocer et al., 2014). PB2358 is highly conserved residue (>99.5% of all influenza A viruses have a glutamic acid (E) at this position) is adjacent to a conserved histidine residue which is reported to interact with the inverted guanidine cap of mRNA (Guilligay et al., 2008). Interestingly, a H357N change in PB2 was found in 2009 pdmH1N1 influenza virus that was experimentally adapted to the murine host (Ping et al., 2011). This data highlights the importance of the cap-binding domain in the fitness of the virus and this information may be used to generate alternate attenuated influenza viruses and serve as an antiviral target (Pautus et al., 2013). Other residues within the domain have also been reported to affect pathogenesis (Liu et al., 2013). Interestingly the E358V in H7N3 had a much greater impact than the reciprocal V358E mutation in H7N9. We speculate that this is due to compensatory mutations elsewhere in the mRNA cap binding domain on the PB2 protein of H7N9 virus, for example position 471 or the adjacent 340. Alternatively, differences in the PA or PB1 protein between H7N3 and H7N9 influenced the effect of position 358 in PB2. The cap binding domain of the PB2 gene of influenza A virus is fairly well conserved and several species specific residues have been identified. The importance of these changes on replication and pathogenesis of human and avian influenza viruses are currently unknown, but should be evaluated in future studies.
Evaluation of the differences in the PA protein between H7N3 and H7N9 virus identified two residues that changed the virulence of H7N3 virus. Residue 400 on the PA protein of influenza virus is highly variable with the majority of North American avian influenza virus PA proteins expressing proline (49%), glutamine (34%), and serine (10%). In contrast, the PA genes from human isolates (n=6025), including the pandemic 2009 H1N1 virus, contain predominantly proline (52%) and leucine (47%) and none had a glutamine at this position. Interestingly, a glutamine at position 400 is often found in North American lineage avian isolates but not in Eurasian avian influenza viruses. Our studies show that a glutamine (Q) at position 400 on the PA protein increases virulence in a mammalian host. It remains to be determined if position 400 affects virus replication and hence pathogenesis, or whether the residue affects the host response to IAV. Position 400 is near the PA-arch (366-397) that is involved in the binding of the 5′ promoter region by the polymerase complex. The double proline at position 396-397 stacks on the bases of the 5th and 6th nucleotide of the viral RNA (Pflug et al., 2014). Several residues within this region have been implicated in host adaptations (Manz et al., 2013). Finally, C-terminal residue changes in the PA protein of WSN virus affected virus replication through several mechanisms (Liang et al., 2012).
A proline at position 190 on PA is relatively unique with less than 1% of all sequenced avian viruses harboring this specific amino-acid residue (Jagger et al., 2012). Position 190 was recently identified to be part of a highly conserved decanucleotide position that was important for frame-shifting and the translation of a newly identified influenza virus protein called PA-X (Desmet et al., 2013; Gao et al., 2015a; Gao et al., 2015b; Gao et al., 2015c; Hayashi et al., 2015; Hu et al., 2015; Jagger et al., 2012; Khaperskyy et al., 2016; Oishi et al., 2015). PA-X is important for host protein shut-off and avian PA-X proteins are more active compared to PA-X from human viruses (Desmet et al., 2013). The effects of PA-X on pathogenesis vary between different influenza viruses. In the 1918 Spanish influenza virus or highly pathogenic H5N1 virus, deletion of PA-X exacerbated disease (Gao et al., 2015b; Hu et al., 2015; Jagger et al., 2012), while in H9N2 and 2009 pandemic H1N1 virus, deletion of PA-X attenuates the virus (Gao et al., 2015c; Hayashi et al., 2015). In the North American H7N3 virus, a proline to serine substitution at position 190 in the PA protein attenuated the virus in vivo. One possibility is that the nucleotides encoding for serine (UCC) or proline (CCA) at this position changed the frameshift efficiency and therefore the production of PA-X protein. A different codon for proline (CCC) was reported to reduce the frame-shift efficiency by 20-30% (Jagger et al., 2012). Alternatively, the proline at this position has an effect on protein activity or it modulates the host response to infection. Interestingly, position 190 was found to mutate from a serine to a phenylalanine or threonine in several mouse-adapted influenza viruses (Ping et al., 2011). The rationale for this change in the context of mouse adaptation is unknown, although it has been reported that more inflammation supports increased virus replication through the recruitment of uninfected cells (Pang et al., 2013).
Previous analysis of virulence determinants in H7 viruses also discovered a role for PB2 and PA protein (de Wit et al., 2010; Munster et al., 2007). Molecular characterization of the amino-acid differences between a H7N7 virus isolated from a fatal case in 2003 in The Netherlands, and a H7N7 virus isolated from an individual with conjunctivitis, revealed that the PB2, PA, HA and NA gene contained molecular determinants of adaptation to the human host. Combined these two studies suggest that the polymerase genes are very important for adaptation and virulence of avian H7 viruses in the mammalian host.
The lung virus titer 2 and 3 days after H7N9 infection spanned several logs of virus. A similar variation in early lung tissue titer between mice was previously observed after intranasal inoculation of C57BL/6 mice with another avian H7N3 virus (Driskell et al., 2010) suggesting that this is a characteristic of the virus and not the mouse model that we used. We associate this variation in lung virus titer with poor growth in and adaptation to the murine host. It is interesting to note that the day 6 virus titer does not have this variation in virus titer. The rationale for this is not known but could imply rapid adaptation of the avian virus to the murine host.
Despite the importance of the polymerase proteins in virulence and pathogenesis in vivo, we and others (Bussey et al., 2010; Bussey et al., 2011) have found that the polymerase activity, measured in 293T cells, does not always correlate with morbidity and mortality in mice. The PB1 protein of H7N9 increased the polymerase activity (∼3-fold) of the H7N3 polymerase complex, but had not impact on virulence. Similarly, the PA gene of A/California/04/2009, increased the polymerase activity of H7N3 proteins 20-fold. The corresponding H7N3 virus containing this PA gene was attenuated in vivo (Williams et al., 2016). This inconsistency can be caused by the differences between human tissue culture and mice, or additional interactions between the polymerase and other viral proteins. Finally, virulence is not always directly associated with virus replication in vivo.
This study is the first of its kind to identify molecular determinants of pathogenesis in North American avian H7 influenza viruses in a mammalian host. We observed that disease severity of avian influenza viruses is modulated by differences in the PB2 and PA protein. The amino-acid differences in PB2 (E358V) and PA (P190S) affected replication and inflammation, resulting in reduced disease and death of the infected host. Emerging influenza viruses should be monitored for these residues and understanding how these residues impact replication and virulence will help develop novel intervention strategies to combat influenza viruses.
Material and Methods
Mice and viruses
Six- to ten-week-old DBA/2J mice were purchased from Jackson Laboratories (Bar Harbor, ME) or bred in-house in a barrier facility at Washington University School of Medicine, St Louis, MO, USA. The mice received food and water ad libitum and all experiments were conducted in accordance with rules of the Institutional Animal Control and Use Committee. Per animal protocol mice that lost more than 30% of their starting body weight were sacrificed to minimize discomfort.
Wild-type and plasmid-derived low pathogenic influenza A viruses (IAV), A/shorebird/Delaware/22/2006 (H7N3) and A/mallard/Alberta/177/2004 (H7N9), were propagated in 10-day-old embryonated chicken eggs (Cackle Hatcheries, IA, USA). After 48 hours at 35°C, the allantoic fluid containing the infectious virus was harvested and stored at -80°C. The viral titer (tissue culture infectious dose 50, TCID50) was determined using MDCK cells. Each viral stock in this study was titrated at least twice to accurately quantify the amount of infectious virus.
Generation of plasmid-derived influenza A viruses
Viral RNA was extracted from an egg (E1) passage of the H7N3 and H7N9 viruses and used to generate cDNA. Individual gene-segments were amplified from the cDNA using gene-specific primers (Hoffmann et al., 2000a; Hoffmann et al., 2000b) and a high fidelity polymerase enzyme (Phusion, New England Biolabs) followed by cloning into the Topo-Blunt vector (Invitrogen). After sequence confirmation, the gene-segments were cloned into the pHW2000 vector using the BsmBI or AarI restriction sites (New England Biolabs). After a second round of DNA sequencing the plasmids were used to generate recombinant H7N3 and H7N9 virus as well as various combinations or viruses containing point mutations according to published methods (Hoffmann et al., 2000b; Neumann et al., 1999). The pHW2000 vectors containing the PB2, PB1, PA, or NP gene-segments of H7N3 or H7N9 virus were used to generate pcDNA3.1+ expression vectors for the mini-genome reporter assay.
Sequence Analysis of North American avian influenza viruses
Genetic differences between H7N3 and H7N9 virus were identified using Lasergene MegAlign (DNAStar). A total of 53 amino-acid differences on 7 gene-segments were identified (NA was excluded because they belong to different serotypes). Four residues on PB2 protein and three residues on PA were selected for further evaluation. These residues were selected based on changes in the chemical properties of the amino-acids involved per NCBI amino-acid explorer. The amino-acid substitutions were introduced into pHW2000 and pcDNA3.1+ vectors containing the PB2 or PA genes of H7N3 or H7N9 virus by site-directed mutagenesis. The consensus sequence was identified by the influenza virus sequence database website using all available North American avian influenza viruses (PB2, n = 6790; PB1, n = 6840; PB1-F2, n = 6146; PA, n = 6918; PA-X, n = 8519; NP, n = 6395; M2, n = 6496; NS1, n = 6721; NS2, n = 6490). For the HA protein, we identified the consensus for H7 HA proteins from North American avian influenza viruses (n = 858). Residues that are found in more than 10% of the available sequences are shown in the consensus in Figure 2.
Multistep Growth Curves of Influenza A virus
MDCK cells were seeded in 24-well plates and infected the next day with 50 TCID50 of the different IAV. MDCK cells were washed once with PBS before adding the inoculum in Minimal Essential Medium (MEM) containing penicillin, streptomycin, glutamine, and vitamins plus 0.1% bovine serum albumin (M0.1B) for one hour at 37°C. After the one hour, the cells were washed with PBS and 1.0 ml of M0.1B with 1μg/ml TPCK-trypsin (Worthington Technologies) was added to each well. Culture supernatants were collected at 8, 24, 48 and 72 hours post infection and the amount of infectious virus was quantified by titration on MDCK cells.
Mini-genome luciferase assay with polymerase genes of influenza A virus
The PB2, PB1, PA and NP genes from H7N3 and H7N9 were cloned into the pcDNA3.1+ (Invitrogen) mammalian expression vector. The pLuci plasmid was kindly provided by Dr. Yen (Hong Kong University, Hong Kong, China) and contains the Firefly luciferase gene flanked by the non-coding regions of NP gene segment in the negative orientation. The transcription of this influenza A virus-like gene segment is under the control of a human polymerase I promoter. A Renilla luciferase gene containing expression plasmid is used to normalize for transfection efficiency. 293T cells are transfected with the expression plasmids containing the PB2, PB1, PA and NP gene segments of IAV plus the two luciferase containing plasmids (500 ng per plasmid) using TransIT LT1 (Mirus Bio LLC). The next day the media was changed and the cells were incubated for 48 hours before the cells were harvested, lysed and used to analyze Firefly and Renilla luciferase activity (Promega). Each condition (set of plasmids) was done in duplicate and repeated independently in separate experiments at least three times. The relative light units (RLU) of Firefly are normalized to the RLU for Renilla and the activity of varying polymerase combinations are then normalized to that of H7N3 or H7N9 IAV. Statistical analysis was done on the average value from a single assay.
Inoculation of mice with influenza A virus
DBA/2J mice were inoculated with IAV intranasally in 30 μl of sterile PBS after sedation with Avertin (2,2,2-tribromoethanol, Sigma-Aldrich, MO, USA). Morbidity and mortality upon intranasal inoculation was monitored for 21 days in female DBA/2J mice. To assess lung viral titers, lungs of male DBA/2J mice were collected on days 2, 3, 6 and 9 post inoculation with 102 or 103 TCID50 of IAV, homogenized in 1.0 ml of MEM, spun for 5 min at 1000×g and stored in aliquots at –80°C. The homogenates were used to quantify the amount of infectious virus (TCID50) present in the lungs using MDCK cells as described previously (Boon et al., 2009). Lung homogenates were also used to assess cytokine and chemokine production after infection with IAV using a 23-plex cytokine array (Bio-Plex Pro™ Mouse Cytokine 23-plex Assay, Biorad) according to manufacturer's protocol. The cytokine screen included IL-1α, IL-1β, IL-2, IL-3 IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17, Eotaxin, G-CSF, GM-CSF, IFN-γ, KC, MCP-1 (CCL2) MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), and TNF-α. The results from the cytokine array are the average of two experiments with three and four different mice per time-point per experiment. The results from the lung titrations are the average of two experiments with four or more different mice per time-point per experiment. The cytokine and chemokine concentrations in mock-infected lung homogenates of C57BL/6 mice was: IL1a (3 pg/ml), IL1b (144 pg/ml), CCL4 (20 pg/ml), IL12p40 (19 pg/ml), CCL3 (1.2 pg/ml), G-CSF (19 pg/ml), GM-CSF (22 pg/ml), KC (10 pg/ml), and CCL2 (< 1 pg/ml).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 software. Differences in mortality were determined using the log-rank (Mantel-Cox) test. The Mann-Whitney U-test was used to analyze differences in lung virus titers between the different virus strains. The Student's T-test was used to determine statistical significance in cytokine and chemokine production, weight loss on day 8 after influenza A virus infection, and in the polymerase activity assay.
Supplementary Material
Highlights.
Polymerase proteins PB2 and PA are important for virulence of avian influenza viruses in mice.
Mutations in PB2 (E358V) and PA (P190S and Q400P) play crucial roles in the pathogenesis of avian influenza viruses in mice.
DBA/2 mice are a suitable animal model to identify virulence factors in low pathogenic avian influenza viruses.
Acknowledgments
We would like to thank Dr. Matthew Sandbulte for critically reviewing the manuscript. APG and GW were supported by a training grant GM: 007067. We thank Mike White for help with colony maintenance.
References
- Belser JA, Davis CT, Balish A, Edwards LE, Zeng H, Maines TR, Gustin KM, Martinez IL, Fasce R, Cox NJ, Katz JM, Tumpey TM. Pathogenesis, transmissibility, and ocular tropism of a highly pathogenic avian influenza A (H7N3) virus associated with human conjunctivitis. J Virol. 2013a;87:5746–5754. doi: 10.1128/JVI.00154-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Gustin KM, Pearce MB, Maines TR, Zeng H, Pappas C, Sun X, Carney PJ, Villanueva JM, Stevens J, Katz JM, Tumpey TM. Pathogenesis and transmission of avian influenza A (H7N9) virus in ferrets and mice. Nature. 2013b;501:556–559. doi: 10.1038/nature12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Lu X, Maines TR, Smith C, Li Y, Donis RO, Katz JM, Tumpey TM. Pathogenesis of avian influenza (H7) virus infection in mice and ferrets: enhanced virulence of Eurasian H7N7 viruses isolated from humans. J Virol. 2007;81:11139–11147. doi: 10.1128/JVI.01235-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser JA, Tumpey TM. Mammalian models for the study of H7 virus pathogenesis and transmission. Curr Top Microbiol Immunol. 2014;385:275–305. doi: 10.1007/82_2014_383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon AC, deBeauchamp J, Hollmann A, Luke J, Kotb M, Rowe S, Finkelstein D, Neale G, Lu L, Williams RW, Webby RJ. Host genetic variation affects resistance to infection with a highly pathogenic H5N1 influenza A virus in mice. Journal of virology. 2009;83:10417–10426. doi: 10.1128/JVI.00514-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon AC, deBeauchamp J, Krauss S, Rubrum A, Webb AD, Webster RG, McElhaney J, Webby RJ. Cross-reactive neutralizing antibodies directed against pandemic H1N1 2009 virus are protective in a highly sensitive DBA/2 mouse influenza model. J Virol. 2010;84:7662–7667. doi: 10.1128/JVI.02444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon AC, Finkelstein D, Zheng M, Liao G, Allard J, Klumpp K, Webster R, Peltz G, Webby RJ. H5N1 influenza virus pathogenesis in genetically diverse mice is mediated at the level of viral load. MBio. 2011;2:e00171–00111. doi: 10.1128/mBio.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013;154:197–212. doi: 10.1016/j.cell.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DF, Smith DJ. A recommended numbering scheme for influenza A HA subtypes. PLoS One. 2014;9:e112302. doi: 10.1371/journal.pone.0112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey KA, Bousse TL, Desmet EA, Kim B, Takimoto T. PB2 residue 271 plays a key role in enhanced polymerase activity of influenza A viruses in mammalian host cells. J Virol. 2010;84:4395–4406. doi: 10.1128/JVI.02642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey KA, Desmet EA, Mattiacio JL, Hamilton A, Bradel-Tretheway B, Bussey HE, Kim B, Dewhurst S, Takimoto T. PA residues in the 2009 H1N1 pandemic influenza virus enhance avian influenza virus polymerase activity in mammalian cells. J Virol. 2011;85:7020–7028. doi: 10.1128/JVI.00522-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yuan H, Gao R, Zhang J, Wang D, Xiong Y, Fan G, Yang F, Li X, Zhou J, Zou S, Yang L, Chen T, Dong L, Bo H, Zhao X, Zhang Y, Lan Y, Bai T, Dong J, Li Q, Wang S, Li H, Gong T, Shi Y, Ni X, Li J, Fan J, Wu J, Zhou X, Hu M, Wan J, Yang W, Li D, Wu G, Feng Z, Gao GF, Wang Y, Jin Q, Liu M, Shu Y. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D, Li Y, Yao X, Zhang Y, Wu H, Zheng S, Diao H, Xia S, Chan KH, Tsoi HW, Teng JL, Song W, Wang P, Lau SY, Zheng M, Chan JF, To KK, Chen H, Li L, Yuen KY. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E, Munster VJ, van Riel D, Beyer WE, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. Molecular determinants of adaptation of highly pathogenic avian influenza H7N7 viruses to efficient replication in the human host. J Virol. 2010;84:1597–1606. doi: 10.1128/JVI.01783-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet EA, Bussey KA, Stone R, Takimoto T. Identification of the N-terminal domain of the influenza virus PA responsible for the suppression of host protein synthesis. J Virol. 2013;87:3108–3118. doi: 10.1128/JVI.02826-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell EA, Jones CA, Stallknecht DE, Howerth EW, Tompkins SM. Avian influenza virus isolates from wild birds replicate and cause disease in a mouse model of infection. Virology. 2010;399:280–289. doi: 10.1016/j.virol.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SA, Munster V, Kuiken T, Rimmelzwaan GF, Schutten M, Van Doornum GJ, Koch G, Bosman A, Koopmans M, Osterhaus AD. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101:1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Sun H, Hu J, Qi L, Wang J, Xiong X, Wang Y, He Q, Lin Y, Kong W, Seng LG, Pu J, Chang KC, Liu X, Liu J, Sun Y. Twenty amino acids at the C-terminus of PA-X are associated with increased influenza A virus replication and pathogenicity. J Gen Virol. 2015a;96:2036–2049. doi: 10.1099/vir.0.000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Sun Y, Hu J, Qi L, Wang J, Xiong X, Wang Y, He Q, Lin Y, Kong W, Seng LG, Sun H, Pu J, Chang KC, Liu X, Liu J. The contribution of PA-X to the virulence of pandemic 2009 H1N1 and highly pathogenic H5N1 avian influenza viruses. Sci Rep. 2015b;5:8262. doi: 10.1038/srep08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Xu G, Sun Y, Qi L, Wang J, Kong W, Sun H, Pu J, Chang KC, Liu J. PA-X is a virulence factor in avian H9N2 influenza virus. J Gen Virol. 2015c;96:2587–2594. doi: 10.1099/jgv.0.000232. [DOI] [PubMed] [Google Scholar]
- Guilligay D, Tarendeau F, Resa-Infante P, Coloma R, Crepin T, Sehr P, Lewis J, Ruigrok RW, Ortin J, Hart DJ, Cusack S. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol. 2008;15:500–506. doi: 10.1038/nsmb.1421. [DOI] [PubMed] [Google Scholar]
- Hatta Y, Hershberger K, Shinya K, Proll SC, Dubielzig RR, Hatta M, Katze MG, Kawaoka Y, Suresh M. Viral replication rate regulates clinical outcome and CD8 T cell responses during highly pathogenic H5N1 influenza virus infection in mice. PLoS Pathog. 2010;6:e1001139. doi: 10.1371/journal.ppat.1001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, MacDonald LA, Takimoto T. Influenza A Virus Protein PA-X Contributes to Viral Growth and Suppression of the Host Antiviral and Immune Responses. J Virol. 2015;89:6442–6452. doi: 10.1128/JVI.00319-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Hobom G, Webster RG, Kawaoka Y. “Ambisense” approach for the generation of influenza A virus: vRNA and mRNA synthesis from one template. Virology. 2000a;267:310–317. doi: 10.1006/viro.1999.0140. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000b;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Mo Y, Wang X, Gu M, Hu Z, Zhong L, Wu Q, Hao X, Hu S, Liu W, Liu H, Liu X. PA-X decreases the pathogenicity of highly pathogenic H5N1 influenza A virus in avian species by inhibiting virus replication and host response. J Virol. 2015;89:4126–4142. doi: 10.1128/JVI.02132-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M, Kawaoka Y. The role of receptor binding specificity in interspecies transmission of influenza viruses. Curr Opin Virol. 2012;2:160–167. doi: 10.1016/j.coviro.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:199–204. doi: 10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonges M, Welkers MR, Jeeninga RE, Meijer A, Schneeberger P, Fouchier RA, de Jong MD, Koopmans M. Emergence of the virulence-associated PB2 E627K substitution in a fatal human case of highly pathogenic avian influenza virus A(H7N7) infection as determined by Illumina ultra-deep sequencing. J Virol. 2014;88:1694–1702. doi: 10.1128/JVI.02044-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph T, McAuliffe J, Lu B, Jin H, Kemble G, Subbarao K. Evaluation of replication and pathogenicity of avian influenza a H7 subtype viruses in a mouse model. J Virol. 2007;81:10558–10566. doi: 10.1128/JVI.00970-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaperskyy DA, Schmaling S, Larkins-Ford J, McCormick C, Gaglia MM. Selective Degradation of Host RNA Polymerase II Transcripts by Influenza A Virus PA-X Host Shutoff Protein. PLoS Pathog. 2016;12:e1005427. doi: 10.1371/journal.ppat.1005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocer ZA, Fan Y, Huether R, Obenauer J, Webby RJ, Zhang J, Webster RG, Wu G. Survival analysis of infected mice reveals pathogenic variations in the genome of avian H1N1 viruses. Sci Rep. 2014;4:7455. doi: 10.1038/srep07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhou L, Zhou M, Chen Z, Li F, Wu H, Xiang N, Chen E, Tang F, Wang D, Meng L, Hong Z, Tu W, Cao Y, Li L, Ding F, Liu B, Wang M, Xie R, Gao R, Li X, Bai T, Zou S, He J, Hu J, Xu Y, Chai C, Wang S, Gao Y, Jin L, Zhang Y, Luo H, Yu H, Wang X, Gao L, Pang X, Liu G, Yan Y, Yuan H, Shu Y, Yang W, Wang Y, Wu F, Uyeki TM, Feng Z. Epidemiology of human infections with avian influenza A(H7N9) virus in China. N Engl J Med. 2014;370:520–532. doi: 10.1056/NEJMoa1304617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Danzy S, Dao LD, Parslow TG. Mutational analyses of the influenza A virus polymerase subunit PA reveal distinct functions related and unrelated to RNA polymerase activity. PLoS One. 2012;7:e29485. doi: 10.1371/journal.pone.0029485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Qiao C, Marjuki H, Bawa B, Ma J, Guillossou S, Webby RJ, Richt JA, Ma W. Combination of PB2 271A and SR polymorphism at positions 590/591 is critical for viral replication and virulence of swine influenza virus in cultured cells and in vivo. J Virol. 2012;86:1233–1237. doi: 10.1128/JVI.05699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Qin K, Meng G, Zhang J, Zhou J, Zhao G, Luo M, Zheng X. Structural and functional characterization of K339T substitution identified in the PB2 subunit cap-binding pocket of influenza A virus. J Biol Chem. 2013;288:11013–11023. doi: 10.1074/jbc.M112.392878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JS, Giotis ES, Moncorge O, Frise R, Mistry B, James J, Morisson M, Iqbal M, Vignal A, Skinner MA, Barclay WS. Species difference in ANP32A underlies influenza A virus polymerase host restriction. Nature. 2016;529:101–104. doi: 10.1038/nature16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz B, Schwemmle M, Brunotte L. Adaptation of avian influenza A virus polymerase in mammals to overcome the host species barrier. J Virol. 2013;87:7200–7209. doi: 10.1128/JVI.00980-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok CK, Lee HH, Lestra M, Nicholls JM, Chan MC, Sia SF, Zhu H, Poon LL, Guan Y, Peiris JS. Amino acid substitutions in polymerase basic protein 2 gene contribute to the pathogenicity of the novel A/H7N9 influenza virus in mammalian hosts. J Virol. 2014;88:3568–3576. doi: 10.1128/JVI.02740-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, de Wit E, van Riel D, Beyer WE, Rimmelzwaan GF, Osterhaus AD, Kuiken T, Fouchier RA. The molecular basis of the pathogenicity of the Dutch highly pathogenic human influenza A H7N7 viruses. J Infect Dis. 2007;196:258–265. doi: 10.1086/518792. [DOI] [PubMed] [Google Scholar]
- Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Yamayoshi S, Kawaoka Y. Mapping of a Region of the PA-X Protein of Influenza A Virus That Is Important for Its Shutoff Activity. J Virol. 2015;89:8661–8665. doi: 10.1128/JVI.01132-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang IK, Pillai PS, Iwasaki A. Efficient influenza A virus replication in the respiratory tract requires signals from TLR7 and RIG-I. Proc Natl Acad Sci U S A. 2013;110:13910–13915. doi: 10.1073/pnas.1303275110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautus S, Sehr P, Lewis J, Fortune A, Wolkerstorfer A, Szolar O, Guilligay D, Lunardi T, Decout JL, Cusack S. New 7-Methylguanine Derivatives Targeting the Influenza Polymerase PB2 Cap-Binding Domain. J Med Chem. 2013;56:8915–8930. doi: 10.1021/jm401369y. [DOI] [PubMed] [Google Scholar]
- Pflug A, Guilligay D, Reich S, Cusack S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature. 2014;516:355–360. doi: 10.1038/nature14008. [DOI] [PubMed] [Google Scholar]
- Pica N, Iyer A, Ramos I, Bouvier NM, Fernandez-Sesma A, Garcia-Sastre A, Lowen AC, Palese P, Steel J. The DBA.2 mouse is susceptible to disease following infection with a broad, but limited, range of influenza A and B viruses. J Virol. 2011;85:12825–12829. doi: 10.1128/JVI.05930-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping J, Keleta L, Forbes NE, Dankar S, Stecho W, Tyler S, Zhou Y, Babiuk L, Weingartl H, Halpin RA, Boyne A, Bera J, Hostetler J, Fedorova NB, Proudfoot K, Katzel DA, Stockwell TB, Ghedin E, Spiro DJ, Brown EG. Genomic and protein structural maps of adaptive evolution of human influenza A virus to increased virulence in the mouse. PLoS One. 2011;6:e21740. doi: 10.1371/journal.pone.0021740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant LA, Kuiken T, Osterhaus AD. Adaptive pathways of zoonotic influenza viruses: from exposure to establishment in humans. Vaccine. 2012;30:4419–4434. doi: 10.1016/j.vaccine.2012.04.049. [DOI] [PubMed] [Google Scholar]
- Salomon R, Franks J, Govorkova EA, Ilyushina NA, Yen HL, Hulse-Post DJ, Humberd J, Trichet M, Rehg JE, Webby RJ, Webster RG, Hoffmann E. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J Exp Med. 2006;203:689–697. doi: 10.1084/jem.20051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Song W, Lau SY, Que TL, Lung DC, Hung IF, Chen H, Yuen KY. Unique reassortant of influenza A(H7N9) virus associated with severe disease emerging in Hong Kong. J Infect. 2014 doi: 10.1016/j.jinf.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kiso M, Fukuyama S, Nakajima N, Imai M, Yamada S, Murakami S, Yamayoshi S, Iwatsuki-Horimoto K, Sakoda Y, Takashita E, McBride R, Noda T, Hatta M, Imai H, Zhao D, Kishida N, Shirakura M, de Vries RP, Shichinohe S, Okamatsu M, Tamura T, Tomita Y, Fujimoto N, Goto K, Katsura H, Kawakami E, Ishikawa I, Watanabe S, Ito M, Sakai-Tagawa Y, Sugita Y, Uraki R, Yamaji R, Eisfeld AJ, Zhong G, Fan S, Ping J, Maher EA, Hanson A, Uchida Y, Saito T, Ozawa M, Neumann G, Kida H, Odagiri T, Paulson JC, Hasegawa H, Tashiro M, Kawaoka Y. Characterization of H7N9 influenza A viruses isolated from humans. Nature. 2013;501:551–555. doi: 10.1038/nature12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SH, Yang JR, Wu HS, Chang MC, Lin JS, Lin CY, Liu YL, Lo YC, Yang CH, Chuang JH, Lin MC, Chung WC, Liao CH, Lee MS, Huang WT, Chen PJ, Liu MT, Chang FY. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respir Med. 2013;1:771–778. doi: 10.1016/S2213-2600(13)70221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GD, Pinto AK, Doll B, Boon AC. A North American H7N3 influenza virus supports reassortment with 2009 pandemic H1N1 and induces disease in mice without prior adaptation. J Virol. 2016 doi: 10.1128/JVI.02761-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamayoshi S, Yamada S, Fukuyama S, Murakami S, Zhao D, Uraki R, Watanabe T, Tomita Y, Macken C, Neumann G, Kawaoka Y. Virulence-affecting amino acid changes in the PA protein of H7N9 influenza A viruses. J Virol. 2014;88:3127–3134. doi: 10.1128/JVI.03155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.