Abstract
Background
Determining the efficacy of human vaccines that induce antigen-specific protective CD4 T cell responses against pathogens can be particularly challenging to evaluate. Surface expression of CD11a and CD49d has been shown to identify antigen-specific CD4 T cells against viral pathogens in mice. We hypothesized that CD11a and CD49d would also serve as markers of human antigen-specific T cells responding to vaccination.
Methods
A phase I vaccine trial enabled us to evaluate a novel gating strategy based on surface expression of CD11a and CD49d as a means of detecting antigen-specific, cytokine producing CD4 and CD8 T cells induced after vaccination of naïve individuals against leishmaniasis. Three study groups received LEISH-F3 recombinant protein combined with either squalene oil-in-water emulsion (SE) alone, SE with the synthetic TLR-4 ligand glucopyranosyl lipid adjuvant (GLA-SE), or SE with Salmonella minnesota-derived monophosphoryl lipid A (MPL-SE). Individuals were given 3 vaccine doses, on days 0, 28 and 168.
Results
Starting after the first vaccine dose, the frequency of both CD11ahiCD49d+ CD4 and CD11ahiCD49d+ CD8 T cells significantly increased over time throughout the 24-week trial. To confirm the role of CD11ahiCD49d+ expression in the identification of the antigen-specific T cells, cytokine production was measured following LEISH-F3 stimulation. All of the IFN-γ, TNF-α, and IL-2 producing cells were found within the CD11ahiCD49d+ population.
Conclusions
Our results suggest that the change in the frequency of CD11ahiCD49d+ T cells can be used to track antigen-specific CD4 and CD8 T cell responses following T cell-targeted vaccination.
Keywords: Adjuvant, Protective immunity, Visceral Leishmaniasis, T cell response, Activation markers
1. Introduction
Development of effective vaccines in humans is hindered by an inability to discern whether a protective immune response has developed. CD4 T cell responses are especially difficult to track because they often target a broad array of pathogen-derived antigens, and the frequency of CD4 T cells responding to individual epitopes is often low [1–5]. Discerning the protective epitopes or cytokine-producing clones is not trivial. Furthermore, CD4 T cells can differentiate into a number of effector cell subsets that produce unique cytokines [6]. Thus, identification of antigen-specific CD4 T cells based on the production of a single cytokine in response to a specific peptide will likely underestimate the number of antigen-specific T cells. These challenges impede the evaluation and validation of new vaccine candidates.
We recently demonstrated that the cell surface integrins CD11a and CD49d can be used to identify total antigen-specific CD4 T cell responses to a specific peptide during murine infection with LCMV [7]. These integrins mediate cell trafficking into sites of inflammation [8–10]. Cell surface expression of CD11a and CD49d is up-regulated on murine antigen-specific CD4 T cells and maintained through memory [7]. Expression of CD11a and CD49d on CD4 T cells is not modulated by inflammation in the absence of cognate antigen. Thus, these markers can be used to identify and track newly generated antigen-specific CD4 T cells following infection [7]. Even though they are expressed on human T cells [11, 12], it is not known whether CD11a and CD49d exhibit similar up-regulation in human T cells responding to antigen, as they do in mice. Therefore, we evaluated the reliability of CD11a and CD49d to identify antigen-specific CD4 T cells following primary vaccination in humans.
The current study took advantage of a phase I clinical trial in which naïve volunteers were immunized with LEISH-F3, a recombinant protein subunit vaccine containing fused open reading frames encoding Leishmania donovani nucleoside hydrolase (NH) and L. infantum sterol 24-c-methyltransferase (SMT). Subjects were chosen because of their lack of prior exposure to Leishmania spp. and the fact that they were unlikely to visit an endemic region through the course of this study. The LEISH-F3 antigen was previously shown to induce antibody and Th1-type CD4 T cell responses in mice and humans; the latter is required for protective immunity [13]. In this clinical trial LEISH-F3 was administered in one of three adjuvant formulations. Glucopyranosyl lipid adjuvant (GLA), a synthetic TLR-4 ligand, and 3-O-desacyl-4’-monophosphoryl lipid A (MPL), a TLR-4 ligand from the lipopolysaccharide of Salmonella minnesota, were separately formulated in a stable oil-in water-emulsion (SE). Both adjuvants induce antigen-specific Th1 responses following vaccination [13–16]. A third group received LEISH-F3 in SE alone (no TLR-4 ligand), which induces a Th2-biased response [14, 15].
We observed that the activated human CD4 and CD8 T cells responding to LEISH-F3 vaccination expressed CD11a and CD49d, and the frequency of cells with these markers increased over time after vaccination. Furthermore, virtually all of the IFN-γ, TNF-α, and IL-2-producing T cells responding to LEISH-F3 were CD11ahiCD49d+. These data suggest the combination of CD11a and CD49d can be used as markers to focus on cytokine-producing human T cells responding to the immunizing antigen after vaccination. This approach might constitute a useful approach to tracking vaccine-induced T cell responses.
2. Materials and Methods
2.1. Study design
The parent study was a randomized, open-label clinical vaccine trial conducted by the Vaccine Treatment and Evaluation Unit (VTEU) at the University of Iowa between March 2013 and August 2014. This was a part of larger trial recorded at https://clinicaltrials.gov/ct2/show/NCT01751048. The study was conducted in compliance with the protocol, International Conference on Harmonization guideline E6: Good Clinical Practice: Consolidated Guideline, the applicable regulatory requirements from US Code of Federal Regulations (CFR) (Title 45 CFR Part 46 and Title 21 CFR including Parts 50 and 56) concerning informed consent and Institutional Review Board regulations, and the NIAID Clinical Terms of Award.
2.2. Vaccine and adjuvants
Recombinant LEISH-F3 protein in adjuvant was provided by IDRI for the trial. Synthetic Glycopyranosyl Lipid A in a stable oil-in-water emulsion (GLA-SE), is a TLR4 agonist. MPL (provided by GSK Biologicals) is a Salmonella minnesota Lipid A in SE. Recombinant LEISH-F3 polypeptide was provided by IDRI for antigen stimulation experiments.
2.3. Study participants
Participants were males and non-pregnant females between 21 and 49 years old. The first 12 consenting subjects in each parent study group were enrolled. All subjects were healthy, and screening laboratory values for hemoglobin, white blood cell count, neutrophil count, platelets, creatinine, AST, ALT and total bilirubin were within normal limits. Exclusion criteria included visiting or living in a Leishmania-endemic region, history of possible Leishmania infection or previous exposure to Leishmania vaccine or GLA-SE. Individuals were vaccinated with 0.5 ml of vaccine plus adjuvant intramuscularly on Days 0, 28 and 168.
2.4. Whole Blood Assay
Blood samples were collected on Days 0 (pre-vaccination), 1, 3, 7, 14, 28 (post-vaccination), 35, 42, 56, 168 (post-vaccination), 182, 196 and 365. Venous whole blood from each individual was stimulated with either 10 µg/ml recombinant LEISH-F3, PBS (negative control), or 75 µg/ml PHA (positive control; Sigma-Aldrich, St. Louis, MO) for 12 hours at 37°C. Brefeldin A (10 µg/ml, Sigma-Aldrich) was added for the final 6 hours. Cells were treated with FACS Lysis Solution (BD Biosciences, San Jose, CA) and stained for surface CD3 (OKT3), CD4 (OKT4), CD8 (SK1), CD49d (9F10) and CD11a (HI111). Cells were permeabilized with eBioscience permeabilization buffer and stained for intracellular IFN-γ (4S.B3), TNF-α (MAb11), IL-2 (MQ1-17H12) and IL-10 (JES3-9D7). All antibodies were obtained from eBioscience. Samples were run on a BD LSR Fortessa (BD Biosciences) and data were analyzed with FlowJo software (Tree Star Inc, Ashland, OR). Supplemental Figures 1 and 2 depict the Day 0 and Day 182 staining controls.
2.5 Statistical Analysis
With the exception of Figures 2C, 2F, 3B and 3C which compare days 0 and 182, statistical analyses considered within- and between- group variability for all data at all time points. Statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC). A linear mixed model analysis for repeated measures was used to compare CD11a and CD49d expression on either CD4 or CD8 T cells among the three vaccine formulations over time. Data were natural log transformed for analyses. Ratios were calculated as the mean differences after back transformation. Based on the fitted mixed model, tests of mean contrasts were performed to assess pairwise differences between the vaccine groups at each time point. P-values were adjusted for multiple tests using Bonferroni’s method. The time effect for each vaccine formulation was tested with Dunnett’s post-test to assess change from Day 0 at each time point.
Figure 2. Increased CD11a and CD49d expression on T cells following vaccination.
Representative CD11a and CD49d expression at day 182 on CD4+ (A) or CD8+ (D) CD3+ T cells are shown. Cell numbers are indicated for times throughout vaccination (B, E). The change in the frequency of CD11ahiCD49d+ CD4+ (B) and CD8+ (E) T cells was calculated by determining the difference between the frequency of CD11ahiCD49d+ cells on day 0 and the indicated day. The natural log changes in the frequency of CD11ahiCD49d+ CD4 (C) and CD8 (F) T cells between study groups were compared at the peak of the response on day 182 (*p<0.01; one-way ANOVA). Data represent the mean ± SEM for 12 subjects per group at each time point.
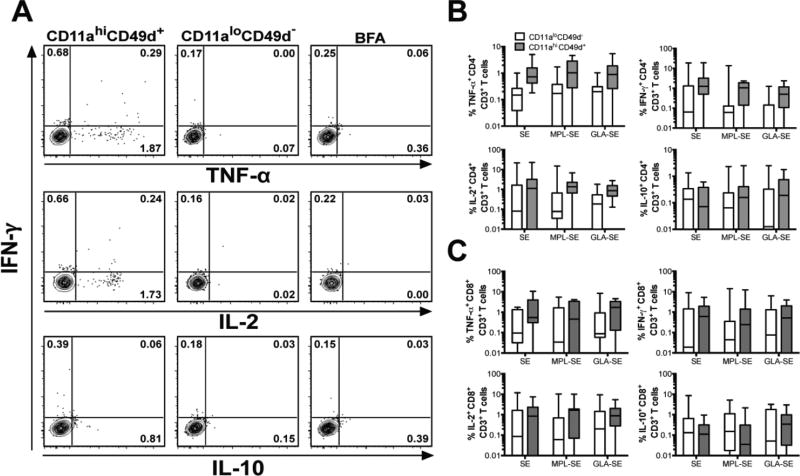
Figure 3. CD11ahiCD49d+ cells represent antigen-experienced CD4 and CD8 T cells.
On day 182 of the study (14 days after the final vaccine dose), whole blood leukocytes were stimulated with LEISH-F3 recombinant protein or left unstimulated, and cells were stained for flow cytometry. (A) Representative plots depict cytokine production by CD11ahiCD49d+ versus CD11aloCD49d− CD4+ CD3+ T cells stimulated with LEISH-F3, or incubated without antigen in the presence of BFA. The cumulative frequency of LEISH-F3-stimulated cytokine-producing CD4 (B) and CD8 (C) T cells are shown on day 182 of the study (14 days after the last vaccine dose) based on CD11ahiCD49d+ (gray) or CD11aloCD49d (white) expression for each treatment group. Data are shown as box plots on a log scale, with whiskers representing the 5th and 95th percentiles.
A linear mixed model analysis for repeated measures was used to consider cytokine expression at all time points in antigen-stimulated CD11ahiCD49d+ versus CD11aloCD49d− cells. Control cells were incubated in media rather than antigen. The fixed effects tested in the model included vaccine formulation, CD11a and CD49d expression, and time after study initiation. This analysis used ln-transformed data with 0.01 added to replace zero values prior to ln transformation. From the fixed model, time effect on antigen-induced cytokine responses was tested for each vaccine type with Dunnett’s test to assess change from Day 0 at each time point. The rationale for using day 0 as a baseline was to detect changes due to vaccine antigen in this previously naïve population. Mean estimates in the original scale were computed by back transformation of the ln means with corresponding standard error calculated using the delta method. The analyses considered all data from antigen-stimulated cells generated in the study. For simplicity, data are shown from only the most relevant times for this study, i.e., 14 days after each vaccine dose (Days 14, 42 and 182).
Each subject’s immune response after the full vaccination series (Day 182) was compared with the response prior to initiating vaccination (Day 0). The difference between the natural log frequency of CD11ahiCD49d+ cells on Day 0 and Day 182 was compared by one-way ANOVA with Tukey’s multiple test correction (GraphPad Software Inc., La Jolla, CA). Wilcoxon matched pairs test was used to compare the differences between the frequency of cytokine producing CD11ahiCD49d+ versus CD11aloCD49d− in CD4 or CD8 T cells on Day 182 post-vaccination.
3. Results
3.1 Changes in CD11a and CD49d cell surface expression on CD4 and CD8 T cells following vaccination
To determine whether they mark antigen-specific T cells, we evaluated CD11a and CD49d on both CD4 and CD8 T cells in peripheral blood of healthy individuals either before or after vaccination. Three groups of individuals received the LEISH-F3 recombinant subunit vaccine on days 0, 28, and 168 of the trial. The vaccine was formulated in SE alone, SE with monophosphoryl lipid A (MPL-SE), or SE with glucopyranosyl lipid adjuvant (GLA-SE) (Figure 1).
Figure 1.
Diagram of trial design, the number of individuals at each follow-up and analysis.
Humans are naturally exposed to many antigens and circulating T cells include a mixture of many antigen-experienced cells. Therefore, we maximized our chance of detecting cells specifically responding to LEISH-F3 antigen by analyzing cell surface phenotypes 14 days after the most recent vaccine exposure, and by omitting individuals with concurrent infections from the study. Considering all data from all groups, the frequency of CD11ahiCD49d+ CD4 (p = 0.0002) and CD11ahiCD49d+ CD8 (p = 0.0267) T cells increased significantly over time after beginning LEISH-F3 vaccination. Figure 2 and Table 1 indicate results of flow cytometry analyses of CD11a and CD49d expression on T cells prior to vaccination, 14 days after each dose of vaccine (days 14, 42, 182) and at a late time point (day 365). Examining the total data set in each of the study groups, the frequency of CD11ahiCD49d+ CD4 T cells significantly increased over time in individuals given the LEISH-F3 vaccine antigen plus either SE or GLA-SE (Table 1: CD4 Test Time effect). Furthermore, the frequency of CD11ahiCD49d+ CD8 T cells significantly increased in individuals vaccinated with LEISH-F3 + GLA-SE (Table 1: CD8 Test Time effect).
Table 1.
The frequency of CD11ahiCD49d+ CD4 and CD8 T cells increases over time following vaccination.
| Cell Type |
Day | SE | MPL + SE | GLA + SE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Ratio* | p-value# | Mean | SEM | Ratio | p-value | Mean | SEM | Ratio | p-value | ||
| CD4 | 0 | 30.2 | 2.6 | 35.9 | 3.1 | 29.5 | 2.5 | ||||||
| 14 | 26.3 | 3.2 | 0.87 | 0.742 | 34.3 | 4.4 | 0.96 | >0.999 | 29.5 | 3.8 | 0.99 | >0.999 | |
| 42 | 35.1 | 3.2 | 1.16 | 0.453 | 39.0 | 3.5 | 1.09 | 0.932 | 36.1 | 3.2 | 1.23 | 0.123 | |
| 182 | 37.5 | 2.9 | 1.24 | 0.063 | 38.9 | 3.1 | 1.09 | 0.913 | 40.2 | 3.2 | 1.36 | 0.003 | |
| 365 | 30.8 | 3.6 | 1.02 | >0.999 | 43.8 | 5.0 | 1.22 | 0.302 | 33.0 | 3.8 | 1.12 | 0.871 | |
| Test Time effect+ | p=.030 | p=0.319 | p=.005 | ||||||||||
| CD8 | 0 | 42.0 | 5.4 | 51.1 | 6.6 | 47.4 | 6.1 | ||||||
| 14 | 33.7 | 4.8 | 0.80 | 0.239 | 47.4 | 7.0 | 0.93 | 0.989 | 50.9 | 7.5 | 1.07 | 0.993 | |
| 42 | 42.7 | 4.6 | 1.02 | >0.999 | 50.9 | 5.5 | 1.00 | >0.999 | 47.9 | 5.1 | 1.01 | >0.999 | |
| 182 | 46.6 | 4.3 | 1.11 | 0.822 | 53.6 | 4.9 | 1.05 | 0.998 | 63.7 | 5.9 | 1.34 | 0.019 | |
| 365 | 41.5 | 4.2 | 0.99 | >0.999 | 51.6 | 5.1 | 1.01 | >0.999 | 49.8 | 5.0 | 1.05 | 0.998 | |
| Test Time effect+ | p=150 | p=0.494 | p=.035 | ||||||||||
Time effect for each study group with a Dunnett’s test to assess change from Day 0 at each time point.
Ratio of CD11ahiCD49d+ expressing cells on indicated day compared to an individual’s day 0 expression.
Based on the fitted mixed model, test of mean contrasts were performed to assess pairwise differences between the vaccines at each time point with p-values adjusted using Bonferroni’s method.
In addition to the overall increase, the highest frequency of CD11ahiCD49d+ expression on CD4 or CD8 T cells was observed on Day 182, 14 days after the final immunization dose (Figure 2; Table 1). These data are consistent with a cumulative effect of sequential vaccine doses on CD11a and CD49d co-expression on CD4 and CD8 T cells. Comparing the change in CD11a and CD49d expression at its peak expression on Day 182, we found a significantly greater increase in CD11a and CD49d expression on both CD4 and CD8 T cells in the study group vaccinated with GLA-SE compared to either MPL-SE or SE alone (Figure 2C and Figure 1F). This is consistent with the hypothesis that the GLA-SE adjuvant induces a more robust antigen-specific activation of both CD4 and CD8 T cells following vaccination.
3.2 CD11a and CD49d identify antigen-specific effector CD4 T cells
Because the above findings do not distinguish between CD11a and CD49d expressing T cells that respond to the vaccine antigen versus other antigens, we investigated whether the CD11a and CD49d co-expressing T cells correspond to the CD4 T cells specifically recognizing LEISH-F3 polypeptide. Whole blood was stimulated with LEISH-F3 recombinant protein, and cytokine production by CD11ahiCD49d+ CD4 T cells was examined by intracellular flow cytometry at the study time points indicated above. Our analyses were performed to consider both all changes in antigen-stimulated T cells from all study groups throughout the entire study, as well as the final immune response after completion of all three vaccine doses. Importantly, CD4 T cells from immunized individuals that were not exposed to antigen (BFA column in Figures 3A, and Supplemental Figures S1 and S2) did not display substantial intracellular cytokine. Thus, the results shown in Figure 3 and Table 2 are based on antigen-exposed T cells from study subjects.
Table 2.
Antigen-induced CD11ahiCD49d+ CD4 and CD8 T cells are the cytokine-producing cells following vaccination.
| Cell Type |
Effect+ | IFN-γ | TNF-α | IL-2 | IL-10 | IFN-γ/ TNF-α |
|---|---|---|---|---|---|---|
| CD4 | CD11a CD49d | <0.0001 | <0.0001 | 0.0788 | <0.0001 | 0.0694 |
| Time | 0.0483 | 0.0337 | 0.0548 | 0.0968 | <0.0001 | |
| Study group | 0.8559 | 0.4797 | 0.0609 | 0.4554 | 0.0805 | |
| CD8 | CD11a CD49d | <0.0001 | 0.0001 | 0.0002 | 0.4938 | <0.0001 |
| Time | 0.2121 | <0.0001 | 0.0239 | 0.1090 | <0.0001 | |
| Study group | 0.4758 | 0.5851 | 0.2396 | 0.8809 | 0.9635 |
Data show the analyses of antigen-exposed CD4 or CD8 T cells from individuals receiving LEISH-F3 vaccine plus adjuvant in the three study groups. Data are compared to the individual’s baseline response on day 0 prior to exposure to the vaccine antigen. A linear mixed model analysis for repeated measures was used to compare the mean levels of the response variable CD11ahiCD49d+ and CD11aloCD49d antigen-exposed CD4 or CD8 T cells among the vaccines over time. The data in different rows show p values considering variability of each cytokine or cytokine combination according to CD11a/CD49d status, time, or membership in the different adjuvant groups. Significant p values indicate the row parameter is associated with significant variability in cytokine producing cells. For this analysis the natural log transformation of the data was used.
The first analysis was performed using a linear mixed model. The data reveal that, in both CD4 and CD8 T cells, co-expression of CD11a and CD49d significantly associates with the antigen-induced production of cytokines (Table 2: CD11a CD49d). In the case of CD4 T cells, analysis of antigen-induced TNF-α, IFN-γ, IL-2, and IL-10 revealed that the frequency of cytokine-producing CD4 T cells was significantly greater in the CD11ahiCD49d+ cell population as compared to the CD11aloCD49d− cells (Table 2: CD11a CD49d).
The second approach entailed examination of the final immune response after all three vaccinations. This analysis showed the frequency of antigen-exposed IFN-γ (p = 0.0001), TNF-α (p = 0.0002), and IL-2 (p = 0.0003) producing cells was significantly increased in the CD11ahiCD49d+ CD4 T cell population compared to CD11aloCD49d− CD4 T cells. However, we did not see a significant difference in the frequency of IL-10-producing cells (p = 0.2021).
A strategy based on detecting antigen-induced cytokine production by total peripheral lymphocytes did not distinguish cytokine positive cells from background. Both the linear mixed model and endpoint comparisons suggest, however, that a gating strategy focused on only the subset of T cells that co-express CD11a and CD49d can be used to enrich the antigen-specific IFN-γ/TNF-α co-producing CD4 T cells responding to LEISH-F3 immunization. In the case of this this phase 1 vaccine trial, this strategy enabled us to detect the response of T cells that were induced by the vaccine antigen.
The frequency of CD4 T cells producing TNF-α, IFN-γ, or the combination of both of these cytokines in response to LEISH-F3 recombinant protein increased significantly over time after vaccination with the greatest significance observed in the combination of cells producing IFN-γ and TNF (Table 2: Time). This indicates that the vaccine in combination with any of the three adjuvants was able to induce a multi-functional antigen-specific CD4 T cell response.
Table 2 documents significant changes in cytokine producing cells based on CD11aCD49d status, and over time of the study. Also shown in Table 2 (Study group), there were no significant differences between study groups in the frequency of cytokine producing CD11ahiCD49d+ CD4 T cells throughout the study, or on Day 182 after the final vaccination (Figure 3B). This suggests that, once induced, the antigen-specific CD11ahiCD49d+ CD4 T cells produced in response to vaccine plus any of the adjuvants do not differ significantly from each other.
3.3 Antigen-specific CD8 T cells are identified by CD11a and CD49d expression
Murine studies inspiring this analysis show that the combined expression of surface CD11a and CD49d identifies antigen-specific murine CD4 T cells [7], whereas antigen-specific CD8 T cells are identified by up regulation of CD11a combined with down regulation of CD8α [17, 18]. In addition to the aforementioned results on CD4 T cells, our data revealed the unanticipated finding that the combined expression of CD11a and CD49d is significantly increased on antigen-specific human CD8 T cells following vaccination of previously naïve donors. Indeed, over the course of a 3-dose vaccination regimen, there were significant increases in the frequencies of CD11ahiCD49d+ CD8 or CD4 T cells that produced IFN-γ, TNF-α or IL-2, or cells that co-produced TNF-α with IFN-γ when stimulated with antigen, compared to CD11aloCD49d− CD8 T cells of the same lineage (see Table 2). The greatest increase in cytokine producers was observed on day 182 following vaccination (Figure 3). Similar to the result with CD4 T cells, the cytokine producing antigen-specific CD11ahiCD49d+ CD8 T cells induced by each adjuvant group did not differ significantly.
Together these data suggest that co-expression of CD11a and CD49d can be used to identify antigen-experienced CD4 and CD8 T cells and detect those that are producing cytokine, and in particular co-producing IFN-γ and TNF-α following primary human vaccination. The data also suggest that different adjuvant regimens alter the frequency of CD11ahiCD49d+ CD4 and CD8 T cells responding to vaccination.
4. Discussion
Methods to identify and track antigen-specific T cells in humans are essential for determining the immunogenicity of T cell-based vaccines. However, antigen-specific T cell responses have been difficult to track in humans. Our results suggest that the co-expressed surface markers CD11a and CD49d may provide a broader means of detecting antigen-specific CD4 and CD8 T cells, and thus avoid the need for more specific knowledge of epitopes and HLA types when evaluating the primary immune response to vaccines.
We recently reported that cell surface markers CD11a and CD49d can be used to identify the full antigen-specific CD4 T cell response following viral infections in mice [7, 19, 20]. Several subsequent studies have demonstrated the utility of this technique to track murine CD4 T cell responses to other microbes including Plasmodium spp. [21], Ehrlichia muris and Listeria monocytogenes [22, 23]. In the current study we questioned whether these markers could also be used to identify and track antigen-specific CD4 T cells in humans responding to primary vaccination with a recombinant protein antigen (LEISH-F3) containing immunogenic epitopes of the protozoa Leishmania. donovani and L. infantum. The study provided an ideal opportunity to examine vaccine-induced immunity because, first, LEISH-F3 has previously been shown to induce a human immune response in a phase 1 study [13] and, second, the study design included only individuals who were naïve to the LEISH-F3 antigen.
Sequential testing of circulating leukocytes by flow cytometry revealed that vaccination induced significant increases in the frequency of CD4 and CD8 T cells that were CD11ahiCD49d+, when tested 14 days after a vaccine dose. The T cells producing cytokines in response to antigen were significantly associated with the CD11ahiCD49d+ surface phenotype. These observations suggest the CD11ahiCD49d+ cells may be used to enhance the detection of vaccine-induced T cells that provide protective immunity. HLA tetramers are not available for a wide range of antigens including the LEISH-F3 antigen utilized in this study, but in the absence of this capacity CD11a and CD49d can be used as markers to gate on lymphocyte responses to a recently encountered antigen, including a vaccine antigen. To our knowledge this is the first study to show that surface expression of both CD11a and CD49d is modulated in human T cells in vivo after initial exposure to antigen.
Studies with recombinant LEISH-F3 antigen revealed that cytokine-producing CD4 and CD8 T cells were greatly enriched within the CD11ahiCD49d+ population suggesting that, similar to mouse models [7], the combination of CD11a and CD49d might be useful markers focused on antigen-specific T cells after vaccination. However, the change in the frequency of CD11ahiCD49d+ T cells was not equal to the frequency of cytokine producing cells following vaccination. Previous studies have shown that cytokine production underestimates the frequency of antigen-specific T cells [7, 24, 25]. In addition, the change in the frequency of CD11ahiCD49d+ T cells is likely to overestimate the population of responding cells because environmental or pathogen-derived antigens should also cause T cell activation and up-regulation of CD11a and CD49d. Individuals were excluded from a study time point if they presented with an infection during the study period, but this would not entirely remove this confounding factor. As such, co-expression of high CD11a and CD49d can be used to help identify recently induced antigen responsive T cells, and specific (cytokine or proliferation) response to the vaccine antigen would present solid evidence of the response to vaccine antigen.
LEISH-F3 + GLA-SE vaccination in mice was previously reported to induce CD4 T cells producing IFN-γ, TNF-α, and IL-2 [13]. Using CD11a and CD49d to focus our gating strategy on antigen-responding T cells, and incubating in the LEISH-F3 vaccine antigen, we compared the ability of three adjuvants (MPL-SE, GLA-SE, and SE alone) to stimulate antigen-specific cytokines T cell cytokines during vaccination of antigen-naïve humans. As anticipated, all three adjuvants induced both CD4 and CD8 T cell activation in CD11ahiCD49d+, measured by cytokine production following LEISH-F3 re-stimulation ex vivo. The GLA-SE adjuvant group exhibited the greatest increase in CD11a- and CD49d–expressing cytokine+ cells two-weeks after the third and final vaccination, although the type of adjuvant did not contribute significantly to the variation in cytokine-producing T cell numbers (Table 2). Importantly, there was no significant antigen-specific cytokine production by cells lacking the CD11ahiCD49d+ surface phenotype. Together, these data suggest that GLA-SE is the most efficient adjuvant at augmenting the frequency of antigen-responsive CD11ahiCD49d+ T cells that produce cytokines.
The above data suggest that the combined surface phenotype CD11a and CD49d with ex vivo antigen stimulation can be used to identify antigen-specific CD4 and CD8 T cells producing cytokines associated with protective immunity in response to primary vaccination. The change in the frequency of CD11ahiCD49d+ cells prior to and post vaccination would allow for evaluation of the total antigen-specific T cell response without needing to know the specific epitope repertoire or HLA restriction of the individual. Although a great deal of validation with other vaccines, protocols and pathogens is required, if these observations prove generalizable the use of surface CD11a and CD49d on antigen-stimulated T cells would constitute a tremendous advance in our ability to determine the efficacy of candidate human vaccines at inducing protective T cell responses against microbial pathogens.
Supplementary Material
Day 0 prior to vaccination, whole blood leukocytes were stimulated with LEISH-F3 recombinant protein or left unstimulated (BFA), and cells were stained for flow cytometry. Representative contour plots depict cytokine production by CD11ahiCD49d+, CD11aloCD49d− and unstimulated CD4+ CD3+ T cells.
Day 182 post-vaccination (14 days after the final vaccine dose), whole blood leukocytes were stimulated PHA to show maximal responses of antigen-responsive T cells in the patients, or left unstimulated (BFA), and cells were stained for flow cytometry. Representative contour plots depict cytokine production by CD11ahiCD49d+, CD11aloCD49d− and unstimulated CD4+ CD3+ T cells.
Acknowledgments
This study was funded as a sub-study through a Vaccine Trials and Evaluation Unit (HHSN272200800008C). This research was also supported with funding from the Bill & Melinda Gates Foundation to SGR under grants #39129 and OPP1055855. We would like to thank the staff at the Vaccine Trials and Evaluation unit especially Dan Zhao as well as Stacey Hartwig, Yani Chen and Hemali Batra for their excellent technical assistance. We also appreciate the comments from Zachary Sagawa, Jill Ashman, Tracey Day, and Randall Howard during preparation of this manuscript.
Abbreviations
- SE
squalene oil-in-water emulsion
- GLA
glucopyranosyl lipid A adjuvant
- MPL
monophosphoryl lipid A
- NH
nucleoside hydrolase
- SMT
sterol 24-c-methyltransferase
- LEISH-F3
recombinant protein subunit vaccine antigen containing NH and SMT
- CFR
Code of Federal Regulations
- BFA
Brefeldin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
AFC, UGD, RNC, AMB, PLW, BMZ, SMV, and MEW have no conflicts of interest to report.
SGR is an inventor on patents owned by IDRI including US patent 8,273,361 and its US and foreign counterparts for GLA and a founder and equity holder of Immune Design, a licensee of certain rights to GLA as a vaccine adjuvant. SGR is also an inventor on US patents and patent applications for LEISH-F3.
References
- 1.Chaves FA, Lee AH, Nayak JL, Richards KA, Sant AJ. The Utility and Limitations of Current Web-Available Algorithms To Predict Peptides Recognized by CD4 T Cells in Response to Pathogen Infection. Journal of Immunology. 2012;188:4235–48. doi: 10.4049/jimmunol.1103640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dow C, Oseroff C, Peters B, Nance-Sotelo C, Sidney J, Buchmeier M, et al. Lymphocytic Choriomeningitis Virus Infection Yields Overlapping CD4 and CD8 T-Cell Responses. Journal of Virology. 2008;82:11734–41. doi: 10.1128/JVI.00435-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mothé BR, Stewart BS, Oseroff C, Bui H-H, Stogiera S, Garcia Z, et al. Chronic Lymphocytic Choriomeningitis Virus Infection Actively Down-Regulates CD4+ T Cell Responses Directed against a Broad Range o f Epitopes. The Journal of Immunology. 2007;179:1058–67. doi: 10.4049/jimmunol.179.2.1058. [DOI] [PubMed] [Google Scholar]
- 4.Varga SM, Welsh RM. Cutting Edge: Detection of a High Frequency of Virus-Specific CD4 T Cells During Acute Infection with Lymphocytic Choriomeningitis Virus. The Journal of Immunology. 1998;161:3215–8. [PubMed] [Google Scholar]
- 5.Leroux-Roels G, Leroux-Roels I, Clement F, Ofori-Anyinam O, Lievens M, Jongert E, et al. Evaluation of the immune response to RTS,S/AS01 and RTS,S/AS02 adjuvanted vaccines: randomized, double-blind study in malaria-naive adults. Human vaccines & immunotherapeutics. 2014;10:2211–9. doi: 10.4161/hv.29375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu J, Yamane H, Paul WE. Differentiation of Effector CD4 T Cell Populations*. Annual Review of Immunology. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott DS, Varga SM. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. Journal of immunology (Baltimore, Md : 1950) 2011;187:5568–76. doi: 10.4049/jimmunol.1102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Bose TO, Pham QM, Jellison ER, Mouries J, Ballantyne CM, Lefrancois L. CD11a regulates effector CD8 T cell differentiation and central memory development in response to infection with Listeria monocytogenes. Infection and immunity. 2013;81:1140–51. doi: 10.1128/IAI.00749-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence CW, Braciale TJ. Activation, Differentiation, and Migration of Naive Virus-Specific CD8 T Cells during Pulmonary Influenza Virus Infection. The Journal of Immunology. 2004;173:1209–18. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 11.Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rotzschke O, et al. CD49d provides access to "untouched" human Foxp3+ Treg free of contaminating effector cells. Blood. 2009;113:827–36. doi: 10.1182/blood-2008-04-150524. [DOI] [PubMed] [Google Scholar]
- 12.Okumura M, Fujii Y, Inada K, Nakahara K, Matsuda H. Both CD45RA+ and CD45RA− subpopulations of CD8+ T cells contain cells with high levels of lymphocyte function-associated antigen-1 expression, a phenotype of primed T cells. The Journal of Immunology. 1993;150:429–37. [PubMed] [Google Scholar]
- 13.Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, et al. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Trans Immunol. 2015;4:e35. doi: 10.1038/cti.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desbien AL, Reed SJ, Bailor HR, Dubois Cauwelaert N, Laurance JD, Orr MT, et al. Squalene emulsion potentiates the adjuvant activity of the TLR4 agonist, GLA, via inflammatory caspases, IL-18, and IFN-gamma. European journal of immunology. 2015;45:407–17. doi: 10.1002/eji.201444543. [DOI] [PubMed] [Google Scholar]
- 15.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PloS one. 2011;6:16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a Recombinant Polyprotein Vaccine That Protects against Visceral Leishmaniasis by Elicitation of CD4+ T Cells. Infection and immunity. 2007;75:4648–54. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rai D, Pham N-LL, Harty JT, Badovinac VP. Tracking the Total CD8 T Cell Response to Infection Reveals Substantial Discordance in Magnitude and Kinetics between Inbred and Outbred Hosts. The Journal of Immunology. 2009;183:7672–81. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masopust D, Murali-Krishna K, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J Virol. 2007;81:2002–11. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knudson CJ, Weiss KA, Hartwig SM, Varga SM. The Pulmonary Localization of Virus-Specific T Lymphocytes Is Governed by the Tissue Tropism of Infection. Journal of Virology. 2014;88:9010–6. doi: 10.1128/JVI.00329-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartwig SM, Holman KM, Varga SM. Depletion of alveolar macrophages ameliorates virus-induced disease following a pulmonary coronavirus infection. PloS one. 2014;9:90720. doi: 10.1371/journal.pone.0090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, et al. Therapeutic PD-L1 and LAG-3 blockade rapidly clears established blood-stage Plasmodium infection. Nature Immunology. 2011;13:188–95. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh AK, Thirumalapura NR. Early Induction of Interleukin-10 Limits Antigen-Specific CD4 T Cell Expansion, Function, and Secondary Recall Responses during Persistent Phagosomal Infection. Infection and immunity. 2014;82:4092–103. doi: 10.1128/IAI.02101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt NW, Khanolkar A, Hancox L, Heusel JW, Harty JT. Perforin plays an unexpected role in regulating T-cell contraction during prolonged Listeria monocytogenes infection. European journal of immunology. 2012;42:1629–40. doi: 10.1002/eji.201141902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolz JC, Rai D, Badovinac VP, Harty JT. Division-linked generation of death-intermediates regulates the numerical stability of memory CD8 T cells. Proceedings of the National Academy of Sciences. 2012;109:6199–204. doi: 10.1073/pnas.1118868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitmire JK, Benning N, Whitton JL. Precursor Frequency, Nonlinear Proliferation, and Functional Maturation of Virus-Specific CD4+ T Cells. The Journal of Immunology. 2006;176:3028–36. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Day 0 prior to vaccination, whole blood leukocytes were stimulated with LEISH-F3 recombinant protein or left unstimulated (BFA), and cells were stained for flow cytometry. Representative contour plots depict cytokine production by CD11ahiCD49d+, CD11aloCD49d− and unstimulated CD4+ CD3+ T cells.
Day 182 post-vaccination (14 days after the final vaccine dose), whole blood leukocytes were stimulated PHA to show maximal responses of antigen-responsive T cells in the patients, or left unstimulated (BFA), and cells were stained for flow cytometry. Representative contour plots depict cytokine production by CD11ahiCD49d+, CD11aloCD49d− and unstimulated CD4+ CD3+ T cells.