Abstract
Objective
Little is known about how women with isolated GnRH deficiency cope with their condition. This study aimed to examine the health and informational needs of women with congenital hypogonadotropic hypogonadism (CHH) and evaluate if their experiences differ from women with more common forms of infertility.
Design
Cross-sectional, multiple methods study using web-based data collection to reach dispersed rare disease patients.
Methods
A community-based participatory research framework was employed to develop an online survey and collect quantitative and qualitative data. Adult women diagnosed with CHH who had received at least one year of hormonal treatment completed the Morisky Medication Adherence Scale, Revised Illness Perception Questionnaire and Zung Self-Rating Depression Scale. Information on health care experiences, treatment outcomes and patient-reported challenges were also collected.
Results
Women (n = 55) were often diagnosed late (20.7 ± 7.4, range: 10–48 years) and 16/20 patients receiving fertility treatment conceived. Poor adherence was frequently observed (34/55) while more than half (27/49) reported a gap in treatment exceeding a year. Low adherence correlated with depressive symptoms (r = 0.3, P > 0.05). Negative illness perceptions were pervasive and 30/55 exhibited some depressive symptoms – significantly greater than women with common female factor infertility (P < 0.01). Symptoms were underappreciated by providers as only 15 of 55 patients had discussions about psychological services. Women identified isolation, need for information and finding expert care as challenges to living with CHH.
Conclusions
Despite being a treatable form of female infertility, the presumable availability of treatment does not necessarily ensure adequate quality of life for women with isolated GnRH deficiency.
Keywords: female infertility, illness perceptions, Kallmann syndrome, medication adherence, patient-centered care, rare diseases
Introduction
Infertility affects ~10–15% of women globally and is a significant health concern (1). Sometimes referred to as the pilot light of reproduction, gonadotropin-releasing hormone (GnRH) secretion is essential for developing and maintaining the reproductive capacity (2). Acquired GnRH deficiency (i.e. hypothalamic amenorrhea) is a common cause of secondary amenorrhea that is reversible (3). More severe forms of GnRH deficiency, such as congenital hypogonadotropic hypogonadism (CHH), are much less common. However, CHH is responsive to hormonal therapy and is a treatable form of infertility that does not necessarily require invasive assisted reproduction techniques (4).
In its most severe form, CHH presents as a complete absence of puberty with undetectable serum gonadotropins and hypogonadal sex steroid levels. Clinical presentation is variable: some patients display partial puberty (5), there is a wide range of associated phenotypes (e.g. absent sense of smell, skeletal anomalies, mirror movements, renal agenesis) and cases of reversal have been reported (6). Similarly, genetic etiology is heterogeneous with more than 25 genes having been identified in relation to CHH and the genetic architecture can sometimes be complex as evidenced by oligogenicity (4). CHH is rare (1:4000–10,000) and there is a striking sexual discordance. Combining three large patient cohorts from the United States (n = 250) (7), United Kingdom (n = 215) (8) and France (n = 334) (9) reveals the male to female ratio to be 3.6–1. Yet, unlike many other rare disorders, effective treatments are available. Hormone replacement in the form of low-dose estradiol (titrated over time) is the standard treatment for younger hypogonadal women to induce secondary sexual characteristics and menses (4). Combined gonadotropin therapy or physiologic treatment with pulsatile GnRH are equally effective for inducing fertility in the vast majority of cases (10).
Compared to fertile counterparts, women with infertility have higher levels of stress, anxiety and depression, and all of which can erode the quality of life (11). However, little is known about the experiences of women with CHH and how they cope with their condition. There is a body of literature on quality of life issues in women with primary infertility (i.e. Turner syndrome, TS) (12). However, differences in terms of timing of diagnosis, phenotype and fertility potential preclude extending findings from women with a hypogonadotropic cause of infertility (TS) to those with a hypogonadotropic etiology (CHH). Notably, conducting research in rare disease patients is challenged by the fact that these patients are dispersed and difficult to reach. Therefore, the purpose of this study was to partner with patients and use web-based data collection to reach women with CHH to conduct a needs assessment to identify targets for developing more patient-centered approaches to care for these endocrine patients.
Subjects and methods
Design and subjects
This cross-sectional needs assessment employed multiple methods within a community-based participatory research framework (13). We engaged patient community leaders in developing content and validating the online survey as well as for recruitment. Patients were identified via patient-oriented social media sites (i.e. Facebook, Rareconnect.org) and the international network studying GnRH deficiency (COST Action BM1105, www.gnrhdeficiency.eu) over a 14-month period (October 2014–December 2015). CHH was defined as previously reported (5) and diagnosis was confirmed in a 40% random sample of respondents as described (14). Adult women (18+ years) with CHH who had been on hormonal treatment for at least one year were included in the analysis. The project was reviewed and approved by the local ethics committee, and all participants provided opt-in electronic consent.
Needs assessment survey
We co-constructed an online survey with patients including items on patient demographics, health literacy (15), medical history, health care interactions, sexuality as well as several validated questionnaires. The selection of questionnaires was based on their relevance, validity and widespread use that facilitate comparison with particular patient populations of interest (i.e. women with infertility and men with CHH and patients with rare endocrine disorders and chronic conditions). The Morisky Medication Adherence Scale (MMAS) is an 8-item instrument that assesses medication taking behavior to determine low, medium or high adherence (16, 17, 18). The Zung Self-Rating Depression Scale (SDS) is a widely used, validated 20-item instrument quantifying the severity of affective, somatic, psychomotor and psychological depressive symptoms (19, 20). The Illness Perception Questionnaire – Revised (IPQ-R) includes 38 statements to assess emotional and cognitive representations of illness spanning 7 dimensions: timeline acute/chronic (beliefs about the chronic nature of the condition), timeline cyclical (beliefs regarding the cyclical nature of the condition), consequences (negative consequences of the disease), personal control (perceived personal controllability of the disease), treatment control (perceived treatment controllability of the disease), emotional representations (the emotional responses generated by the illness) and illness coherence (personal understanding of the disease) (21). Additionally, patients had the opportunity to provide a free text response identifying what they perceive to be the most challenging aspects of living with CHH.
Reference populations
To provide context for the depressive symptoms in women with CHH, SDS scores were compared to: (a) men with CHH (n = 101) (22), (b) women seeking assisted fertility treatment (n = 872) (11) and (c) community-based rates in a healthy, non-psychiatric population (n = 292) who completed the SDS monthly over the course of one full year (23). Because there are no normative scores for the IPQ-R for the general population (i.e. healthy adults), comparisons were made to patients with acute or chronic pain (24), men with CHH (22) and patients with acromegaly (24) to provide a clinical context for these data. Age at CHH diagnosis was compared to a population-based sample for age of menarche (25).
Analysis
Descriptive statistics were used to present survey data. Comparisons between groups were performed using Student’s t-test or Mann–Whitney rank-sum test as appropriate. Categorical values were compared using chi-square test while Pearson product–moment correlations were performed to assess the associations between survey data. IPQ-R subscales were compared across the CHH and reference groups using ANOVA with Bonferroni post hoc correction for multiple comparisons. Z-scores were used to assess the differences in the proportion of patients exhibiting depressive symptoms compared to community base rates. Survey data were analyzed using PASW Statistics, version 17.0.2 (SPSS Inc., Chicago, IL). All data are presented as mean ± s.d. unless otherwise noted and a P ≤ 0.05 was considered statistically significant. Open-ended responses were analyzed using NVivo11 (QSR International PSY Ltd., Melbourne Australia). Deductive thematic analysis (deductive coding) was conducted by two independent investigators (SD and AD). Meaningful units were coded. These codes were sorted into categories and themes in an iterative process and consensus was achieved by discussion. The most frequent themes were given particular emphasis (26).
Results
The web-based survey was online for 14 months during which time 68 women responded. After removing incomplete survey responses and those not meeting inclusion criteria, 55 surveys were included for analysis. Given the rarity of women with CHH (7, 8, 9), it appears that the combination of community partnerships and social media recruitment were effective for reaching these dispersed patients (14). Patient sociodemographic characteristics are depicted in Table 1. The women ranged in age from 18 to 68 years (mean 35 ± 10, median 34), were well-educated (44/55, university or higher) with adequate health literacy and the majority of women were employed (41/55).
Table 1.
Sociodemographic information of women with CHH (n = 55).
| n (%) | |
|---|---|
| Age (years) | |
| 18–29 | 16 (29%) |
| 30–39 | 25 (46%) |
| 40–49 | 9 (16%) |
| 50–59 | 4 (7%) |
| 60+ | 1 (2%) |
| Education | |
| High school/vocational | 10 (18%) |
| University | 28 (51%) |
| Post-graduate | 16 (29%) |
| No response | 1 (2%) |
| Health literacy (25) | |
| Adequate literacy | 45/55 (82%) |
| Inadequate literacy | 10/55 (18%) |
| Employment | |
| Working full-time | 32 (58%) |
| Working part-time | 9 (16%) |
| Not working/unemployed | 8 (15%) |
| Retired | 1 (2%) |
| Student | 5 (9%) |
| Relationship status | |
| Married | 21 (38%) |
| In a relationship | 14 (25%) |
| Single | 9 (16%) |
| Never been in a relationship | 4 (7%) |
| Divorced | 7 (13%) |
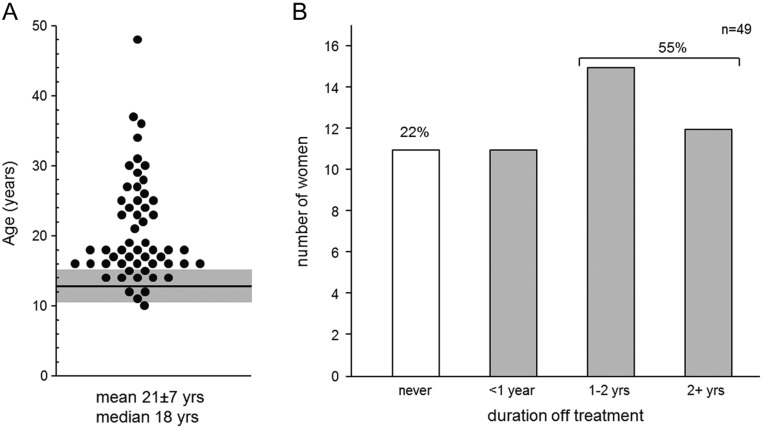
Given that menarche is an important single-event signpost of puberty and that 90% of CHH women present with primary amenorrhea (5), earlier diagnosis and treatment would be expected compared to male counterparts who lack such a hallmark. However, we found no such pattern (females: n = 55; 95% CI: 18.7–22.7, males: n = 101; 17.6–20.2 years; P = 0.16) (22). The women were diagnosed between 10 and 48 years of age (Fig. 1A), and more than half (n = 32) had received any meaningful treatment prior to age 18 years (95% CI: 17.2–20.6 years). Nearly two-thirds (34/55) of survey respondents had been seen at a specialized academic medical center (Table 2). Twenty women underwent fertility-inducing treatment, two-thirds (13/20) of whom at an academic medical center and the vast majority (16/20) successfully conceived, consistent with rates previously reported (27, 28), and in line with male CHH counterparts (95% CI: 60–85%) (29). In total, nearly half (25/55) the women had undergone genetic testing yet significantly fewer (11/55, P < 0.005) received genetic counseling. Among surveyed patients, women receiving fertility-inducing treatment were not more likely to have genetic testing (12/20, P = 0.10) or counseling (5/20, P = 0.48) compared to the larger group.
Figure 1.
Age at CHH diagnosis and self-reported adherence to treatment. (A) Age at CHH diagnosis for 55 women ranged from 10 to 48 years. The mean age at menarche for Caucasian females is shown as a horizontal line, and the shaded region depicts ± two s.d. (36). Only 11/55 women were diagnosed by age 15 years. (B) Self-reported adherence to treatment (n = 49). Approximately one-quarter of respondents reported never having a gap in treatment. In total, more than half (27/49) reported a gap in treatment of a year or longer. Similarly 20/46 women reported having a lapse in health care exceeding one year (data not shown). Age at diagnosis was moderately correlated with duration of gap in health care (r = 0.56, P < 0.001).
Table 2.
Health care experiences of women with CHH (n = 55).
| n (%) | |
|---|---|
| Medical history | |
| Seen at a specialized academic medical center | 34 (62%) |
| Genetic testing performed | 25 (45%) |
| Genetic counseling received | 11 (20%) |
| Sought psychological counseling | 16 (29%) |
| Health care interactions | |
| Provider understands medical aspects of CHH | 28 (51%) |
| Provider understands patient’s feelings of living with CHH | 14 (25%) |
| Provider discussed or gave referral for counseling | 15 (27%) |
| Experienced discrimination in the health care system | 15 (27%) |
| Treatment and adherence | |
| Duration of treatment: mean ± s.d. (range, median) | 16 ± 10 years (1–42, 17) |
| MMAS low adherence | 34 (62%) |
| Medium adherence | 12 (22%) |
| High adherence | 9 (16%) |
| Fertility outcomes | |
| Received fertility-inducing treatment | 20/55 (36%) |
| Biologic children | 16/20 (80%) |
MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E. Morisky, ScD, ScM, MSPH, Professor, 294 Lindura Ct., Las Vegas, NV 89138-4632.
All respondents included in the analysis had been on treatment for at least one year. Women completed the MMAS to assess adherence behavior (Table 2) and provided self-reported longest duration off treatment (Fig. 1B). Notably, MMAS scores indicated low adherence in nearly two-thirds (34/55) of women. Lifetime duration of treatment was weakly correlated with MMAS (r = 0.29, P < 0.05). Only 11/49 reported never having a lapse in treatment – a proportion similar to those exhibiting the highest level of adherence on the MMAS (Table 1). However, more than half (27/49) reported long gaps in treatment (i.e. 12 months or longer). Similarly, 18/43 claimed to have gone 2 years or longer without seeing a health care provider (data not shown).
Across IPQ-R dimensions, women and men had comparable perceptions of their CHH with scores indicating significant emotional impact and negative consequences (22) (Table 3). These findings extend the findings of a small qualitative study including interviews with 5 women with CHH (30). Women with CHH perceived more negative consequences of their illness compared to both patients with acute pain and patients with acromegaly (both P < 0.01) yet less than patients with chronic pain. The negative emotional impact of CHH was larger than that in patients with acromegaly (P < 0.01) but not different from patients with acute or chronic pain. Both negative consequences and emotional impact were modestly correlated with poorer medication adherence (r = 0.298 and r = 0.33 respectively, both P < 0.05). Furthermore, females with CHH perceived less personal control over their illness than patients with acute pain (P < 0.01), but did not differ from patients with chronic pain or acromegaly. In terms of treatment control, females with CHH perceived less treatment control than patients with acute pain or acromegaly (both P < 0.01), but more treatment control than patients with chronic pain (P = 0.01). Lastly, women with CHH exhibited the highest score on illness coherence compared to patients with acute or chronic pain (both P < 0.01). This better understanding of their illness could be related to the congenital nature of CHH.
Table 3.
Comparison of IPQ-R scores between female CHH patients and other patient groups.
| IPQ-R | CHH (women) (n = 55) | CHH (men) (n = 101) | acute pain (n = 35) | chronic pain (n = 63) | Acromegaly (n = 81) |
|---|---|---|---|---|---|
| Timeline (acute/chronic) | 27.2 (3.6) | 26.7 (3) | 13.4 (5)** | 23.1 (4)** | 22.9 (6)** |
| Timeline (cyclical) | 9.2 (3.9) | 9.7 (4) | 9.4 (3) | 12.9 (4)** | 10.1 (4) |
| Consequences | 20.0 (5.1) | 21.3 (4) | 14.2 (4)** | 23.5 (4)** | 16.9 (5)** |
| Emotional representations | 17.8 (6.2) | 19.2 (6) | 16.1 (4) | 19.8 (4) | 12.6 (4)** |
| Personal control | 19.6 (4.9) | 19.9 (5) | 22.9 (4)** | 18.4 (4) | 17.5 (5) |
| Treatment control | 16.1 (3.3) | 15.5 (4) | 19.4 (3)** | 14.2 (3)* | 18.1 (3)** |
| Illness coherence | 16.5 (4.7) | 18.1 (4) | 9.3 (3)** | 13.4 (5)** | 17.5 (3) |
Data are mean (s.d.), *P < 0.05 compared with CHH (women), **P < 0.01 compared with CHH (women).
More than half the women (30/55) exhibited some depressive symptoms. This is significantly increased compared to the 9% observed in a community dwelling non-psychiatric population (P < 0.001) (23) yet similar to their male CHH counterparts (64/100, P = N.S.) (22). Compared to those presenting with more common female infertility (14.7% of 872 women) (11), women with CHH were more likely to exhibit depressive symptoms (P < 0.01), and this relationship persisted when we limited the analysis to only those patients (25.6% of 193 women) with female factor infertility (P < 0.01). In total, 14/55 women with CHH exhibited mild depressive symptoms, 9/55 had moderated symptoms similar to the type of depression treated in an ambulatory setting while 7/55 had severe depressive symptoms akin to major depressive disorder. We found depressive symptoms were moderately correlated with poorer medication adherence (r = 0.3, P < 0.05) consistent with prior studies in patients with chronic diseases (31). Depressive symptoms were also correlated with illness perception dimensions. We observed a strong correlation between depressive symptoms and negative emotional impact of CHH (r = 0.6, P < 0.0001), a moderate relationship with consequences (r = 0.42, P < 0.01) and a weak association with illness coherence – how one makes sense of their condition (r = 0.35, P < 0.01).
Importantly, the increased depressive symptoms appear to be underappreciated as only 15 women stated that their provider had discussed or provided a referral for psychological counseling. Half the patients (28/55) perceived that their health care provider well understood the medical aspects of their GnRH deficiency yet significantly fewer felt that their provider understood how patients feel about living with CHH (14/55, P < 0.01). Women with CHH were more likely than their male counterparts to have been in a relationship and sexually active (both, P < 0.05) (32). Despite this, nearly all women (51/55) cited issues of body image concerns, with 44/55 reporting feelings of shame or embarrassment about their body and over half (32/55) found intimate relationships difficult and had experienced teasing or ridicule about their lack of development (31/55).
Qualitative data were also collected as part of the survey. Patients were asked to describe the most challenging aspect of living with CHH and approximately two-thirds (36/55) responded. Responses were coded and the 61 topics thematically clustered in three categories: (i) isolation and insecurity (n = 24), (ii) need for information and support (n = 24) and (iii) delayed diagnosis and finding expert care (n = 13). A table with representative quotes is provided in the online supplemental file (see section on supplementary data given at the end of this article). We also asked patients where they sought information about their condition. Nearly all women (52/55) reported that they found information about their condition on the internet, 46/55 via online community and social media (i.e. Facebook, Rareconnect.org) and 44/55 were informed by health care professionals. Despite high education and health literacy levels, approximately half (27/55) sought information from the medical literature. Patients rated health care professionals as the most important source of information followed by online community and the Internet. Ratings of importance were not statistically significant between these sources (P = 0.13).
Discussion
We found that women with CHH are often diagnosed late and experience significant physical, social and psychological consequences in relation to their condition. These findings have implications for developing more patient-centered approaches to care for these women. This cross-sectional study of 55 women is perhaps the most robust portrayal of this patient population to date. Data on women with CHH are scant with only a handful of single-center studies reporting on very small samples (i.e. n < 15). Additionally, patients were involved in developing the study – this strengthens our confidence that the identified targets for improving care are aspects that matter to patients.
Some aspects of this study may limit the ultimate transferability of the findings to all women with CHH. First, these patients were well educated with high levels of health literacy. Second, the inherent Anglophone bias should engender caution in considering cultural equivalence. Third, given the sample size, we may be underpowered in some of our analyses. Indeed, difficulty in recruiting adequate numbers of patients with rare diseases is widely acknowledged. This challenge informed our strategy of using the Internet and patient-oriented social media to reach these dispersed patients, which we acknowledge as a potential source of sampling bias. Finally, self-report nature of the instruments employed has its own limitations, albeit our goal was to better understand patient perspectives to identify the unmet needs as an initial step in developing more patient-centered approaches to care.
Data on the age of diagnosis in CHH women comprise a single article (n = 5; mean: 23 ± 9, range: 12–35, median 21 years) (30) along with some unpublished historic data (females: n = 38; mean: 18.2 ± 5, range: 10–53 years) (33). We found women with CHH are often diagnosed quite late and only 58% had received treatment prior to age 18 years. It is well established that later induction of puberty for adolescent girls with TS is associated with poor self-esteem, difficult social adjustment and diminished sex life (34, 35). Further, late diagnosis (and initiation of treatment) negatively impacts psychosexual development in men with CHH (32). Thus, greater attention to earlier detection and timely initiation of sex steroid therapy seems warranted.
The vast majority (16 of 20) of women who underwent fertility treatment were able to conceive, in line with published literature (27, 28). Notably, relatively few (11/55) women had received genetic counseling. This is surprising given that the American College of Medical Genetics considers abnormal pubertal timing a clinical presentation requiring referral to a medical genetics professional (i.e. genetic counselor) (36). Further, both the European Society of Human Genetics and the European Society of Human Reproduction and Embryology consider genetic counseling necessary when genetic factors are related to the cause of infertility (37). However, in our cohort, women receiving fertility-inducing treatment were no more likely to have genetic testing or counseling. These data suggest either lack of awareness of the importance of genetic counseling for these patients or may reflect inadequate access to specialists with sufficient understanding of the sometimes complex genetics of CHH (38). Improved access to genetic counseling appears to be a relevant target for enhancing patient care.
Long-term adherence to treatment was problematic as more than half of women had a gap in care of a year or longer. Without treatment, these women rapidly become hypogonadal, with deleterious impact on mood, well-being, sex life and bone health. A Finnish cohort of 24 men and 9 women found patients with the longest gaps in treatment exhibited the most impaired bone density (39). Additionally, risk for osteopenia/osteoporosis may be further compounded by late diagnosis, as up to 90% of adult bone mass is accumulated during adolescence (40). Delays in diagnosis (and treatment initiation) could prolong estrogen deficiency thus impairing bone mineralization. These data highlight the importance of adequate hormone replacement and ongoing follow-up to monitor bone density and adherence as well as the role for a coordinated transition to adult services to facilitate continuity of care (4, 39, 41).
Women had negative illness perceptions yet exhibited relatively high scores on illness coherence. Interestingly, CHH women who had received fertility treatment (and were able to conceive) had significantly lower ratings of negative consequences (P < 0.05) and emotional impact (P < 0.05) compared to those without children. While the sample is small, this may represent a psychological buffering effect of successful fertility treatment. We did not employ a formal health-related quality of life (HR-QoL) instrument in this study; yet, the negative illness perceptions and increased depressive symptoms observed in women with CHH are consistent with impaired HR-QoL. A recent study of women with TS showed impaired HR-QoL compared to controls (35). Interestingly, the study included 21 women with other types of congenital hypogonadism (14 with CHH, 7 with 46XX gonadal dysgenesis) whose SF-36 scores were quite similar. A subset of these patients completed the Female Sexual Function Index revealing impaired sexual desire, arousal, lubrication, orgasm and global satisfaction compared to controls (35). Similarly in our cohort, body image concerns were pervasive and the majority of women stated that intimate relationships were difficult. A recent study of young adults with CHH during transition found that few young women felt adequately informed about sexuality (2/7), intimate relationships (1/7), potential future fertility (1/7) or intercourse/potential discomfort with sex (0/7) (42). These current data underscore the need for appropriate anticipatory guidance on the topics of sexuality and intimate relationships.
The qualitative data analysis suggests that women struggle with feelings of isolation and finding expert care. They frequently reported having used the Internet and social media to learn about their condition and to find support from other patients (14). The Pew Foundation identified patients with a rare disease as ‘Internet power-users’ (43), which aligns with our findings. We have previously shown that Web-based modalities are used by and acceptable to patients with CHH (14). This may present a novel avenue for reaching these dispersed patients, engaging them and promoting health and self-management. The Institute of Medicine defines patient-centered care as being guided by patient values and is both respectful of, and responsive to individual preferences, needs and values (44). The Picker Principles of patient-centered care (45) provide a useful framework for developing more patient-centered approaches to care. Accordingly, we have charted the findings from the present study onto this framework along with suggested avenues for translating the results of this study into improved care for women with CHH (see supplemental file).
In summary, this participatory, multiple methods needs assessment identifies that women with CHH frequently have lengthy gaps in treatment/care, perceive their condition to have a significant psychosocial impact on their life and exhibit increased depressive symptoms. Care for these women could be improved by earlier diagnosis and timely initiation of treatment, greater access to genetic counseling and providing accurate information about CHH (and fertility treatment) as well as offering professional and peer-to-peer psychological support. The Internet is effective for reaching and connecting dispersed patients, and Web-based platforms may hold promise for delivering patient-centered interventions to empower patients for improved self-management and adherence.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
A A D, R Q, N P and D M conceived and designed the study. A A D collected the data. S D, J T and A A D analyzed the data and drafted the manuscript. R Q, N P and D M critically revised the manuscript. All authors approved the final manuscript version.
Acknowledgements
The authors wish to thank the patients for their generous participation and acknowledge Neil Smith and the other patient community leaders who contributed to this work. Use of the MMAS is protected by US copyright laws. Permission for use is required. A license agreement is available from: Donald E Morisky, ScD, ScM, MSPH, Prof., 294 Lindura Ct., Las Vegas, NV 89138-4632. This project was registered at www.clinicaltrials.gov (Nbib1914172).
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLOS Medicine 2012. 9 e1001356 ( 10.1371/journal.pmed.1001356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramanian R, Crowley WF., Jr. Isolated GnRH deficiency: a disease model serving as a unique prism into the systems biology of the GnRH neuronal network. Molecular and Cellular Endocrinology 2011. 346 4–12. ( 10.1016/j.mce.2011.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caronia LM, Martin C, Welt CK, Sykiotis GP, Quinton R, Thambundit A, Avbelj M, Dhruvakumar S, Plummer L, Hughes VA, et al. A genetic basis for functional hypothalamic amenorrhea. New England Journal of Medicine 2011. 364 215–225. ( 10.1056/NEJMoa0911064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehm U, Bouloux PM, Dattani MT, de Roux N, Dode C, Dunkel L, Dwyer AA, Giacobini P, Hardelin JP, Juul A, et al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism-pathogenesis, diagnosis and treatment. Nature Reviews Endocrinology 2015. 11 547–564. ( 10.1038/nrendo.2015.112) [DOI] [PubMed] [Google Scholar]
- 5.Shaw ND, Seminara SB, Welt CK, Au MG, Plummer L, Hughes VA, Dwyer AA, Martin KA, Quinton R, Mericq V, et al. Expanding the phenotype and genotype of female GnRH deficiency. Journal of Clinical Endocrinology and Metabolism 2011. 96 E566–E576. ( 10.1210/jc.2010-2292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dwyer AA, Raivio T, Pitteloud N. Management of endocrine disease: reversible hypogonadotropic hypogonadism. European Journal of Endocrinology 2016. 174 R267–R274. ( 10.1530/EJE-15-1033) [DOI] [PubMed] [Google Scholar]
- 7.Seminara SB, Hayes FJ, Crowley WF., Jr. Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): pathophysiological and genetic considerations. Endocrine Reviews 1998. 19 521–539. [DOI] [PubMed] [Google Scholar]
- 8.Quinton R, Duke VM, Robertson A, Kirk JM, Matfin G, de Zoysa PA, Azcona C, MacColl GS, Jacobs HS, Conway GS, et al. Idiopathic gonadotrophin deficiency: genetic questions addressed through phenotypic characterization. Clinical Endocrinology 2001. 55 163–174. ( 10.1046/j.1365-2265.2001.01277.x) [DOI] [PubMed] [Google Scholar]
- 9.Bry-Gauillard H, Trabado S, Bouligand J, Sarfati J, Francou B, Salenave S, Chanson P, Brailly-Tabard S, Guiochon-Mantel A, Young J. Congenital hypogonadotropic hypogonadism in females: clinical spectrum, evaluation and genetics. Annales d'Endocrinologie 2010. 71 158–162. ( 10.1016/j.ando.2010.02.024) [DOI] [PubMed] [Google Scholar]
- 10.Martin KA, Hall JE, Adams JM, Crowley WF., Jr. Comparison of exogenous gonadotropins and pulsatile gonadotropin-releasing hormone for induction of ovulation in hypogonadotropic amenorrhea. Journal of Clinical Endocrinology and Metabolism 1993. 77 125–129. [DOI] [PubMed] [Google Scholar]
- 11.Chiaffarino F, Baldini MP, Scarduelli C, Bommarito F, Ambrosio S, D’Orsi C, Torretta R, Bonizzoni M, Ragni G. Prevalence and incidence of depressive and anxious symptoms in couples undergoing assisted reproductive treatment in an Italian infertility department. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011. 158 235–241. ( 10.1016/j.ejogrb.2011.04.032) [DOI] [PubMed] [Google Scholar]
- 12.Garrido Oyarzun MF, Castelo-Branco C. Sexuality and quality of life in congenital hypogonadisms. Gynecological Endocrinology 2016. 32 947–950. ( 10.1080/09513590.2016.1241229) [DOI] [PubMed] [Google Scholar]
- 13.Wallerstein N, Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. American Public Health 2010. 100 (Supplement 1) S40–S46. ( 10.2105/AJPH.2009.184036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwyer AA, Quinton R, Morin D, Pitteloud N. Identifying the unmet health needs of patients with congenital hypogonadotropic hypogonadism using a web-based needs assessment: implications for online interventions and peer-to-peer support. Orphanet Journal of Rare Diseases 2014. 9 83 ( 10.1186/1750-1172-9-83) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, Bradley KA, Nugent SM, Baines AD, Vanryn M. Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of General Internal Medicine 2008. 23 561–566. ( 10.1007/s11606-008-0520-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. American Journal of Managed Care 2009. 15 59–66. [PMC free article] [PubMed] [Google Scholar]
- 17.Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. Journal of Clinical Epidemiology 2011. 64 255–257. ( 10.1016/j.jclinepi.2010.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of Clinical Hypertension 2008. 10 348–354. ( 10.1111/j.1751-7176.2008.07572.x) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Zung WW. A self-rating depression scale. Archives of General Psychiatry 1965. 12 63–70. ( 10.1001/archpsyc.1965.01720310065008) [DOI] [PubMed] [Google Scholar]
- 20.Zung WW. The role of rating scales in the identification and management of the depressed patient in the primary care setting. Journal of Clinical Psychiatry 1990. 51 (Supplement) 72–76. [PubMed] [Google Scholar]
- 21.Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, Buick D. The revised illness perception quesionnaire (IPQ-R). Psychology and Health 2002. 17 1–16. ( 10.1080/08870440290001494) [DOI] [Google Scholar]
- 22.Dwyer AA, Tiemensma J, Quinton R, Pitteloud N, Morin D. Adherence to treatment in men with hypogonadotrophic hypogonadism. Clinical Endocrinology 2017. 86 377–383. ( 10.1111/cen.13236) [DOI] [PubMed] [Google Scholar]
- 23.Barrett J, Hurst MW, DiScala C, Rose RM. Prevalence of depression over a 12-month period in a nonpatient population. Archives of General Psychiatry 1978. 35 741–744. ( 10.1001/archpsyc.1978.01770300083009) [DOI] [PubMed] [Google Scholar]
- 24.Tiemensma J, Kaptein AA, Pereira AM, Smit JW, Romijn JA, Biermasz NR. Affected illness perceptions and the association with impaired quality of life in patients with long-term remission of acromegaly. Journal of Clinical Endocrinology and Metabolism 2011. 96 3550–3558. ( 10.1210/jc.2011-1645) [DOI] [PubMed] [Google Scholar]
- 25.Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatric Research in Office Settings network. Pediatrics 1997. 99 505–512. ( 10.1542/peds.99.4.505) [DOI] [PubMed] [Google Scholar]
- 26.Saldana J. Coding Manual for Qualitative Researchers. Thousand Oaks, CA, USA: Sage, 2009. [Google Scholar]
- 27.Filicori M, Flamigni C, Dellai P, Cognigni G, Michelacci L, Arnone R, Sambataro M, Falbo A. Treatment of anovulation with pulsatile gonadotropin-releasing hormone: prognostic factors and clinical results in 600 cycles. Journal of Clinical Endocrinology and Metabolism 1994. 79 1215–1220. [DOI] [PubMed] [Google Scholar]
- 28.Gronier H, Peigne M, Catteau-Jonard S, Dewailly D, Robin G. Ovulation induction by pulsatile GnRH therapy in 2014: literature review and synthesis of current practice. Gynécologie Obstétrique & Fertilité 2014. 42 732–740. ( 10.1016/j.gyobfe.2014.07.017) [DOI] [PubMed] [Google Scholar]
- 29.Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology 2014. 2 794–808. ( 10.1111/andr.262) [DOI] [PubMed] [Google Scholar]
- 30.Hofmann J, Watzlawik M, Richter-Appelt H. Living with Kallmann syndrome – analysis of subjective experience reports from women. Geburtshilfe Frauenheilkd 2013. 73 1112–1120. ( 10.1055/s-0033-1350881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine 2000. 160 2101–2107. ( 10.1001/archinte.160.14.2101) [DOI] [PubMed] [Google Scholar]
- 32.Dwyer AA, Quinton R, Pitteloud N, Morin D. Psychosexual development in men with congenital hypogonadotropic hypogonadism on long-term treatment: a mixed methods study. Sexual Medicine 2015. 3 32–41. ( 10.1002/sm2.50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinton R. Phenotypic Aspects of Kallmann Syndrome. Cambridge, UK: University of Cambridge, 2001. [Google Scholar]
- 34.Carel JC, Elie C, Ecosse E, Tauber M, Leger J, Cabrol S, Nicolino M, Brauner R, Chaussain JL, Coste J. Self-esteem and social adjustment in young women with Turner syndrome – influence of pubertal management and sexuality: population-based cohort study. Journal of Clinical Endocrinology and Metabolism 2006. 91 2972–2979. ( 10.1210/jc.2005-2652) [DOI] [PubMed] [Google Scholar]
- 35.Ros C, Alobid I, Balasch J, Mullol J, Castelo-Branco C. Turner’s syndrome and other forms of congenital hypogonadism impair quality of life and sexual function. American Journal of Obstetrics and Gynecology 2013. 208 e481–e486. ( 10.1016/j.ajog.2012.10.876) [DOI] [PubMed] [Google Scholar]
- 36.Pletcher BA, Toriello HV, Noblin SJ, Seaver LH, Driscoll DA, Bennett RL, Gross SJ. Indications for genetic referral: a guide for healthcare providers. Genetics in Medicine 2007. 9 385–389. ( 10.1097/GIM.0b013e318064e70c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harper J, Geraedts J, Borry P, Cornel MC, Dondorp WJ, Gianaroli L, Harton G, Milachich T, Kaariainen H, Liebaers I, et al. Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. Human Reproduction 2014. 29 1603–1609. ( 10.1093/humrep/deu130) [DOI] [PubMed] [Google Scholar]
- 38.Au MG, Crowley WF, Jr, Buck CL. Genetic counseling for isolated GnRH deficiency. Molecular and Cellular Endocrinology 2011. 346 102–109. ( 10.1016/j.mce.2011.05.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laitinen EM, Hero M, Vaaralahti K, Tommiska J, Raivio T. Bone mineral density, body composition and bone turnover in patients with congenital hypogonadotropic hypogonadism. International Journal of Andrology 2012. 35 534–540. ( 10.1111/j.1365-2605.2011.01237.x) [DOI] [PubMed] [Google Scholar]
- 40.Divasta AD, Gordon CM. Hormone replacement therapy and the adolescent. Current Opinion in Obstetrics and Gynecology 2010. 22 363–368. ( 10.1097/GCO.0b013e32833e4a35) [DOI] [PubMed] [Google Scholar]
- 41.Dwyer AA, Phan-Hug F, Hauschild M, Elowe-Gruau E, Pitteloud N. Transition in endocrinology: hypogonadism in adolescence. European Journal of Endocrinology 2015. 173 R15–R24. ( 10.1530/EJE-14-0947) [DOI] [PubMed] [Google Scholar]
- 42.Godbout A, Tejedor I, Malivoir S, Polak M, Touraine P. Transition from pediatric to adult healthcare: assessment of specific needs of patients with chronic endocrine conditions. Hormone Research in Paediatrics 2012. 78 247–255. ( 10.1159/000343818) [DOI] [PubMed] [Google Scholar]
- 43.Fox S. Peer-to-peer healthcare: many people – especially those living with chronic or rare diseases – use online connections to supplement professional medical advice. In Pew Internet and Americal Life Project, p 26 Washington DC, USA: Pew Research Center, 2011. [Google Scholar]
- 44.Committee on Quality Health Care in America, Institute of Medicine. Crossing The Quality Chasm: a New Health System for the 21st Century. Washington DC, USA: National Academy Press, 2001. [Google Scholar]
- 45.Frampton S, Guastello S, Brady C, Hale M, Horowitz S, Bennett-Smith S, Stone S. Picker Institute Patient-centered Care Improvement Guide. Derby, UK: CT Planetree, Inc., 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a