Abstract
BACKGROUND
Blacks have higher coronary heart disease (CHD) mortality compared with whites. However, a previous study suggests that nonfatal CHD risk may be lower for black versus white men.
METHODS
We compared fatal and nonfatal CHD incidence, and CHD case-fatality among blacks and whites in the Atherosclerosis Risk In Communities (ARIC), Cardiovascular Health Study (CHS), and REasons for Geographic And Racial Differences in Stroke (REGARDS) study, by gender. Participants 45–64 years of age in ARIC (men=6,479, women=8,488) and REGARDS (men=5,296, women=7,822), and ≥65 years of age in CHS (men=1,836, women=2,790) and REGARDS (men=3,381, women=4,112), all without a history of CHD, were analyzed. Fatal and nonfatal CHD incidence was assessed from baseline (ARIC=1987–1989, CHS=1989–1990, REGARDS=2003–2007) through up to 11 years of follow-up.
RESULTS
Age-adjusted hazard ratios (HR) comparing black versus white men 45–64 years of age in ARIC and REGARDS were 2.09 (95%CI 1.42–3.06) and 2.11 (1.32–3.38), respectively for fatal CHD, and 0.82 (0.64–1.05) and 0.94 (0.69–1.28), respectively for nonfatal CHD. After adjustment for social determinants of health and cardiovascular risk factors, HRs in ARIC and REGARDS were 1.19 (95%CI 0.74–1.92) and 1.09 (0.62–1.93), respectively for fatal CHD, and 0.64 (0.47–0.86) and 0.67 (0.48–0.95), respectively for nonfatal CHD. Similar patterns were present among men ≥65 years of age in CHS and REGARDS. Among women 45–64 years of age in ARIC and REGARDS, age-adjusted HRs comparing blacks versus whites were 2.61 (95%CI 1.57–4.34) and 1.79 (1.06–3.03), respectively for fatal CHD, and 1.47 (1.13–1.91) and 1.29 (0.91–1.83), respectively for nonfatal CHD. After multivariable adjustment, HRs in ARIC and REGARDS were 0.67 (95%CI 0.36–1.24) and 1.00 (0.54–1.85), respectively for fatal CHD, and 0.70 (0.51–0.97) and 0.70 (0.46–1.06), respectively for nonfatal CHD. Racial differences in CHD incidence were attenuated among older women. CHD case-fatality was higher among black versus white men and women, and the difference remained similar after multivariable adjustment.
CONCLUSIONS
After accounting for social determinants of health and risk factors, black men and women have similar risk for fatal CHD compared with white men and women, respectively. However, the risk for nonfatal CHD is consistently lower for black versus white men and women.
Keywords: Coronary heart disease, Continental Population Groups, Mortality, Survival
Introduction
Blacks have higher coronary heart disease (CHD) mortality compared with whites.1–4 However, black-white differences in CHD incidence have been less well investigated. A prior analysis of participants ≥45 years of age from the nationwide REasons for Geographic And Racial Differences in Stroke (REGARDS) study showed that black men have twice the risk for incident fatal CHD compared with white men, but lower nonfatal CHD incidence.5 In contrast, incidence rates for fatal and nonfatal CHD were consistently higher among black versus white women.
The origin of the lower incidence of nonfatal CHD among black versus white men is unclear. The prior analysis of the REGARDS cohort only provided up to 7 years of follow-up.5 Also, the REGARDS study uses participant self-report without active surveillance to identify nonfatal CHD events, which may result in some events not being detected.
We compared the incidence of fatal, nonfatal, and total CHD and CHD case-fatality among black versus white men and women in REGARDS with up to 11 years of follow-up. The extended follow-up provided a larger number of events to investigate racial differences in CHD incidence and case-fatality by gender. We also conducted similar analyses in the Atherosclerosis Risk In Communities (ARIC) study, and the Cardiovascular Health Study (CHS). ARIC and CHS included active surveillance and serial electrocardiograms to detect CHD events that might be missed through participant self-report. We also repeated analyses in REGARDS using Medicare claims (i.e., administrative data collected for reimbursement) to identify CHD events which were not detected through study procedures. Analyses in ARIC and CHS, and using Medicare claims in REGARDS, present little threat for spurious findings due to differential reporting of events by race.
Methods
Study populations
The ARIC study enrolled 15,792 black and non-black participants 45–64 years of age in 1987–1989 from Forsyth County NC, Jackson MS, suburbs of Minneapolis MN, and Washington County MD.6 CHS enrolled 5,201 participants ≥65 years of age in 1989–1990 from the Health Care Financing Administration’s Medicare eligibility list in Forsyth County NC, Sacramento County CA, Washington County MD, and Pittsburgh PA.7,8 An additional group of 687 blacks were enrolled in 1992–1993 from counties in NC, CA and PA.9 The REGARDS study enrolled 30,239 blacks and whites ≥45 years of age from all 48 contiguous US states and the District of Columbia in 2003–2007.10 Data from REGARDS study participants were linked with Medicare claims using social security number with linkages confirmed by birthdate and gender.11
We used ARIC and CHS publicly available datasets, which exclude 60 and 93 participants, respectively, who did not allow their data to be released.12 The REGARDS dataset excludes 56 participants because of anomalies in their informed consent. We further excluded 39 CHS participants who were non-black/non-white. As reported elsewhere, very few ARIC participants (<1%) were from a race/ethnicity other than black or white.13 Whites and non-black/non-white participants are defined as non-black in the ARIC publicly available dataset, preventing their identification. For the current analysis, all non-black participants in ARIC were included as whites. REGARDS study participants ≥65 years without Medicare Part A (hospitalization insurance) fee-for-service coverage at baseline were excluded. Medicare provides insurance coverage for US adults ≥65 years of age, or with disability or end-stage renal disease. Therefore, we did not require Medicare coverage for REGARDS participants <65 years of age as this represent a select population. Finally, we excluded ARIC, CHS and REGARDS participants with a baseline history of CHD, as defined below, and those without follow-up for incident CHD. After these criteria were applied, 14,967 ARIC participants, 13,118 REGARDS participants 45–64 years of age, 4,626 CHS participants and 7,493 REGARDS participants ≥65 years of age were included in the analyses (Supplemental figures 1–2).
ARIC and CHS analyses were approved by the Institutional Review Board (IRB) at the University of Alabama at Birmingham.14 The REGARDS study was approved by the IRBs governing research in human subjects at the participating centers and all participants provided written informed consent.10
Baseline assessment
Methods for baseline assessment in ARIC,6 CHS,15,16 and REGARDS10,17 have been described elsewhere. In brief, an in-home interview and an in-clinic examination were conducted at baseline in ARIC and CHS. In REGARDS, a telephone interview and an in-home examination were conducted. Self-reported information collected in each study interview included age, race, gender, education, annual household income, alcohol consumption, physical activity, current smoking, history of diabetes, atrial fibrillation (except in ARIC), CHD and stroke, medication use including antihypertensive medication, and health insurance. We defined having no alcohol consumption in ARIC, CHS and REGARDS as reporting 0 drinks per week, moderate alcohol consumption as >0 to 7 drinks per week for women and >0 to 14 drinks per week for men, and heavy alcohol consumption as >7 drinks per week for women and >14 drinks per week for men. In ARIC, low physical activity was defined by self-reporting not engaging in any exercise or sport. In CHS, participants were asked if they participated in any of 15 leisure-time activities over the past two weeks and about their usual pace of walking when outside home.16 For this analysis, CHS participants were defined as having low physical activity if they reported not participating in any of the leisure-time activities and walking for exercise at a casual or strolling pace (<2 mph or <3.2 kmph). Low physical activity in REGARDS was defined by self-reporting not engaging in any weekly activity intense enough to work up a sweat. In ARIC, current smoking was defined as having smoked more than 400 cigarettes in lifetime and currently smoking cigarettes. In CHS, current smoking was defined as having smoked more than 100 cigarettes in lifetime and having smoked in the past 30 days.18 In REGARDS, current smoking was defined as having smoked more than 100 cigarettes in lifetime and currently smoking cigarettes, even occasionally.
During examinations, health professionals measured participants’ waist circumference, performed blood pressure measurements which were averaged, obtained blood samples and an electrocardiogram, and conducted a medication inventory. History of CHD was defined by a self-report of a prior myocardial infarction (MI), coronary artery bypass or coronary angioplasty during the study interview, or evidence of a previous MI on the study electrocardiogram. History of CHD in CHS also included a self-reported history of angina and was confirmed by medical records and medication review.15,19 Total and high-density lipoprotein (HDL) cholesterol, glucose and creatinine were measured using blood samples. We defined diabetes by a fasting glucose ≥126 mg/dL, a non-fasting glucose ≥200 mg/dL, or self-report of a prior diagnosis with current antidiabetes medication use.20 Creatinine in ARIC and CHS was calibrated as described elsewhere.21 Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation based on creatinine,22 with eGFR <60 ml/min/1.73 m2 defined as reduced. Left ventricular hypertrophy (LVH) was defined using the study electrocardiogram and the Cornell definition in ARIC and CHS. In REGARDS, LVH was defined using the Cornell definition for participants with a 12-lead electrocardiogram and a modified Cornell definition for about a third of participants with a 7-lead electrocardiogram.17 Atrial fibrillation was defined using the study electrocardiogram or self-report in CHS and REGARDS. As described elsewhere, very few ARIC participants (n=37) had atrial fibrillation on their baseline study electrocardiogram.23 These data were not included in the ARIC publicly available dataset and were not analyzed.
Follow-up assessment
Participants or their proxies were contacted once a year in ARIC and twice a year in CHS and REGARDS to identify CHD-related hospitalizations and to confirm vital status.5,6,8 CHD-related hospitalizations and deaths were also detected by active surveillance through field center investigations in ARIC and CHS.6,24–26 The National Death Index and online sources (e.g., Social Security Death Index) were used for death detection in REGARDS.5 CHD-related hospitalizations and cause of death were adjudicated by experts in ARIC, CHS and REGARDS following similar approaches.5,6,25
For the main analysis, the primary definition of fatal CHD includes a definite or probable fatal MI (i.e., expert-adjudicated definite or probable MI followed by death within 28 days) or CHD death (i.e., death from MI not meeting the criteria for definite or probable, or sudden death preceded by cardiac symptoms or signs without evidence of non-coronary causes [e.g., stroke]).27 The primary definition of nonfatal CHD includes a definite or probable nonfatal MI. Among REGARDS participants ≥65 years of age, a secondary definition of fatal and nonfatal CHD was also used, including CHD events by the primary definition, or a Medicare claim for an overnight hospitalization with an International Classification of Disease, Ninth revision diagnosis code of 410.XX, except 410.X2, in any position.26 Events detected through Medicare claims were classified as fatal if participants died within 28 days after admission.
Some participants have MIs with no or mild symptoms which may not be clinically recognized and therefore, may not be detected through study procedures (i.e., unrecognized MIs). Unrecognized MIs are associated with coronary artery disease and a higher risk for future cardiac events and mortality.28,29 Follow-up electrocardiograms to detect unrecognized MIs were obtained in 1990–1992, 1993–1995, and 1996–1998 in ARIC, and annually through 1999 in CHS. For secondary analyses in ARIC and CHS, we defined nonfatal CHD as a definite or probable nonfatal MI or an unrecognized MI detected through follow-up electrocardiograms.
For secondary analyses in REGARDS, fatal CHD events were classified as out-of-hospital if the death occurred before hospital admission (e.g., in the emergency department), or as post-admission if the death occurred during or after hospitalization. Information on whether CHD deaths occurred before or after hospital admission was not available in the ARIC and CHS publicly available datasets. Follow-up data in ARIC, CHS and REGARDS were available through December 31, 2001, June 30, 2010 and December 31, 2013, respectively.
Statistical analysis
Analyses described below were conducted stratified by gender and among ARIC participants, REGARDS participants 45–64 years of age, CHS participants, and REGARDS participants ≥65 years of age, separately. We calculated baseline characteristics by race. Also, we conducted time-to-event analyses to compare the incidence of fatal, nonfatal and total (i.e., fatal and nonfatal) CHD among blacks and whites. Restricted to participants with incident CHD, we also compared the risk for having their incident event classified as fatal (i.e., case-fatality) among blacks and whites. Secondary analyses were conducted using different analytical approaches and including supplementary CHD outcomes (e.g., unrecognized MIs) to assess the robustness of the main results. Further details about statistical analyses are provided below.
For the main analysis, participants were followed through the first CHD event by the primary definition (and secondary definition among REGARDS participants ≥65 years of age), or non-CHD death. Participants lost to follow-up without a CHD event were censored on the last day known to be alive. The maximum follow-up available in REGARDS was 11 years. Therefore, we censored ARIC and CHS participants who remained alive and free of CHD after 11 years of follow-up to obtain comparable estimates across studies.
In time-to-event analyses, we calculated the incidence rate for fatal, nonfatal and total CHD, and non-CHD mortality by race. We also calculated the cumulative incidence function for fatal, nonfatal and total CHD by race considering competing risk as described by Fine and Gray.30 Fatal CHD analyses included competing risk for nonfatal CHD and non-CHD death (i.e., death due to cardiovascular causes other than CHD or non-cardiovascular causes). Analyses of nonfatal CHD included competing risk for fatal CHD and non-CHD death. Total CHD analyses included competing risk for non-CHD death. Using competing risk regression, we estimated hazard ratios (HR) and 95% confidence intervals (CI) for fatal, nonfatal and total CHD among blacks versus whites. Regression models included progressive adjustment for age, education, annual household income, region of residence (in REGARDS), alcohol consumption, physical activity, waist circumference, smoking, diabetes, eGFR, history of stroke, systolic blood pressure (SBP), antihypertensive medication, total and HDL cholesterol, lipid-lowering medication use, and health insurance. LVH is associated with a higher risk for arrhythmias including atrial fibrillation,31–34 which can be potentiated by ischemia.35 LVH is more common among blacks compared with whites36,37 and could contribute to the racial differences in fatal CHD.38 Therefore, a final model included adjustment for covariates listed above plus LVH and atrial fibrillation.
Restricted to participants with incident CHD, we calculated the case-fatality by race. Case-fatality was calculated as the number of participants with incident fatal CHD divided by the total number of participants with incident CHD (fatal or nonfatal). We used Poisson regression with robust variance estimates and progressive adjustment for covariates described above to calculate the case-fatality ratio and 95% CIs comparing blacks versus whites.39 We compared HRs for incident fatal, nonfatal and total CHD and case-fatality ratios associated with black race among men and women by including interaction terms between gender and race in regression models.
We conducted secondary analyses in ARIC, CHS and REGARDS to explore whether results would remain similar after removing competing risk. Specifically, we used Cox-regression to estimate HRs for incident fatal, nonfatal and total CHD censoring participants on the date of a competing event. We also conducted secondary analyses in ARIC and CHS to estimate HRs for incident CHD accounting for competing risks, and case-fatality ratios using all available follow-up and separately, including unrecognized MIs in the definition of nonfatal CHD. In REGARDS, we conducted secondary analyses to estimate HRs for out-of-hospital and post-admission fatal CHD. Analyses were conducted using competing risk regression to account for post-admission and out-of-hospital fatal CHD as appropriate, and nonfatal CHD and non-CHD death.
We used multiple imputation by chained equations to impute missing covariates (Supplemental table 1).40,41 All analyses were performed in Stata 13 (Stata Corp, College Station, TX) using and a two-sided level of significance alpha <0.05.
Results
Table 1 and Supplemental table 2 show participant characteristics with and without multiple imputation, respectively. Within each study, black men and women were more likely to have less than high school education, <$25,000 annual income, low physical activity, diabetes, reduced eGFR, history of stroke and higher SBP levels compared with white men and women, respectively. The prevalence of current smoking and HDL cholesterol levels were higher for blacks versus whites among men, but similar among women. Waist circumference was higher for blacks versus whites among women, but similar among men.
Table 1.
Baseline characteristics of participants included in the analysis by race and gender.
| Baseline characteristics | 45–64 years of age | ≥65 years of age | ||||||
|---|---|---|---|---|---|---|---|---|
| ARIC | REGARDS | CHS | REGARDS | |||||
| Blacks | Whites | Blacks | Whites | Blacks | Whites | Blacks | Whites | |
| MEN, N | 1,534 | 4,945 | 2,044 | 3,252 | 277 | 1,559 | 997 | 2,384 |
|
| ||||||||
| Age in years, mean (SE) | 53.7 (0.15) | 54.6 (0.08) | 57.2 (0.11) | 57.6 (0.08) | 72.2 (0.35) | 72.7 (0.15) | 71.9 (0.18) | 72.4 (0.12) |
|
| ||||||||
| Region of residence,* % | ||||||||
|
| ||||||||
| Stroke belt (buckle states) | NA | NA | 18.6 | 20.3 | NA | NA | 14.5 | 21.3 |
|
| ||||||||
| Stroke belt (non-buckle states) | NA | NA | 36.7 | 34.0 | NA | NA | 31.8 | 37.1 |
|
| ||||||||
| Other contiguous US states | NA | NA | 44.7 | 45.8 | NA | NA | 53.6 | 41.7 |
|
| ||||||||
| Less than high school education, % | 43.8 | 17.0 | 10.8 | 4.0 | 46.2 | 27.6 | 26.2 | 7.5 |
|
| ||||||||
| Annual household income <$25,000, % | 61.2 | 20.3 | 27.5 | 10.8 | 71.3 | 50.5 | 40.1 | 16.6 |
|
| ||||||||
| Alcohol consumption, % | ||||||||
|
| ||||||||
| None | 55.1 | 45.6 | 56.5 | 41.9 | 51.3 | 39.5 | 63.7 | 52.1 |
|
| ||||||||
| Moderate | 34.3 | 43.2 | 39.4 | 51.6 | 39.4 | 48.0 | 32.9 | 42.8 |
|
| ||||||||
| Heavy | 10.5 | 11.2 | 4.1 | 6.5 | 9.4 | 12.5 | 3.4 | 5.1 |
|
| ||||||||
| Low physical activity, % | 52.4 | 29.3 | 26.7 | 23.4 | 58.1 | 50.4 | 31.7 | 25.6 |
|
| ||||||||
| Current smoking, % | 38.1 | 24.6 | 24.7 | 15.1 | 21.7 | 10.4 | 12.5 | 7.9 |
|
| ||||||||
| Diabetes, % | 16.4 | 8.3 | 26.3 | 13.0 | 23.5 | 15.3 | 31.7 | 16.1 |
|
| ||||||||
| Reduced eGFR, % | 2.9 | 2.2 | 5.2 | 2.7 | 23.7 | 27.5 | 14.9 | 13.8 |
|
| ||||||||
| History of stroke, % | 3.4 | 1.2 | 5.8 | 2.8 | 10.8 | 4.0 | 10.8 | 5.9 |
|
| ||||||||
| Waist circumference (cm), mean (SE) | 96.7 (0.31) | 99.5 (0.14) | 99.6 (0.34) | 100.1 (0.24) | 96.6 (0.64) | 97.6 (0.25) | 98.8 (0.43) | 99.8 (0.25) |
|
| ||||||||
| SBP (mmHg), mean (SE) | 130.3 (0.55) | 120.2 (0.23) | 130.3 (0.36) | 124.6 (0.25) | 138.6 (1.26) | 136.2 (0.54) | 133.6 (0.55) | 129.4 (0.32) |
|
| ||||||||
| Taking antihypertensive medication, % | 32.9 | 18.2 | 53.1 | 33.0 | 48.9 | 35.9 | 62.3 | 45.6 |
|
| ||||||||
| Total cholesterol (mg/dL), mean (SE) | 210.9 (1.13) | 210.3 (0.55) | 189.5 (0.89) | 192.1 (0.67) | 192.2 (2.19) | 201.6 (0.90) | 183.8 (1.27) | 181.7 (0.71) |
|
| ||||||||
| HDL cholesterol (mg/dL), mean (SE) | 50.9 (0.45) | 43.1 (0.18) | 47.8 (0.32) | 44.2 (0.23) | 52.1 (0.83) | 48.0 (0.32) | 49.2 (0.48) | 45.3 (0.28) |
|
| ||||||||
| Taking lipid-lowering medications, % | 1.1 | 2.9 | 28.6 | 31.6 | 3.2 | 3.1 | 35.9 | 37.6 |
|
| ||||||||
| Health insurance, % | 76.5 | 95.4 | 84.0 | 92.5 | NA | NA | NA | NA |
|
| ||||||||
| Left ventricular hypertrophy, % | 5.6 | 1.0 | 3.6 | 0.7 | 5.8 | 3.3 | 4.4 | 2.2 |
|
| ||||||||
| Atrial fibrillation,† % | NA | NA | 4.6 | 4.7 | 5.8 | 6.0 | 5.3 | 9.0 |
|
| ||||||||
| WOMEN, N | 2,551 | 5,937 | 3,729 | 4,093 | 448 | 2,342 | 1,590 | 2,522 |
|
| ||||||||
| Age in years, mean (SE) | 53.3 (0.11) | 53.9 (0.07) | 56.9 (0.08) | 57.0 (0.08) | 72.3 (0.27) | 71.6 (0.11) | 72.1 (0.15) | 72.6 (0.12) |
|
| ||||||||
| Region of residence, * % | ||||||||
|
| ||||||||
| Stroke belt (buckle states) | NA | NA | 20.1 | 25.9 | NA | NA | 21.8 | 26.4 |
|
| ||||||||
| Stroke belt (non-buckle states) | NA | NA | 37.1 | 36.0 | NA | NA | 33.0 | 38.1 |
|
| ||||||||
| Other contiguous US states | NA | NA | 42.8 | 38.0 | NA | NA | 45.1 | 35.5 |
|
| ||||||||
| Less than high school, % | 40.0 | 16.3 | 12.9 | 5.0 | 43.3 | 24.3 | 26.8 | 8.3 |
|
| ||||||||
| Annual household income <$25,000, % | 75.9 | 31.5 | 41.3 | 19.0 | 83.7 | 61.1 | 59.6 | 36.7 |
|
| ||||||||
| Alcohol consumption, % | ||||||||
|
| ||||||||
| None | 84.9 | 66.4 | 75.1 | 57.8 | 72.9 | 51.1 | 84.1 | 68.8 |
|
| ||||||||
| Moderate | 12.4 | 26.0 | 22.9 | 36.6 | 24.2 | 37.5 | 14.4 | 27.1 |
|
| ||||||||
| Heavy | 2.7 | 7.6 | 2.0 | 5.6 | 2.9 | 11.5 | 1.6 | 4.1 |
|
| ||||||||
| Low physical activity, % | 59.4 | 31.9 | 37.4 | 34.3 | 66.3 | 58.1 | 45.6 | 39.3 |
|
| ||||||||
| Current smoking, % | 24.6 | 24.9 | 19.1 | 16.2 | 13.0 | 12.8 | 10.5 | 8.5 |
|
| ||||||||
| Diabetes, % | 18.5 | 6.9 | 26.4 | 10.2 | 21.7 | 10.5 | 30.0 | 12.8 |
|
| ||||||||
| Reduced eGFR, % | 3.9 | 2.4 | 5.2 | 2.7 | 21.6 | 20.2 | 19.8 | 16.9 |
|
| ||||||||
| History of stroke, % | 1.9 | 0.9 | 4.9 | 2.8 | 4.0 | 2.3 | 8.0 | 4.9 |
|
| ||||||||
| Waist circumference (cm), mean (SE) | 100.4 (0.32) | 93.1 (0.19) | 97.8 (0.27) | 89.1 (0.25) | 98.3 (0.61) | 90.5 (0.25) | 95.9 (0.36) | 88.0 (0.29) |
|
| ||||||||
| SBP (mmHg), mean (SE) | 127.9 (0.42) | 117.0 (0.23) | 127.8 (0.28) | 119.8 (0.23) | 143.9 (1.14) | 135.5 (0.44) | 132.2 (0.45) | 127.2 (0.32) |
|
| ||||||||
| Taking antihypertensive medication, % | 44.0 | 19.0 | 61.9 | 33.5 | 63.2 | 38.9 | 72.2 | 50.8 |
|
| ||||||||
| Total cholesterol (mg/dL), mean (SE) | 217.3 (0.93) | 218.1 (0.55) | 199.3 (0.67) | 204.2 (0.62) | 212.4 (1.96) | 224.9 (0.78) | 199.1 (1.10) | 200.6 (0.77) |
|
| ||||||||
| HDL cholesterol (mg/dL), mean (SE) | 58.0 (0.36) | 57.5 (0.22) | 56.3 (0.26) | 57.6 (0.26) | 61.6 (0.76) | 59.4 (0.33) | 58.8 (0.43) | 58.3 (0.35) |
|
| ||||||||
| Taking lipid-lowering medication, % | 1.5 | 3.1 | 27.7 | 25.6 | 6.9 | 5.0 | 39.0 | 36.6 |
|
| ||||||||
| Health insurance, % | 76.3 | 95.0 | 82.5 | 90.9 | NA | NA | NA | NA |
|
| ||||||||
| Left ventricular hypertrophy, % | 5.2 | 0.9 | 7.9 | 2.3 | 9.2 | 4.7 | 13.3 | 6.3 |
|
| ||||||||
| Atrial fibrillation,† % | NA | NA | 7.4 | 5.5 | 3.3 | 4.4 | 5.5 | 9.9 |
ARIC: Atherosclerosis Risk In Communities; CHS: Cardiovascular Health Study; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; NA: non-applicable; REGARDS: REasons for Geographic And Racial Differences in Stroke; SBP: systolic blood pressure; SE: standard error; US: United States.
Stroke belt (buckle states) includes coastal North Carolina, South Carolina and Georgia. Stroke belt (non-buckle states) includes the remaining parts of the stroke buckle states and Tennessee, Mississippi, Alabama, Louisiana and Arkansas.
Data on atrial fibrillation at baseline was not included in the ARIC publicly available dataset.
Analyses were conducted using multiple imputation for missing data.
CHD incidence and case-fatality among men
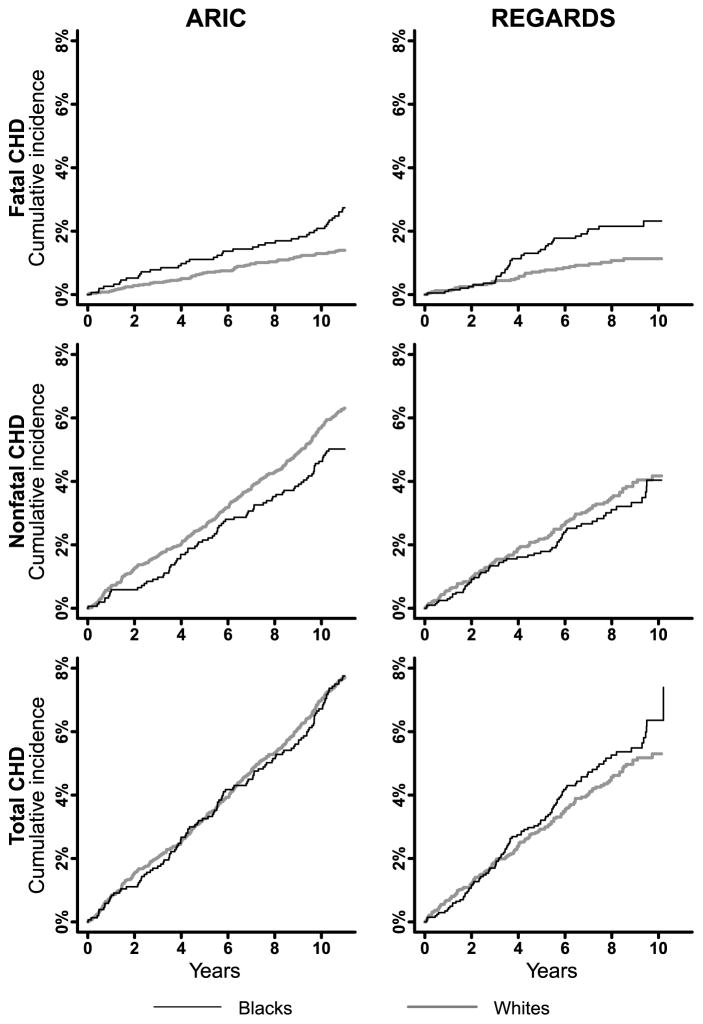
Among men 45–64 years of age in ARIC and REGARDS, blacks had higher incidence of fatal CHD and lower incidence of nonfatal CHD compared with whites (Figure 1 and Supplemental table 3, left panel). Incidence of total CHD was higher for black versus white men in REGARDS, but similar in ARIC. Non-CHD mortality was higher for black versus white men in both studies. Among men ≥65 years of age in CHS and REGARDS, blacks had higher incidence of fatal CHD, lower incidence of nonfatal CHD, and similar incidence of total CHD compared with whites (Supplemental figure 3 and Supplemental table 3, right panel). Non-CHD mortality was higher for black versus white men ≥65 years of age in CHS and REGARDS.
Figure 1.
Cumulative incidence of fatal, nonfatal and total CHD among black and white men 45–64 years of age in ARIC and REGARDS. ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; REGARDS: REasons for Geographic And Racial Differences in Stroke. The maximum follow-up for all analyses was 11 years. Mean follow-up was 10.2 years in ARIC and 7.3 years in REGARDS.
After age adjustment, black men had higher risk for fatal CHD compared with white men in all analyses (Table 2), although the association was numerically lower and not statistically significant in CHS (HR 1.32; 95% CI 0.86–2.03). Black men also had similar or lower age-adjusted risk for nonfatal CHD, and similar age-adjusted risk for total CHD compared with white men. After multivariable adjustment, black men had similar risk for fatal CHD compared with white men in all analyses, but lower risk for nonfatal CHD (which was not statistically significant in CHS; HR 0.83; 95% CI 0.57–1.21). There was a trend for a lower risk for total CHD among black versus white men after multivariable adjustment which was statistically significant in ARIC (HR 0.74; 95% CI 0.57–0.95).
Table 2.
Hazard ratios for fatal, nonfatal and total CHD among black versus white men.
| 45–64 years of age | ≥65 years of age | ||||
|---|---|---|---|---|---|
| ARIC | REGARDS | CHS | REGARDS* | REGARDS† | |
| (N=6,479) | (N=5,296) | (N=1,836) | (N=3,381) | (N=3,381) | |
| Fatal CHD, events | 111 | 71 | 145 | 118 | 120 |
|
| |||||
| Hazard ratio (95% CI) | |||||
|
| |||||
| Model 1 | 2.09 (1.42–3.06) | 2.11 (1.32–3.38) | 1.32 (0.86–2.03) | 1.72 (1.19–2.48) | 1.67 (1.16–2.40) |
|
| |||||
| Model 2 | 1.90 (1.26–2.87) | 1.65 (1.01–2.69) | 1.18 (0.76–1.83) | 1.38 (0.94–2.03) | 1.33 (0.90–1.95) |
|
| |||||
| Model 3 | 1.88 (1.25–2.83) | 1.57 (0.95–2.57) | 1.17 (0.76–1.81) | 1.35 (0.93–1.97) | 1.30 (0.89–1.89) |
|
| |||||
| Model 4 | 1.30 (0.82–2.04) | 1.16 (0.66–2.03) | 1.02 (0.64–1.62) | 1.16 (0.78–1.71) | 1.13 (0.76–1.68) |
|
| |||||
| Model 5 | 1.22 (0.76–1.97) | 1.14 (0.65–2.00) | NA | NA | NA |
|
| |||||
| Model 6 | 1.19 (0.74–1.92) | 1.09 (0.62–1.93) | 0.99 (0.63–1.57) | 1.21 (0.81–1.80) | 1.17 (0.78–1.75) |
|
| |||||
| Nonfatal CHD, events | 389 | 172 | 249 | 187 | 238 |
|
| |||||
| Hazard ratio (95% CI) | |||||
|
| |||||
| Model 1 | 0.82 (0.64–1.05) | 0.94 (0.69–1.28) | 0.85 (0.59–1.22) | 0.55 (0.38–0.80) | 0.71 (0.53–0.97) |
|
| |||||
| Model 2 | 0.67 (0.51–0.89) | 0.84 (0.60–1.18) | 0.85 (0.58–1.24) | 0.59 (0.39–0.89) | 0.69 (0.49–0.97) |
|
| |||||
| Model 3 | 0.65 (0.49–0.86) | 0.80 (0.57–1.12) | 0.85 (0.59–1.25) | 0.58 (0.38–0.88) | 0.68 (0.49–0.96) |
|
| |||||
| Model 4 | 0.64 (0.47–0.86) | 0.70 (0.50–0.99) | 0.83 (0.57–1.21) | 0.54 (0.35–0.83) | 0.64 (0.45–0.91) |
|
| |||||
| Model 5 | 0.64 (0.48–0.87) | 0.70 (0.49–0.98) | NA | NA | NA |
|
| |||||
| Model 6 | 0.64 (0.47–0.86) | 0.67 (0.48–0.95) | 0.83 (0.57–1.21) | 0.53 (0.35–0.82) | 0.63 (0.44–0.90) |
|
| |||||
| Total CHD, events | 500 | 243 | 394 | 305 | 358 |
|
| |||||
| Hazard ratio (95% CI) | |||||
|
| |||||
| Model 1 | 1.04 (0.85–1.28) | 1.20 (0.93–1.56) | 1.02 (0.77–1.35) | 0.92 (0.71–1.18) | 0.98 (0.78–1.24) |
|
| |||||
| Model 2 | 0.88 (0.70–1.11) | 1.03 (0.79–1.35) | 0.97 (0.73–1.30) | 0.86 (0.65–1.13) | 0.88 (0.68–1.13) |
|
| |||||
| Model 3 | 0.85 (0.68–1.07) | 0.98 (0.75–1.29) | 0.96 (0.72–1.29) | 0.85 (0.64–1.11) | 0.87 (0.68–1.11) |
|
| |||||
| Model 4 | 0.76 (0.59–0.97) | 0.81 (0.60–1.08) | 0.88 (0.66–1.19) | 0.76 (0.57–1.01) | 0.79 (0.61–1.02) |
|
| |||||
| Model 5 | 0.75 (0.58–0.96) | 0.80 (0.60–1.07) | NA | NA | NA |
|
| |||||
| Model 6 | 0.74 (0.57–0.95) | 0.78 (0.58–1.04) | 0.88 (0.65–1.18) | 0.76 (0.57–1.01) | 0.79 (0.60–1.02) |
ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; CHS: Cardiovascular Health Study; CI: confidence interval; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; NA: not applicable; MI: myocardial infarction; REGARDS: REasons for Geographic And Racial Differences in Stroke; SBP: systolic blood pressure.
Using the primary definition of CHD.
Using the secondary definition of CHD which includes MI hospitalizations detected through Medicare claims.
Analyses were conducted using competing risk regression and multiple imputation for missing data. The maximum follow-up for all analyses was 11 years. Mean follow-up was 10.2 years in ARIC, 7.3 years among participants 45–64 years of age in REGARDS, 8.3 years in CHS, and 7.1 years among participants ≥65 years of age in REGARDS when using the primary and secondary definitions of CHD.
Model 1 adjusts for age.
Model 2 adjusts for age, education and income levels (and region of residence in REGARDS).
Model 3 adjusts for covariates in Model 2 plus alcohol consumption, physical activity, waist circumference and current smoking.
Model 4 adjusts for covariates in Model 3 plus diabetes, reduced eGFR, stroke, SBP, use of antihypertensive medications, total and HDL cholesterol, and use of lipid-lowering medication.
Model 5 adjusts for covariates in Model 4 plus health insurance among participants <65 years of age (all participants ≥65 years of age had Medicare).
Model 6 adjusts for covariates in Model 5 plus left ventricular hypertrophy and atrial fibrillation (except in ARIC).
Among men with incident CHD, blacks had higher age-adjusted case-fatality compared with whites (Table 3), although the difference was not statistically significant in CHS (case-fatality ratio 1.27; 95% CI 0.93–1.74). The case-fatality ratio for blacks versus whites remained higher and statistically significant after multivariable adjustment in ARIC and among REGARDS participants 45–64 and ≥65 years of age. In CHS, the multivariable-adjusted case fatality ratio was 1.03 (95% CI 0.73–1.45).
Table 3.
Case-fatality among black versus white men with incident CHD.
| 45–64 years of age | ≥65 years of age | ||||
|---|---|---|---|---|---|
| ARIC | REGARDS | CHS | REGARDS* | REGARDS† | |
| Case-fatality (fatal CHD/total CHD) | |||||
|
| |||||
| Blacks | 35.3% (42/119) | 39.0% (39/100) | 44.1% (26/59) | 57.8% (48/83) | 47.1% (48/102) |
|
| |||||
| Whites | 18.1% (69/381) | 22.4% (32/143) | 35.5% (119/335) | 31.5% (70/222) | 28.1% (72/256) |
|
| |||||
| Case-fatality ratio (95% CI)‡ | |||||
|
| |||||
| Model 1 | 1.97 (1.42–2.71) | 1.77 (1.19–2.62) | 1.27 (0.93–1.74) | 1.84 (1.41–2.41) | 1.67 (1.26–2.23) |
|
| |||||
| Model 2 | 2.09 (1.47–2.97) | 1.69 (1.13–2.52) | 1.21 (0.88–1.66) | 1.59 (1.19–2.12) | 1.49 (1.10–2.02) |
|
| |||||
| Model 3 | 2.17 (1.52–3.09) | 1.77 (1.18–2.65) | 1.16 (0.84–1.61) | 1.58 (1.19–2.09) | 1.49 (1.11–2.01) |
|
| |||||
| Model 4 | 1.70 (1.16–2.48) | 1.61 (1.03–2.51) | 1.06 (0.75–1.49) | 1.49 (1.09–2.03) | 1.44 (1.05–1.99) |
|
| |||||
| Model 5 | 1.64 (1.11–2.42) | 1.60 (1.02–2.51) | NA | NA | NA |
|
| |||||
| Model 6 | 1.63 (1.10–2.40) | 1.60 (1.03–2.50) | 1.03 (0.73–1.45) | 1.49 (1.09–2.04) | 1.44 (1.04–2.00) |
ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; CHS: Cardiovascular Health Study; CI: confidence interval; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; NA: not applicable; MI: myocardial infarction; REGARDS: REasons for Geographic And Racial Differences in Stroke; SBP: systolic blood pressure.
Using the primary definition of CHD.
Using the secondary definition of CHD which includes MI hospitalizations detected through Medicare claims.
Case-fatality ratios were calculated comparing blacks versus whites (reference group).
Analyses were conducted using multiple imputation for missing data.
Model 1 adjusts for age.
Model 2 adjusts for age, education and income levels (and region of residence in REGARDS).
Model 3 adjusts for covariates in Model 2 plus alcohol consumption, physical activity, waist circumference and current smoking.
Model 4 adjusts for covariates in Model 3 plus diabetes, reduced eGFR, stroke, SBP, use of antihypertensive medications, total and HDL cholesterol, and use of lipid-lowering medication.
Model 5 adjusts for covariates in Model 4 plus health insurance among participants <65 years of age (all participants ≥65 years of age had Medicare).
Model 6 adjusts for covariates in Model 5 plus left ventricular hypertrophy and atrial fibrillation (except in ARIC).
CHD incidence and case-fatality among women
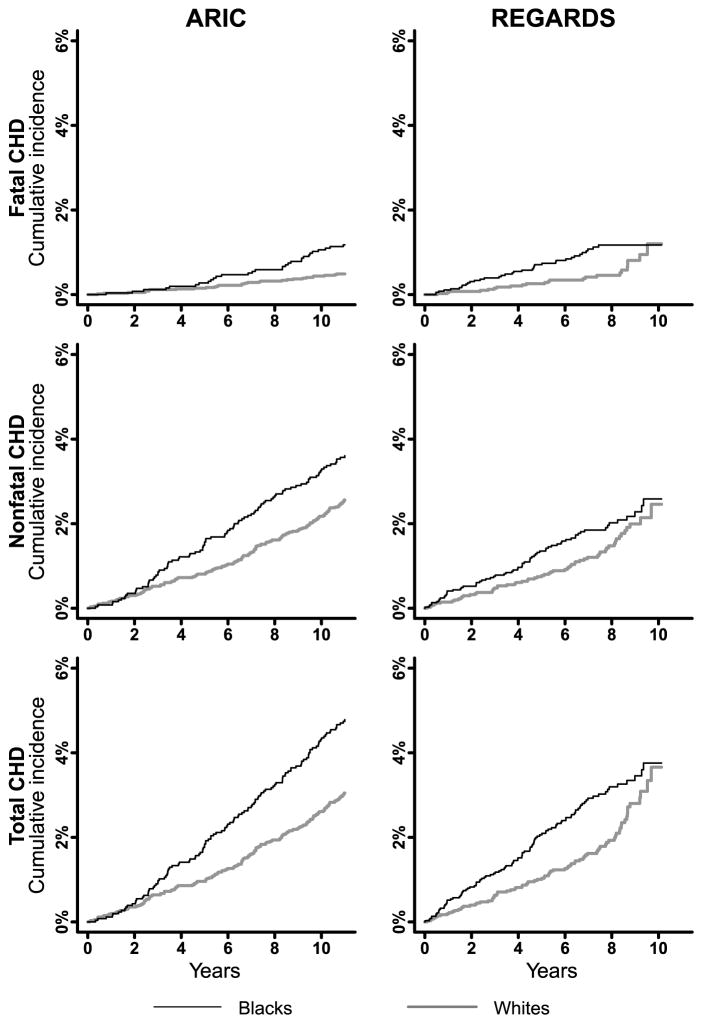
Among women 45–64 years of age, blacks had higher incidence of fatal, nonfatal and total CHD, and higher non-CHD mortality compared with whites (Figure 2 and Supplemental table 4, left panel). A similar pattern was present among black and white women ≥65 years of age in CHS and REGARDS (Supplemental figure 4 and Supplemental table 4, right panel).
Figure 2.
Cumulative incidence of fatal, nonfatal and total CHD among black and white women 45–64 years of age in ARIC and REGARDS. ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; REGARDS: REasons for Geographic And Racial Differences in Stroke. The maximum follow-up for all analyses was 11 years. Mean follow-up was 10.5 years in ARIC and 7.0 years in REGARDS.
After age adjustment, black women 45–64 years of age in ARIC and REGARDS had higher risk for fatal and total CHD compared with white women (Table 4, left panel). Black women also had a higher age-adjusted risk for nonfatal CHD compared with white women, which was not statistically significant in REGARDS (HR 1.29; 95% CI 0.91–1.83). After multivariable adjustment, HRs (95% CI) comparing black versus white women in ARIC and REGARDS were 0.67 (0.36–1.24) and 1.00 (0.54–1.85), respectively for fatal CHD, 0.70 (0.51–0.97) and 0.70 (0.46–1.06), respectively for nonfatal CHD, and 0.69 (0.51–0.92) and 0.78 (0.55–1.09), respectively for total CHD. Age-adjusted HRs for fatal, nonfatal and total CHD comparing black versus white women ≥65 years of age were not statistically significant, except for fatal CHD in REGARDS (Table 4, right panel). Specifically, the age-adjusted HR for fatal CHD among black versus white women ≥65 years of age in REGARDS was 1.57 (95% CI 1.01–2.43) when using the primary definition of CHD. HRs for fatal, nonfatal and total CHD comparing black versus white women ≥65 years of age in CHS and REGARDS were not statistically significant after multivariable adjustment.
Table 4.
Hazard ratios for fatal, nonfatal and total CHD among black versus white women.
| 45–64 years of age | ≥65 years of age | ||||
|---|---|---|---|---|---|
| ARIC | REGARDS | CHS | REGARDS* | REGARDS† | |
| (N=8,488) | (N=7,822) | (N=2,790) | (N=4,112) | (N=4,112) | |
| Fatal CHD, events | 59 | 59 | 145 | 81 | 81 |
|
| |||||
| Hazard ratios (95% CI) | |||||
|
| |||||
| Model 1 | 2.61 (1.57–4.34) | 1.79 (1.06–3.03) | 1.23 (0.82–1.84) | 1.57 (1.01–2.43) | 1.41 (0.91–2.19) |
|
| |||||
| Model 2 | 1.35 (0.82–2.24) | 1.38 (0.79–2.41) | 1.00 (0.66–1.54) | 1.37 (0.85–2.23) | 1.26 (0.77–2.05) |
|
| |||||
| Model 3 | 1.26 (0.73–2.16) | 1.08 (0.62–1.88) | 0.82 (0.53–1.26) | 1.19 (0.74–1.91) | 1.06 (0.65–1.71) |
|
| |||||
| Model 4 | 0.77 (0.42–1.40) | 1.00 (0.54–1.83) | 0.74 (0.46–1.19) | 1.07 (0.65–1.76) | 0.98 (0.59–1.63) |
|
| |||||
| Model 5 | 0.72 (0.40–1.31) | 1.00 (0.55–1.84) | NA | NA | NA |
|
| |||||
| Model 6 | 0.67 (0.36–1.26) | 1.00 (0.54–1.85) | 0.75 (0.46–1.20) | 1.08 (0.65–1.82) | 0.99 (0.59–1.66) |
|
| |||||
| Nonfatal CHD, events | 244 | 128 | 201 | 129 | 192 |
|
| |||||
| Hazard ratios (95% CI) | |||||
|
| |||||
| Model 1 | 1.47 (1.13–1.91) | 1.29 (0.91–1.83) | 1.21 (0.85–1.72) | 0.86 (0.60–1.23) | 1.14 (0.85–1.52) |
|
| |||||
| Model 2 | 0.98 (0.73–1.31) | 1.04 (0.71–1.53) | 1.09 (0.76–1.56) | 0.76 (0.53–1.10) | 0.98 (0.73–1.31) |
|
| |||||
| Model 3 | 0.93 (0.68–1.25) | 0.95 (0.65–1.41) | 0.96 (0.67–1.39) | 0.69 (0.47–0.99) | 0.90 (0.67–1.22) |
|
| |||||
| Model 4 | 0.74 (0.54–1.02) | 0.74 (0.49–1.11) | 0.97 (0.66–1.44) | 0.67 (0.45–0.98) | 0.84 (0.61–1.15) |
|
| |||||
| Model 5 | 0.72 (0.52–0.99) | 0.74 (0.49–1.11) | NA | NA | NA |
|
| |||||
| Model 6 | 0.70 (0.51–0.97) | 0.70 (0.46–1.06) | 0.96 (0.65–1.42) | 0.68 (0.46–1.01) | 0.86 (0.63–1.19) |
|
| |||||
| Total CHD, events | 303 | 187 | 346 | 210 | 273 |
|
| |||||
| Hazard ratios (95% CI) | |||||
|
| |||||
| Model 1 | 1.66 (1.32–2.09) | 1.44 (1.08–1.92) | 1.23 (0.94–1.60) | 1.10 (0.83–1.45) | 1.22 (0.96–1.55) |
|
| |||||
| Model 2 | 1.05 (0.81–1.36) | 1.14 (0.83–1.56) | 1.06 (0.81–1.39) | 0.97 (0.72–1.29) | 1.06 (0.82–1.36) |
|
| |||||
| Model 3 | 0.99 (0.76–1.29) | 0.99 (0.72–1.35) | 0.90 (0.68–1.19) | 0.86 (0.64–1.14) | 0.95 (0.73–1.22) |
|
| |||||
| Model 4 | 0.74 (0.56–0.98) | 0.81 (0.58–1.14) | 0.87 (0.65–1.18) | 0.81 (0.60–1.09) | 0.88 (0.68–1.15) |
|
| |||||
| Model 5 | 0.71 (0.53–0.95) | 0.81 (0.58–1.14) | NA | NA | NA |
|
| |||||
| Model 6 | 0.69 (0.51–0.92) | 0.78 (0.55–1.09) | 0.87 (0.64–1.17) | 0.82 (0.61–1.12) | 0.90 (0.69–1.18) |
ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; CHS: Cardiovascular Health Study; CI: confidence interval; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; NA: not applicable; MI: myocardial infarction; REGARDS: REasons for Geographic And Racial Differences in Stroke; SBP: systolic blood pressure.
Using the primary definition of CHD.
Using the secondary definition of CHD which includes MI hospitalizations detected through Medicare claims.
Analyses were conducted using competing risk regression and multiple imputation for missing data. The maximum follow-up for all analyses was 11 years. Mean follow-up was 10.5 years in ARIC, 7.0 years among participants 45–64 years of age in REGARDS, 9.5 years in CHS, and 7.0 years among participants ≥65 years of age in REGARDS when using the primary and secondary definition of CHD.
Model 1 adjusts for age.
Model 2 adjusts for age, education and income levels (and region of residence in REGARDS).
Model 3 adjusts for covariates in Model 2 plus alcohol consumption, physical activity, waist circumference and current smoking.
Model 4 adjusts for covariates in Model 3 plus diabetes, reduced eGFR, stroke, SBP, use of antihypertensive medications, total and HDL cholesterol, and use of lipid-lowering medication.
Model 5 adjusts for covariates in Model 4 plus health insurance among participants <65 years of age (all participants ≥65 years of age had Medicare).
Model 6 adjusts for covariates in Model 5 plus left ventricular hypertrophy and atrial fibrillation (except in ARIC).
Among women with incident CHD in ARIC, blacks had higher age-adjusted case-fatality compared with whites (case-fatality ratio 1.57; 95% CI 1.00–2.46; Table 5), which was attenuated after multivariable adjustment (case-fatality ratio 1.09, 95% CI 0.65–1.82). Crude case-fatality was higher among black versus white women 45–64 and ≥65 years of age in REGARDS. Case-fatality ratios in REGARDS remained numerically similar but were not statistically significant after progressive adjustment for social determinants of health and cardiovascular risk factors. No racial differences in case-fatality were present in CHS.
Table 5.
Case-fatality among black versus white women with incident CHD.
| 45–64 years of age | ≥65 years of age | ||||
|---|---|---|---|---|---|
| ARIC | REGARDS | CHS | REGARDS* | REGARDS† | |
| Case-fatality (fatal CHD/total CHD) | |||||
|
| |||||
| Blacks | 24.6% (30/122) | 34.6% (36/104) | 44.1% (30/68) | 47.0% (39/83) | 32.5% (37/114) |
|
| |||||
| Whites | 16.0% (29/181) | 27.7% (23/83) | 41.4% (115/278) | 33.1% (42/127) | 27.7% (44/159) |
|
| |||||
| Case-fatality ratio (95% CI)‡ | |||||
|
| |||||
| Model 1 | 1.57 (1.00–2.46) | 1.25 (0.81–1.93) | 0.99 (0.73–1.35) | 1.48 (1.06–2.08) | 1.18 (0.82–1.71) |
|
| |||||
| Model 2 | 1.27 (0.81–2.00) | 1.19 (0.76–1.86) | 0.95 (0.69–1.30) | 1.46 (1.00–2.12) | 1.21 (0.79–1.84) |
|
| |||||
| Model 3 | 1.27 (0.79–2.04) | 1.18 (0.74–1.87) | 0.91 (0.66–1.26) | 1.41 (0.96–2.06) | 1.14 (0.76–1.71) |
|
| |||||
| Model 4 | 1.09 (0.67–1.76) | 1.29 (0.81–2.06) | 0.87 (0.63–1.21) | 1.44 (0.98–2.13) | 1.12 (0.74–1.69) |
|
| |||||
| Model 5 | 1.12 (0.68–1.84) | 1.30 (0.82–2.06) | NA | NA | NA |
|
| |||||
| Model 6 | 1.09 (0.65–1.82) | 1.40 (0.88–2.21) | 0.90 (0.64–1.26) | 1.43 (0.97–2.12) | 1.11 (0.73–1.68) |
ARIC: Atherosclerosis Risk In Communities; CHD: coronary heart disease; CHS: Cardiovascular Health Study; CI: confidence interval; eGFR: estimated glomerular filtration rate; HDL: high-density lipoprotein; NA: not applicable; MI: myocardial infarction; REGARDS: REasons for Geographic And Racial Differences in Stroke; SBP: systolic blood pressure.
Using the primary definition of CHD.
Using the secondary definition of CHD which includes MI hospitalizations detected through Medicare claims.
Case-fatality ratios were calculated comparing blacks versus whites (reference group).
Analyses were conducted using multiple imputation for missing data.
Note: REGARDS study participants ≥65 years of age with incident fatal CHD by the primary definition can have incident nonfatal CHD by the secondary definition if they had a Medicare claim for a MI hospitalization at least 28 days before their death date.
Model 1 adjusts for age.
Model 2 adjusts for age, education and income levels (and region of residence in REGARDS).
Model 3 adjusts for covariates in Model 2 plus alcohol consumption, physical activity, waist circumference and current smoking.
Model 4 adjusts for covariates in Model 3 plus diabetes, reduced eGFR, stroke, SBP, use of antihypertensive medications, total and HDL cholesterol, and use of lipid-lowering medication.
Model 5 adjusts for covariates in Model 4 plus health insurance among participants <65 years of age (all participants ≥65 years of age had Medicare).
Model 6 adjusts for covariates in Model 5 plus left ventricular hypertrophy and atrial fibrillation (except in ARIC).
Black-white disparities in CHD incidence and case-fatality by gender
Within each cohort, age- and multivariable-adjusted HRs for fatal CHD associated with black race were similar among men and women (Supplemental figure 5, top panel). Age-adjusted HRs for nonfatal and total CHD associated with black race tended to be lower for men compared with women within each cohort, but gender differences disappeared after multivariable adjustment. Case-fatality ratios associated with black race were consistent among men and women with incident CHD within each cohort (Supplemental figure 5, bottom panel).
Secondary analyses
Black-white differences in CHD incidence among men and women were consistent with the main results in secondary analyses removing competing risk (Supplemental tables 5 and 6). Results in ARIC and CHS were also consistent with the main analysis when using all available follow-up (Supplemental tables 7 and 8) and including unrecognized MIs (Supplemental tables 9 and 10). In REGARDS, out-of-hospital and post-admission fatal CHD incidence were consistently higher among black versus white men, and differences were attenuated after multivariable adjustment (Supplemental table 11). Incidence of out-of-hospital fatal CHD was higher among black versus white women 45–64 years of age in REGARDS, while incidence of post-admission fatal CHD was higher for black versus white women ≥65 years of age (Supplemental table 12). Differences in out-of-hospital and post-admission fatal CHD among black versus white women 45–64 and ≥65 years of age in REGARDS were not statistically significant after multivariable adjustment. Black-white differences in case fatality among men and women in ARIC and CHS were consistent with the main analysis when using all available follow-up (Supplemental table 13 and 14), and including unrecognized MIs (Supplemental tables 15 and 16).
Discussion
We compared the incidence of fatal, nonfatal, and total CHD among black and white men and women in three US cohorts. After age adjustment, black men had higher risk for fatal CHD, but similar or lower risk for nonfatal and total CHD compared with white men. In contrast, black women had a higher risk for fatal, nonfatal and total CHD versus white women, particularly among those <65 years of age. After multivariable adjustment including social determinants of health and cardiovascular risk factors, black men and women had a similar risk for fatal CHD with a lower risk for nonfatal and total CHD compared with white men and women, respectively. Results from the current analysis also suggest that among men and women with incident CHD, blacks have a higher case-fatality compared with whites, which is not completely explained by social determinants of health and cardiovascular risk factors.
The similar or lower risk for nonfatal and total CHD comparing black versus white men after age adjustment appears inconsistent with the higher burden of unfavorable social determinants of health and cardiovascular risk factors among the former. Also, this finding appears inconsistent with the higher age-adjusted risk for fatal CHD among black versus white men, and for fatal, nonfatal and total CHD among black versus white women. Initially, we considered that the current results could have been attributed to black men being more likely to have undetected nonfatal MIs compared with white men. In REGARDS, nonfatal MIs may not be detected if CHD-related hospitalizations are not reported by participants. We previously showed that, like other large cohorts, the REGARDS study did not detect up to 25% of non-adjudicated events present in Medicare claims.42,43 However, results among REGARDS participants ≥65 years of age were similar after including unreported events detected through Medicare claims. Also, a similar pattern was found in ARIC and CHS, which include an active surveillance component for the detection of unreported events. Nonfatal MIs could have also been undetected if these events were not clinically recognized. Black men have been reported to be less engaged in healthcare than women or white men, especially at younger ages.44–46 Also, in a prior analysis in ARIC, the incidence of clinically unrecognized MIs from baseline through visit 4 (1996–1998) was higher among black versus white men, although the difference was not statistically significant.47 In the current analysis, results in ARIC and CHS remained similar after including clinically unrecognized MIs. Taken together, results from the current study suggest that the similar or lower risk for nonfatal and total CHD comparing black versus white men after age adjustment is unlikely to be explained by racial differences in the occurrence of undetected nonfatal MIs.
After multivariable adjustment, black men and women had a lower risk for nonfatal and total CHD compared with white men and women, respectively. Mechanisms leading to this finding warrant further investigation. Notably, multivariable-adjusted HRs for nonfatal and total CHD associated with black race were numerically very similar comparing men and women. This suggests that differences in age-adjusted HRs for nonfatal and total CHD between men and women could be attributed to different confounding effects by social determinants of health and cardiovascular risk factors by gender.
Consistent with prior studies, we found a higher case-fatality among black versus white men with incident CHD which was statistically significant in ARIC, but not in CHS.48,49 We also found a higher case-fatality among black versus white men 45–64 and ≥65 years of age in REGARDS. We hypothesized that LVH could have been associated with a higher case-fatality among blacks versus whites through a higher risk for fatal arrhythmias. However, case-fatality ratios in ARIC and REGARDS remained statistically significant after multivariable adjustment including LVH and atrial fibrillation (in REGARDS). Case-fatality ratios comparing black versus white women were consistent with results among men. In secondary analyses in REGARDS, blacks had higher incidence of out-of-hospital fatal CHD compared with whites, which is consistent with the higher risk for sudden cardiac death among the former.50 We also found a higher incidence of post-admission fatal CHD among blacks versus whites in REGARDS. Prior studies have shown that blacks are less likely to receive short-term antiplatelet therapies, reperfusion therapy within 24 hours, and diagnostic cardiac catheterization and revascularization after a MI hospitalization compared with whites.51,52 Although some hospital-based studies suggest that short-term mortality after an MI admission may be lower for blacks versus whites,51–53 this seems to have changed over time.54,55 In the National Registry of Myocardial Infarction, in-hospital mortality was lower comparing blacks versus whites in 1994–1999, but higher in 2003–2006.54 Future studies are needed to further elucidate the mechanisms leading to the higher case-fatality among blacks versus whites with incident CHD, including both out-of-hospital and post-admission mortality, and identify potential targets for interventions to reduce racial disparities.
The numerically lower HR for fatal CHD associated with black race in CHS versus ARIC and REGARDS needs to be considered in the context of epidemiological changes occurred since the 1960’s in the US. Studies using mortality data from the 1960’s and 1970’s reported a “black-white mortality age crossover” for CHD.56–58 This phenomenon, which consisted of CHD mortality being higher for blacks versus whites at younger ages, but lower in older populations, was attributed to a survivor bias.56–58 CHD mortality persisted higher for blacks versus whites at younger ages in more contemporary analyses.56 However, CHD mortality among older US adults declined more for whites versus blacks since the 1970s.56 This resulted in CHD mortality being similar among older blacks and whites by the 1980s, just before ARIC and CHS started.56 In the current study, age-adjusted HRs for fatal CHD among blacks versus whites ≥65 years of age were numerically higher in REGARDS versus CHS. This suggests that black-white disparities in fatal CHD incidence may be becoming wider among older US adults. Continued surveillance of CHD incidence and mortality among US adults by race, age and gender is warranted. In addition, targeted interventions may need to be integrated into population health management strategies if disparities are to be eliminated, or at a minimum, if an inadvertent widening of disparities is to be prevented.
Our analysis has several strengths including the use of data from cohorts with a large sample size, adequate representation of blacks and whites, long follow-up and a rigorous CHD event adjudication process. We used a comparable follow-up and CHD definition, and the same analytical approach accounting for missing data and competing risks across studies. When possible, we recreated variables in ARIC and CHS to be consistent with the REGARDS definition. Our study also has potential and known limitations. ARIC, CHS and REGARDS used different methods for data collection and not all the variables could be reconciled. Also, very few non-black/non-white participants in ARIC were included in the analysis as these cannot be differentiated from whites using the publicly available dataset. ARIC and CHS have a limited geographic representation and results from these studies may not be generalizable to the overall US population. In REGARDS, adjudication of nonfatal CHD events is triggered by participants’ self-report of a CHD-related hospitalization, which may result in underestimation of incidence rates. LVH was defined using electrocardiography, which may lead to a differential detection by race and gender as compared with echocardiography.59 Also, LVH and atrial fibrillation were measured at baseline. Some participants may have developed LVH or atrial fibrillation by the time of their incident CHD event. However, data on the presence of LVH and atrial fibrillation at the time of the event was not available in ARIC, CHS and REGARDS. Finally, we were not able to adjust CHD incidence across studies for changes in diagnostic methods, including more sensitive biomarkers.
In the current analysis, black men had consistently lower incidence of nonfatal CHD compared with white men, although having a higher burden of unfavorable social determinants of health and cardiovascular risk factors, and a higher incidence of fatal CHD. This black paradox on CHD incidence does not seem to be explained by racial differences in undetected MIs among men. Indeed, black men and women had a consistently lower risk for nonfatal and total CHD compared with white men and women, respectively after adjusting for social determinants of health and cardiovascular risk factors. Results from the current study also suggest that black men and women with incident CHD have higher case-fatality compared with white men and women, respectively which remained largely unexplained by social determinants of health and cardiovascular risk factors. These findings highlight the importance of primary prevention among blacks as they are more likely to die after their incident CHD event compared with whites.
Supplementary Material
Clinical Perspective.
What is new?
The incidence of nonfatal CHD is consistently lower among black versus white men, although the former have a higher burden of unfavorable social determinants of health and cardiovascular risk factors, and higher fatal CHD incidence.
After adjustment for social determinants of health and cardiovascular risk factors, black men and women have a similar risk for fatal CHD but lower risk for nonfatal CHD compared with white men and women, respectively.
Blacks with incident CHD have a higher case-fatality compared with whites, and the difference remains similar after adjustment for social determinants of health and cardiovascular risk factors.
What are the clinical implications?
The mechanisms leading to the apparent lower risk for nonfatal CHD among black versus white men and women need to be further elucidated.
Blacks have a higher risk for their initial CHD event being fatal compared with whites, highlighting the need for reinforcing primary prevention in this population.
Acknowledgments
Sources of Funding: This research project is supported by a cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. Additional support was provided by grants R01-HL080477 and K24-HL111154 from the National Heart, Lung, and Blood Institute. Representatives of the National Institutes of Health have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Disclosures: Dr. Levitan has received grant support from Amgen Inc. and served on an advisory board for Amgen Inc. outside the submitted work. Dr. Safford has been consultant for diaDexus and received grant support from Amgen Inc. outside the submitted work. Colantonio, Gamboa, Richman, Soliman and Howard have no disclosures.
Additional Acknowledgements: We thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions and further information about the study can be found at http://www.regardsstudy.org.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie CD, Wigington C, Hong Y. Coronary heart disease and stroke deaths - United States, 2009. MMWR Surveill Summ. 2013;62(Suppl 3):157–160. [PubMed] [Google Scholar]
- 3.National Heart, Lung, and Blood Institute. Morbidity & Mortality: 2012 Chartbook on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: NHLBI, Office of Science and Technology; 2013. [Cited: April 22, 2017]. Available in: https://www.nhlbi.nih.gov/research/reports/2012-mortality-chart-book. [Google Scholar]
- 4.Movahed MR, John J, Hashemzadeh M, Jamal MM, Hashemzadeh M. Trends in the age adjusted mortality from acute ST segment elevation myocardial infarction in the United States (1988–2004) based on race, gender, infarct location and comorbidities. Am J Cardiol. 2009;104:1030–1034. doi: 10.1016/j.amjcard.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 5.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. doi: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 7.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG MPH for the Cardiovascular Health Study Research Group (CHS) The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre RN, Furberg CD, Newman AB, Hulley SB, Gordon DJ, Gottdiener JS, McDonald RH, Jr, Psaty BM. Time trends in the use of cholesterol-lowering agents in older adults: the Cardiovascular Health Study. Arch Intern Med. 1998;158:1761–1768. doi: 10.1001/archinte.158.16.1761. [DOI] [PubMed] [Google Scholar]
- 10.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The REasons for Geographic And Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 11.Xie F, Colantonio LD, Curtis JR, Safford MM, Levitan EB, Howard G, Muntner P. Linkage of a Population-Based Cohort With Primary Data Collection to Medicare Claims: The Reasons for Geographic and Racial Differences in Stroke Study. Am J Epidemiol. 2016;184:532–544. doi: 10.1093/aje/kww077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geller NL, Sorlie P, Coady S, Fleg J, Friedman L. Limited access data sets from studies funded by the National Heart, Lung, and Blood Institute. Clin Trials. 2004;1:517–524. doi: 10.1191/1740774504cn050oa. [DOI] [PubMed] [Google Scholar]
- 13.Jones DW, Chambless LE, Folsom AR, Heiss G, Hutchinson RG, Sharrett AR, Szklo M, Taylor HA., Jr Risk factors for coronary heart disease in African Americans: the atherosclerosis risk in communities study, 1987–1997. Arch Intern Med. 2002;162:2565–2571. doi: 10.1001/archinte.162.22.2565. [DOI] [PubMed] [Google Scholar]
- 14.Levitan EB, Tanner RM, Zhao H, Muntner P, Thacker EL, Howard G, Glasser SP, Bittner V, Farkouh ME, Rosenson RS, Safford MM. Secular changes in rates of coronary heart disease, fatal coronary heart disease, and out-of-hospital fatal coronary heart disease. Int J Cardiol. 2014;174:436–439. doi: 10.1016/j.ijcard.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ettinger WH, Wahl PW, Kuller LH, Bush TL, Tracy RP, Manolio TA, Borhani NO, Wong ND, O’Leary DH. Lipoprotein lipids in older people. Results from the Cardiovascular Health Study. The CHS Collaborative Research Group. Circulation. 1992;86:858–869. doi: 10.1161/01.cir.86.3.858. [DOI] [PubMed] [Google Scholar]
- 16.Siscovick DS, Fried L, Mittelmark M, Rutan G, Bild D, O’Leary DH. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 17.Soliman EZ, Howard G, Prineas RJ, McClure LA, Howard VJ. Calculating Cornell voltage from nonstandard chest electrode recording site in the Reasons for Geographic And Racial Differences in Stroke study. J Electrocardiol. 2010;43:209–214. doi: 10.1016/j.jelectrocard.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tell GS, Polak JF, Ward BJ, Kittner SJ, Savage PJ, Robbins J. Relation of smoking with carotid artery wall thickness and stenosis in older adults. The Cardiovascular Health Study. The Cardiovascular Health Study (CHS) Collaborative Research Group. Circulation. 1994;90:2905–2908. doi: 10.1161/01.cir.90.6.2905. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 20.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 21.Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44:198–206. doi: 10.1053/j.ajkd.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 25.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 26.Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, McKnight B, Ives D, Gottdiener JS, Kuller LH, Longstreth WT., Jr Study of Cardiovascular Health Outcomes in the Era of Claims Data: The Cardiovascular Health Study. Circulation. 2016;133:156–164. doi: 10.1161/CIRCULATIONAHA.115.018610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 28.Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–896. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbier CE, Themudo R, Bjerner T, Johansson L, Lind L, Ahlstrom H. Long-term prognosis of unrecognized myocardial infarction detected with cardiovascular magnetic resonance in an elderly population. J Cardiovasc Magn Reson. 2016;18:43. doi: 10.1186/s12968-016-0264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 31.Proietti M, Marra AM, Tassone EJ, De Vuono S, Corrao S, Gobbi P, Perticone F, Corazza GR, Basili S, Lip GY, Violi F, Raparelli V. Frequency of Left Ventricular Hypertrophy in Non-Valvular Atrial Fibrillation. Am J Cardiol. 2015;116:877–882. doi: 10.1016/j.amjcard.2015.05.060. [DOI] [PubMed] [Google Scholar]
- 32.Chrispin J, Jain A, Soliman EZ, Guallar E, Alonso A, Heckbert SR, Bluemke DA, Lima JA, Nazarian S. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63:2007–2013. doi: 10.1016/j.jacc.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatterjee S, Bavishi C, Sardar P, Agarwal V, Krishnamoorthy P, Grodzicki T, Messerli FH. Meta-analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol. 2014;114:1049–1052. doi: 10.1016/j.amjcard.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Shenasa M, Shenasa H, El-Sherif N. Left ventricular hypertrophy and arrhythmogenesis. Card Electrophysiol Clin. 2015;7:207–220. doi: 10.1016/j.ccep.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2:216–223. doi: 10.1053/eupc.2000.0110. [DOI] [PubMed] [Google Scholar]
- 36.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 37.Okwuosa TM, Soliman EZ, Lopez F, Williams KA, Alonso A, Ferdinand KC. Left ventricular hypertrophy and cardiovascular disease risk prediction and reclassification in blacks and whites: the Atherosclerosis Risk in Communities Study. Am Heart J. 2015;169:155–161. e155. doi: 10.1016/j.ahj.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK. The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA. 1995;273:1592–1597. [PubMed] [Google Scholar]
- 39.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 42.Muntner P, Colantonio LD, Cushman M, Goff DC, Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd-Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hlatky MA, Ray RM, Burwen DR, Margolis KL, Johnson KC, Kucharska-Newton A, Manson JE, Robinson JG, Safford MM, Allison M, Assimes TL, Bavry AA, Berger J, Cooper-DeHoff RM, Heckbert SR, Li W, Liu S, Martin LW, Perez MV, Tindle HA, Winkelmayer WC, Stefanick ML. Use of Medicare data to identify coronary heart disease outcomes in the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes. 2014;7:157–162. doi: 10.1161/CIRCOUTCOMES.113.000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eaton LA, Driffin DD, Kegler C, Smith H, Conway-Washington C, White D, Cherry C. The role of stigma and medical mistrust in the routine health care engagement of black men who have sex with men. Am J Public Health. 2015;105:e75–82. doi: 10.2105/AJPH.2014.302322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammond WP, Matthews D, Mohottige D, Agyemang A, Corbie-Smith G. Masculinity, medical mistrust, and preventive health services delays among community-dwelling African-American men. J Gen Intern Med. 2010;25:1300–1308. doi: 10.1007/s11606-010-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams DR. The health of men: structured inequalities and opportunities. Am J Public Health. 2003;93:724–731. doi: 10.2105/ajph.93.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang ZM, Rautaharju PM, Prineas RJ, Rodriguez CJ, Loehr L, Rosamond WD, Kitzman D, Couper D, Soliman EZ. Race and Sex Differences in the Incidence and Prognostic Significance of Silent Myocardial Infarction in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016;133:2141–2148. doi: 10.1161/CIRCULATIONAHA.115.021177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearte CA, Furberg CD, O’Meara ES, Psaty BM, Kuller L, Powe NR, Manolio T. Characteristics and baseline clinical predictors of future fatal versus nonfatal coronary heart disease events in older adults: the Cardiovascular Health Study. Circulation. 2006;113:2177–2185. doi: 10.1161/CIRCULATIONAHA.105.610352. [DOI] [PubMed] [Google Scholar]
- 49.White AD, Rosamond WD, Chambless LE, Thomas N, Conwill D, Cooper LS, Folsom AR. Sex and race differences in short-term prognosis after acute coronary heart disease events: the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 1999;138:540–548. doi: 10.1016/s0002-8703(99)70158-4. [DOI] [PubMed] [Google Scholar]
- 50.Soliman EZ, Prineas RJ, Case LD, Russell G, Rosamond W, Rea T, Sotoodehnia N, Post WS, Siscovick D, Psaty BM, Burke GL. Electrocardiographic and clinical predictors separating atherosclerotic sudden cardiac death from incident coronary heart disease. Heart. 2011;97:1597–1601. doi: 10.1136/hrt.2010.215871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, Manhapra A, Mallik S, Krumholz HM. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353:671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathews R, Chen AY, Thomas L, Wang TY, Chin CT, Thomas KL, Roe MT, Peterson ED. Differences in short-term versus long-term outcomes of older black versus white patients with myocardial infarction: findings from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of American College of Cardiology/American Heart Association Guidelines (CRUSADE) Circulation. 2014;130:659–667. doi: 10.1161/CIRCULATIONAHA.113.008370. [DOI] [PubMed] [Google Scholar]
- 53.Polsky D, Lave J, Klusaritz H, Jha A, Pauly MV, Cen L, Xie H, Stone R, Chen Z, Volpp K. Is lower 30-day mortality posthospital admission among blacks unique to the Veterans Affairs health care system? Med Care. 2007;45:1083–1089. doi: 10.1097/MLR.0b013e3180ca960e. [DOI] [PubMed] [Google Scholar]
- 54.Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, Pollack CV, Jr, Gore JM, Chandra-Strobos N, Peterson ED, French WJ. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1026–1034. doi: 10.1016/j.ahj.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Wellenius GA, Mittleman MA. Disparities in myocardial infarction case fatality rates among the elderly: the 20-year Medicare experience. Am Heart J. 2008;156:483–490. doi: 10.1016/j.ahj.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams JE, Massing M, Rosamond WD, Sorlie PD, Tyroler HA. Racial disparities in CHD mortality from 1968–1992 in the state economic areas surrounding the ARIC study communities. Atherosclerosis Risk in Communities. Ann Epidemiol. 1999;9:472–480. doi: 10.1016/s1047-2797(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 57.Sung JF, Harris-Hooker SA, Schmid G, Ford E, Simmons B, Reed JW. Racial differences in mortality from cardiovascular disease in Atlanta, 1979–1985. J Natl Med Assoc. 1992;84:259–263. [PMC free article] [PubMed] [Google Scholar]
- 58.Roig E, Castaner A, Simmons B, Patel R, Ford E, Cooper R. In-hospital mortality rates from acute myocardial infarction by race in U.S. hospitals: findings from the National Hospital Discharge Survey. Circulation. 1987;76:280–288. doi: 10.1161/01.cir.76.2.280. [DOI] [PubMed] [Google Scholar]
- 59.Rautaharju PM, Park LP, Gottdiener JS, Siscovick D, Boineau R, Smith V, Powe NR. Race- and sex-specific ECG models for left ventricular mass in older populations. Factors influencing overestimation of left ventricular hypertrophy prevalence by ECG criteria in African-Americans. J Electrocardiol. 2000;33:205–218. doi: 10.1054/jelc.2000.7667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.