Abstract
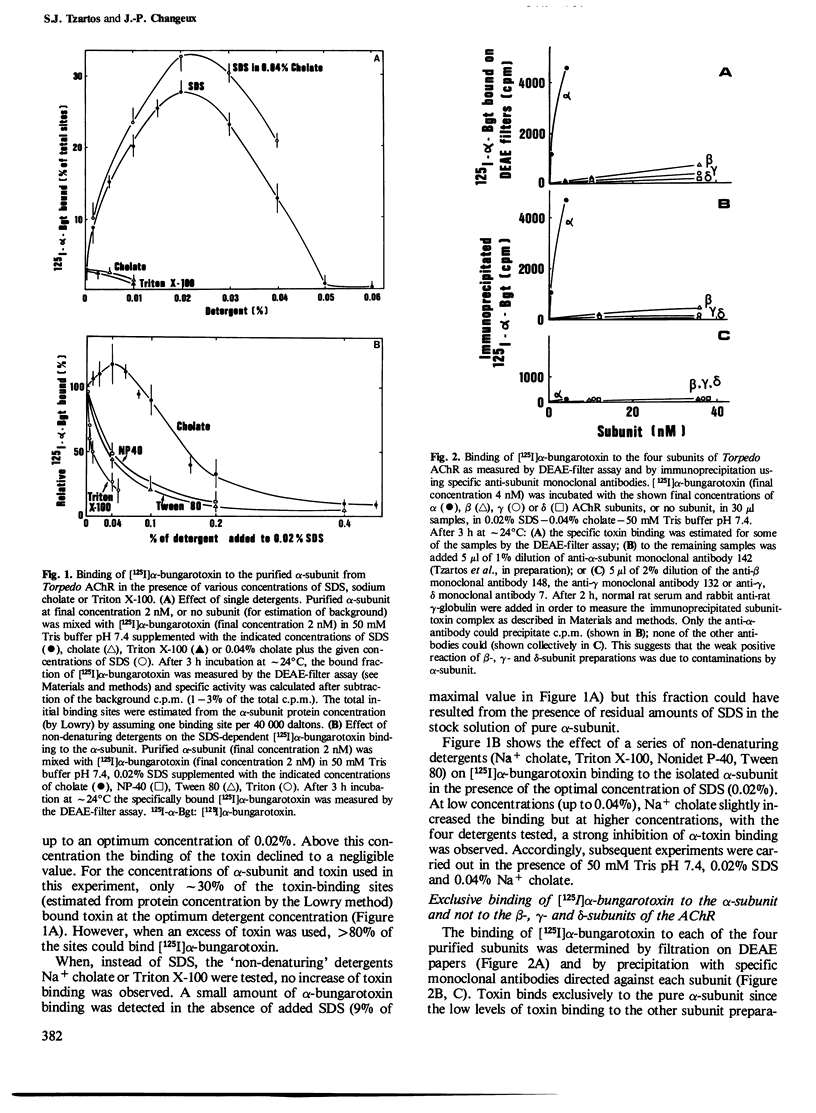
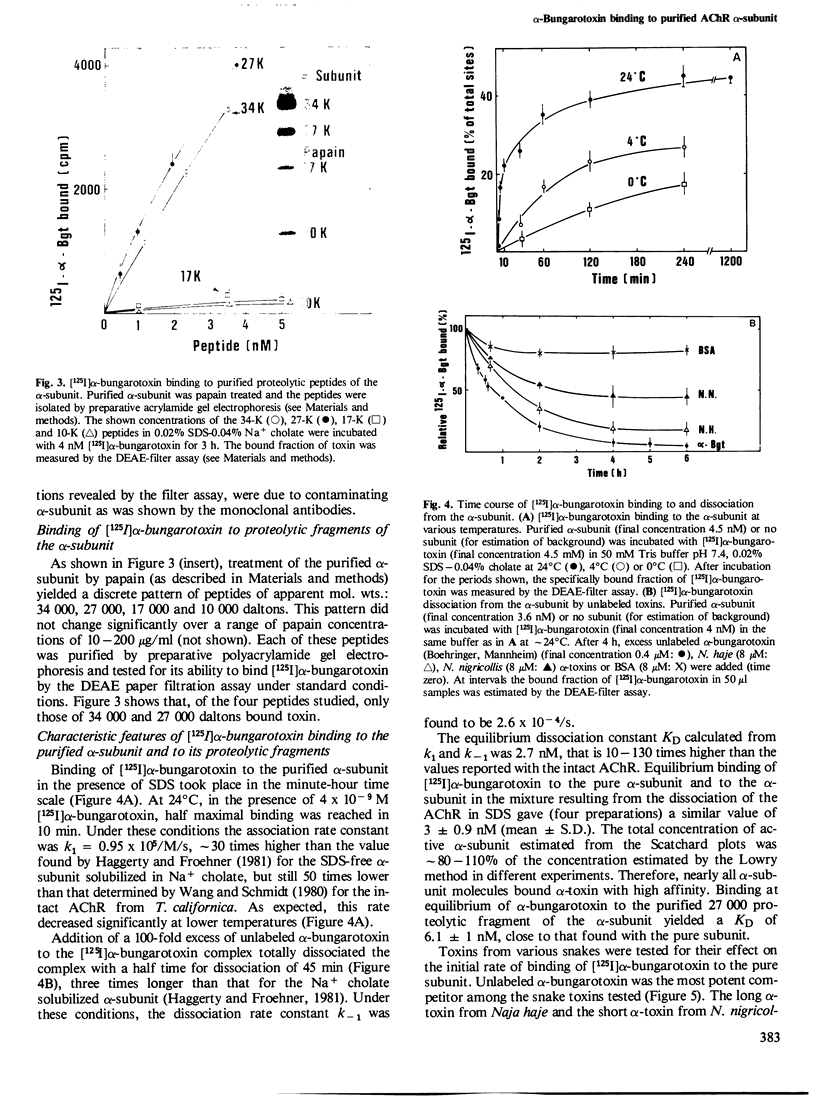
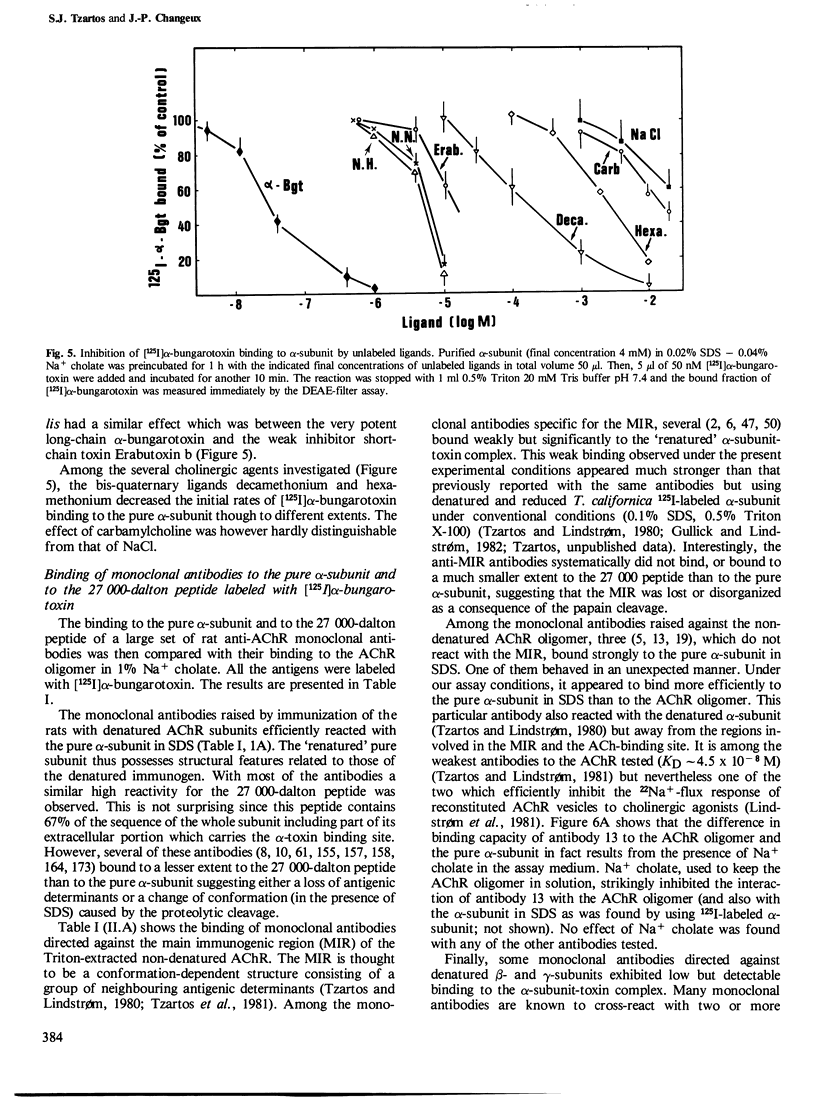
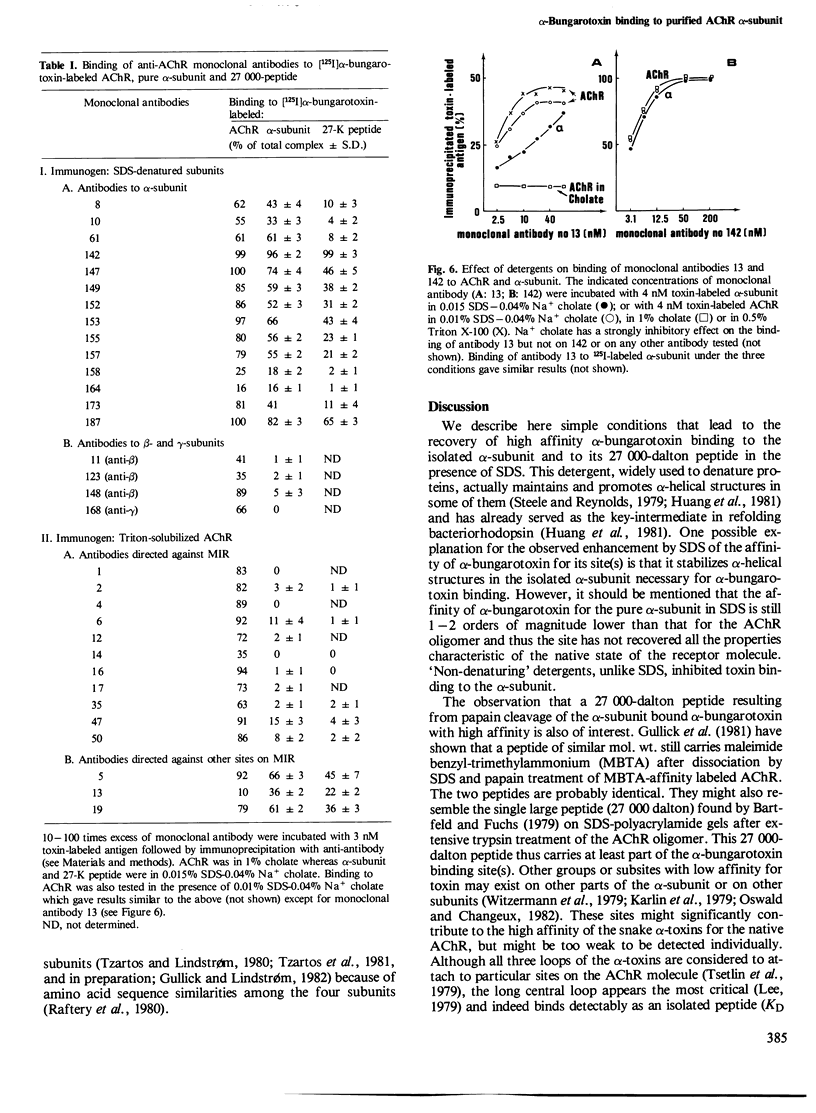
Intact nicotinic acetylcholine receptor (AChR) tightly binds alpha-bungarotoxin. The two toxin-binding sites are presumed to be on the two alpha-subunits, either on or near the ACh-binding sites. Isolated alpha-subunits have been found to maintain weak binding to alpha-bungarotoxin (KD approximately 0.2 microM). We describe here conditions under which the alpha-subunit and a 27,000-dalton proteolytic peptide bound alpha-bungarotoxin with high affinity. The four subunits of Torpedo marmorata AChR, as well as several proteolytic peptides of the alpha-subunit, were first purified by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. We found that the purified alpha-subunit (but not the beta-, gamma- or delta-subunits) and its 27,000-dalton peptide specifically bound 125I-labeled alpha-bungarotoxin with KD approximately 3 and 6 nM, i.e., about two orders of magnitude lower than the intact AChR. Nearly 100% of the sites were recovered. The recovery of this high affinity binding required the presence of SDS (approximately 0.02%) but non-denaturing detergents had a strongly inhibitory effect. Unlabeled alpha-toxins competed with labeled alpha-bungarotoxin, alpha-bungarotoxin being more effective than all the other toxins tested. Decamethonium and hexamethonium competed efficiently with alpha-bungarotoxin binding but carbamylcholine had only a weak effect. The main immunogenic region of the AChR was only partially preserved since conformation-dependent monoclonal antibodies to this region bound the alpha subunit-toxin complexes, but much less efficiently than the intact AChR. We conclude that SDS can be advantageous to the recovery of high toxin binding to the alpha subunit which still has not completely recovered its native conformation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5598–5602. doi: 10.1073/pnas.78.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt R., Lindstrom J., Montal M. Functional equivalence of monomeric and dimeric forms of purified acetylcholine receptors from Torpedo californica in reconstituted lipid vesicles. Eur J Biochem. 1980 Aug;109(2):481–487. doi: 10.1111/j.1432-1033.1980.tb04819.x. [DOI] [PubMed] [Google Scholar]
- Bartfeld D., Fuchs S. Active acetylcholine receptor fragment obtained by tryptic digestion of acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1979 Jul 27;89(2):512–519. doi: 10.1016/0006-291x(79)90659-4. [DOI] [PubMed] [Google Scholar]
- Brisson A. D., Scandella C. J., Bienvenüe A., Devaux P. F., Cohen J. B., Changeux J. P. Interaction of a spin-labeled long chain acylcholine with the cholinergic receptor protein in its membrane environment. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1087–1091. doi: 10.1073/pnas.72.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Heidmann T., Popot J. L., Sobel A. Reconstitution of a functional acetylcholine regulator under defined conditions. FEBS Lett. 1979 Sep 1;105(1):181–187. doi: 10.1016/0014-5793(79)80913-8. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Kasai M., Lee C. Y. Use of a snake venom toxin to characterize the cholinergic receptor protein. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1241–1247. doi: 10.1073/pnas.67.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Podleski T. R., Wofsy L. Affinity labeling of the acetylcholine-receptor. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2063–2070. doi: 10.1073/pnas.58.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. The acetylcholine receptor: an "allosteric" membrane protein. Harvey Lect. 1979 1980;75:85–254. [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Raftery M. A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Auffray C., Rougeon F., Changeux J. P. Identification of a cDNA clone coding for the acetylcholine binding subunit of Torpedo marmorata acetylcholine receptor. EMBO J. 1982;1(6):713–717. doi: 10.1002/j.1460-2075.1982.tb01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick W. J., Lindstrom J. M. The antigenic structure of the acetylcholine receptor from Torpedo californica. J Cell Biochem. 1982;19(3):223–230. doi: 10.1002/jcb.240190303. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Tzartos S., Lindstrom J. Monoclonal antibodies as probes of acetylcholine receptor structure. 1. Peptide mapping. Biochemistry. 1981 Apr 14;20(8):2173–2180. doi: 10.1021/bi00511a016. [DOI] [PubMed] [Google Scholar]
- Haggerty J. G., Froehner S. C. Restoration of 125I-alpha-bungarotoxin binding activity to the alpha subunit of Torpedo acetylcholine receptor isolated by gel electrophoresis in sodium dodecyl sulfate. J Biol Chem. 1981 Aug 25;256(16):8294–8297. [PubMed] [Google Scholar]
- Heidmann T., Sobel A., Popot J. L., Changeux J. P. Reconstitution of a functional acetylcholine receptor. Conservation of the conformational and allosteric transitions and recovery of the permeability response; role of lipids. Eur J Biochem. 1980 Sep;110(1):35–55. doi: 10.1111/j.1432-1033.1980.tb04839.x. [DOI] [PubMed] [Google Scholar]
- Huang K. S., Bayley H., Liao M. J., London E., Khorana H. G. Refolding of an integral membrane protein. Denaturation, renaturation, and reconstitution of intact bacteriorhodopsin and two proteolytic fragments. J Biol Chem. 1981 Apr 25;256(8):3802–3809. [PubMed] [Google Scholar]
- Huganir R. L., Schell M. A., Racker E. Reconstitution of the purified acetylcholine receptor from Torpedo californica. FEBS Lett. 1979 Dec 1;108(1):155–160. doi: 10.1016/0014-5793(79)81199-0. [DOI] [PubMed] [Google Scholar]
- Juillerat M. A., Schwendimann B., Hauert J., Fulpius B. W., Bargetzi J. P. Specific binding to isolated acetylcholine receptor of a synthetic peptide duplicating the sequence of the presumed active center of a lethal toxin from snake venom. J Biol Chem. 1982 Mar 25;257(6):2901–2907. [PubMed] [Google Scholar]
- Karlin A., Cowburn D. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of Electrophorus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3636–3640. doi: 10.1073/pnas.70.12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Damle V., Hamilton S., McLaughlin M., Valderamma R., Wise D. Acetylcholine receptors in and out of membranes. Adv Cytopharmacol. 1979;3:183–189. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y. Recent advances in chemistry and pharmacology of snake toxins. Adv Cytopharmacol. 1979;3:1–16. [PubMed] [Google Scholar]
- Lindstrom J., Cooper J., Tzartos S. Acetylcholine receptors from Torpedo and Electrophorus have similar subunit structures. Biochemistry. 1980 Apr 1;19(7):1454–1458. doi: 10.1021/bi00548a029. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Tzartos S., Gullick W. Structure and function of the acetylcholine receptor molecule studied using monoclonal antibodies. Ann N Y Acad Sci. 1981;377:1–19. doi: 10.1111/j.1749-6632.1981.tb33721.x. [DOI] [PubMed] [Google Scholar]
- Mendez B., Valenzuela P., Martial J. A., Baxter J. D. Cell-free synthesis of acetylcholine receptor polypeptides. Science. 1980 Aug 8;209(4457):695–697. doi: 10.1126/science.7394526. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Tzartos S., Lindstrom J. Inhibition of glycosylation with tunicamycin blocks assembly of newly synthesized acetylcholine receptor subunits in muscle cells. J Biol Chem. 1982 Mar 10;257(5):2694–2701. [PubMed] [Google Scholar]
- Meunier J. C., Olsen R. W., Menez A., Fromageot P., Boquet P., Changeux J. P. Some physical properties of the cholinergic receptor protein from Electrophorus electricus revealed by a tritiated alpha-toxin from Naja nigricollis venom. Biochemistry. 1972 Mar 28;11(7):1200–1210. doi: 10.1021/bi00757a014. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Raftery M. A. Studies of reversible and irreversible interactions of an alkylating agonist with Torpedo californica acetylcholine receptor in membrane-bound and purified states. Biochemistry. 1979 May 15;18(10):1862–1867. doi: 10.1021/bi00577a003. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald R. E., Changeux J. P. Crosslinking of alpha-bungarotoxin to the acetylcholine receptor from Torpedo marmorata by ultraviolet light irradiation. FEBS Lett. 1982 Mar 22;139(2):225–229. doi: 10.1016/0014-5793(82)80857-0. [DOI] [PubMed] [Google Scholar]
- Oswald R., Changeux J. P. Ultraviolet light-induced labeling by noncompetitive blockers of the acetylcholine receptor from Torpedo marmorata. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3925–3929. doi: 10.1073/pnas.78.6.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Oswald R., Wennogle L. P., Changeux J. P. Conditions for the selective labelling of the 66 000 dalton chain of the acetylcholine receptor by the covalent non-competitive blocker 5-azido-[3H]trimethisoquin. FEBS Lett. 1980 Jul 11;116(1):30–36. doi: 10.1016/0014-5793(80)80522-9. [DOI] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Steele J. C., Jr, Reynolds J. A. Characterization of the apolipoprotein B polypeptide of human plasma low density lipoprotein in detergent and denaturation solutions. J Biol Chem. 1979 Mar 10;254(5):1633–1638. [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Emtage J. S., Richards B. M., Barnard E. A. Active multi-subunit ACh receptor assembled by translation of heterologous mRNA in Xenopus oocytes. Nature. 1981 Aug 27;292(5826):862–864. doi: 10.1038/292862a0. [DOI] [PubMed] [Google Scholar]
- Tsetlin V. I., Karlsson E., Arseniev A. S., Utkin Y. N., Surin A. M., Pashkov V. S., Pluzhnikov K. A., Ivanov V. T., Bystrov V. F., Ovchinnikov Y. A. EPR and fluorescence study of interaction of Naja naja oxiana neurotoxin II and its derivatives with acetylcholine receptor protein from Torpedo marmorata. FEBS Lett. 1979 Oct 1;106(1):47–52. doi: 10.1016/0014-5793(79)80692-4. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Rand D. E., Einarson B. L., Lindstrom J. M. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981 Aug 25;256(16):8635–8645. [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. K., Schmidt J. Primary structure and binding properties of iodinated derivatives of alpha-bungarotoxin. J Biol Chem. 1980 Dec 10;255(23):11156–11162. [PubMed] [Google Scholar]
- Weber M., Changeux J. P. Binding of Naja nigricollis (3H)alpha-toxin to membrane fragments from Electrophorus and Torpedo electric organs. 3. Effects of local anaesthetics on the binding of the tritiated alpha-neurotoxin. Mol Pharmacol. 1974 Jan;10(1):35–40. [PubMed] [Google Scholar]
- Witzemann V., Muchmore D., Raftery M. A. Affinity-directed cross-linking of membrane-bound acetylcholine receptor polypeptides with photolabile alpha-bungarotoxin derivatives. Biochemistry. 1979 Nov 27;18(24):5511–5518. doi: 10.1021/bi00591a039. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Raftery M. A. Carbamylcholine-induced rapid cation efflux from reconstituted membrane vesicles containing purified acetylcholine receptor. Biochem Biophys Res Commun. 1979 Jul 12;89(1):26–35. doi: 10.1016/0006-291x(79)90938-0. [DOI] [PubMed] [Google Scholar]




