Summary
The surgical management of atrial fibrillation (AF) is an evolving field with a history of testing various lesion sets and ablation technologies. Prior animal models of AF require a chronic intervention to make AF reliably inducible. Our objective was to create an acute, reliable and reproducible porcine model of sustained AF. To accomplish this, twenty-one adult domestic pigs underwent median sternotomy. Methods to induce AF were then performed sequentially: manual stimulation, rapid pacing (200 beats per minute), then rapid pacing of 8 beats with a cycle length of 300 msecs, followed by an extra stimulus at decreasing cycle lengths. If AF was not induced, burst pacing was done at a cycle length of 90 msec for 30 seconds. If AF was still not induced, IV neostigmine was administered and the process was repeated. AF was considered sustained after 1 minute. Attempts at AF induction were successful in 18 of 21 (86%). AF was induced during manual stimulation in 4 (19%), during rapid pacing in 5 (24%), during burst pacing in 5 (24%) and after the administration of neostigmine in 4 (19%). Mean duration of AF was 3.6 ± 2.6 minutes. Fourteen of the 18 (78%) reverted to sinus rhythm spontaneously and 4 (22%) required an antiarrhythmic. This technique of inducing AF can easily be used to evaluate new technologies and lesion sets without the need for creating a chronic animal model.
Keywords: atrial fibrillation, animal model, arrhythmia surgery, porcine
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, occurring in about 1% of the general population, and about 10% of patients over 80 years of age[1, 2]. The gold standard for the surgical treatment of AF, the Cox-Maze procedure, has a cure rate of over 90% in our experience.[3] Because the operation is technically challenging, groups have attempted to use various energy sources to create ablation lines to replace the surgical lesions of the Cox-Maze procedure. These include cryoablation, radiofrequency energy (both unipolar and bipolar), ultrasound, laser, and microwave[4–11]. Alternative lesion sets have been proposed as well. Furthermore, catheter based interventions are becoming mainstream[12]. These new and promising technologies have the potential to change the approach to the treatment of AF.
Ideally, prior to clinical implementation of a novel ablation technique for the treatment of AF, the efficacy of the therapy should first be evaluated in an animal model. Several animal models exist, but they require chronic atrial pacing, induction of heart failure, or surgical atrial enlargement[13, 14]. Such models, while adequate, represent a large cost and time burden. A simple and reliable method of sustained AF is needed to test new lesion sets and ablation technologies. We describe here an acute porcine model fulfilling these criteria that is reliably reproducible and does not require long-term alteration of atrial tissues.
Methods
Twenty-one adult domestic pigs weighing 45 to 114 kg were utilized in this study. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals,” published by the National Institutes of Health (NIH publication revised 1996). This study was performed according to our institutional policy and was approved by our Animal Studies Committee.
Preoperatively, each animal was premedicated with intramuscularly administered telazol (4.4 mg/kg), ketamine (2.2mg/kg), and xylozene (2.2mg/kg), intubated, and anesthetized with 2–4% isoflurane. Initial intravenous access was obtained by ear vein. The animals were monitored continuously during the procedure with an electrocardiogram, pulse oximeter placed on the tail, end-tidal CO2 and a temperature probe. A femoral cutdown was performed and arterial access was obtained for blood pressure monitoring and a femoral central venous catheter was placed for medication administration and central venous pressure monitoring. An arterial blood sample was obtained approximately every 30 minutes and an arterial blood gas analysis was performed and the ventilator rate and tidal volume were adjusted accordingly to maintain a physiologic pH. Electrolyte values were checked with each blood sample and supplemented to maintain a potassium level greater than 3.0 mmol/L and an ionized calcium level greater than 1.0 mmol/L.
A median sternotomy was created, and the pericardial sac was opened. The pericardial fluid was removed by suction to expose the atria to air, and the pericardial edges were elevated with silk sutures, creating a pericardial sling. Atrial fibrillation induction was attempted by three sequential maneuvers. First, the heart was manually stimulated by retracting the heart to expose the atria. Retraction to view the posterior lateral right atrium and then the posterior lateral left atrium was done with the hand, wearing standard surgical gloves. This would simulate AF that is induced due to mechanical stimulation as in postoperative AF.
For the second intervention, if AF did not occur with removal of pericardial fluid and exposure to air and manual stimulation, a pacing electrode (20 mAmp, 300 msec or 200 bpm) was placed on the superior right atrial free wall and paced for up to 30 seconds to induce AF. An S1S2 protocol was utilized, initially, 8 S1s at 300ms interval with an S2 starting at approximately 180ms and decreasing by 5 ms until AF or the effective refractory period was reached. Then, this value minus 10 ms was used to burst pace for 30 seconds. This was attempted 3 times or until AF was induced.
If the animal still did not fibrillate after burst pacing, 2.5mg of intravenous neostigmine was administered and the above steps were repeated. Neostigmine was given to inhibit acetylcholine esterase, which increased vagal tone without significantly reducing systemic pressure. The increased vagal tone reduces atrial refractory periods increasing the inducibility of AF. The pacing and neostigmine maneuvers simulate alterations in autonomic activation, vagal innervation and catecholamine release.
Sustained fibrillation was defined as AF for greater than 1 minute, confirmed by the continuous rhythm tracing. After AF was sustained, animals either spontaneously returned to normal sinus rhythm or were chemically cardioverted after 10 minutes with administration of an intravenous antiarrhythmic drug, bretylium (4mg/kg) or amiodarone (10mg/kg). At the end of the procedure all animals were euthanized with a lethal dose of potassium injected into the ascending aorta or left ventricle.
Results
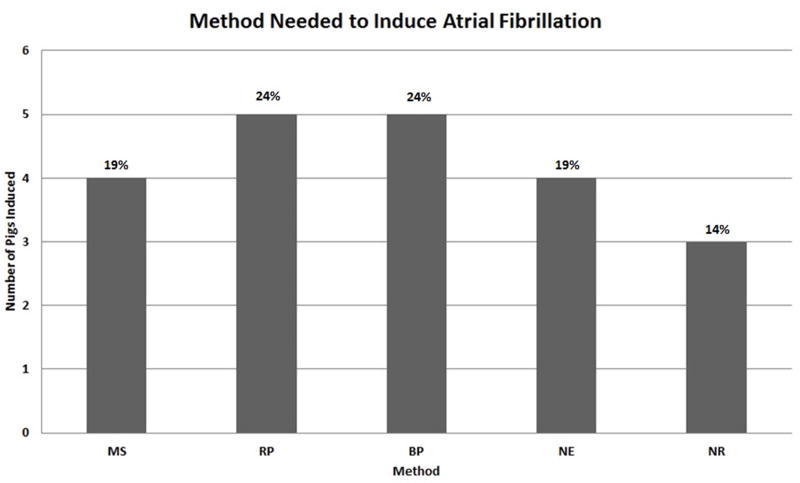
All 21 animals survived to the end of the procedure. AF was sustained in all but three animals, representing an 86% (18/21) success rate. Four (19%) animals spontaneously developed AF with manual stimulation alone. Five (24%) animals required rapid pacing on the right atrial free wall to induce AF. Five (24%) additional animals developed sustained AF with burst pacing. Four (19%) animals did not respond to the above steps and but developed sustained AF after neostigmine (Figure 1). Atrial fibrillation was sustained for 3.6 ± 2.6 minutes (range 1–10 minutes). Fourteen (78%) animals reverted to sinus rhythm spontaneously, while 4 (22%) animals required the administration of an antiarrhythmic. No animals required electrical cardioversion for hemodynamic instability.
Figure 1.
Success rate at inducing atrial fibrillation of each intervention. MS: Manual stimulation alone, RP: Rapid pacing, BP: Burst pacing, NE: Neostigmine, NR: Non responder
In the animals that did not sustain AF, one fibrillated transiently for 20 seconds. This animal did not receive neostigmine. The remaining two animals maintained sinus rhythm despite the administration of neostigmine. There was no association found when evaluating the impact of weight on inability to induce AF. The animals which did not develop AF were 45, 81 and 91 kg in size.
Discussion
The success of this study represents an acute model of atrial fibrillation. Nearly all the animals in this study sustained atrial fibrillation without pharmacologic intervention. Of the 21 animals in the study, AF was able to be induced in 14 without neostigmine. In the remaining 7 animals, 4 went into atrial fibrillation with the administration of neostigmine. One animal, not counted as a success, fibrillated for 20 seconds but did not receive neostigmine. It is possible that this animal, if given neostigmine, would have maintained atrial fibrillation for greater than one minute, thus making it an acceptable test subject for an antiarrhythmic intervention. Overall, AF was maintained for an average of 3.6 minutes. Our data shows that the porcine atria represents a stable substrate for the induction and maintenance of atrial fibrillation and are, therefore, a good model for the acute screening of ablation technology and lesions sets. This study represents an easily reproducible, reliable protocol for inducible AF, furthermore, this is an acute protocol, requiring no prior intervention or chronic therapy.
With the introduction of new technologies and lesion sets to replace some or all of the surgical incisions of the Cox-Maze procedure, a model to test the effectiveness of these interventions needs to be utilized. Several substitutions can be used, including computer-based simulation, canine, caprine, ovine and porcine models. Atrial fibrillation has been induced in both canine cardiac tissue and an ovine animal model[15, 16]. However, the porcine model has several advantages. These include the similarity of the size of the heart, the relative likeness of anatomy to humans, as well as the overall robustness of health in this animal. A porcine model in which AF is induced reliably is a valuable resource for testing the success of a new intervention for the treatment of AF. We have used this porcine model to test the efficacy of a new technology[17]. In one example, this was done by performing a modified Cox-Maze procedure, with minor modifications, with all lesions created with a bipolar radiofrequency ablation device[17]. We then confirmed success of the ablation by demonstrating inability to induce AF using the method described. Additionally, multiple other technologies have been evaluated in a similar fashion including radiofrequency, microwave, laser and cryothermal[18]. This acute model permits the testing of many devices without the need for a chronic model.
Limitations
Though this experimental model demonstrated a high success rate, there were some important limitations. Multiple modes of inducing AF were utilized. Though these were treated similarly, they may actually simulate different mechanisms of AF and therefore, have unique attributes impacting their preventability or treatability. Further, the inability to achieve a 100% success rate may post logistic problems as it may necessitate a higher sample size to show a statistically significant difference if this model is used to evaluate a preventative therapy. Other modifications could be attempted to increase the success rate, such as left atrial stimulation. This may induce AF due to the shorter refractory period within the left atrium. Lastly, while a chronic animal model is more tedious and expensive, it likely offers a more representative model of the pathology and reversibility of this chronic disease in humans.
Conclusions
This series shows that the porcine heart will fibrillate reliably without chronic stimulation. In the majority of animals, rapid or burst pacing caused AF. The porcine heart is sensitive to stimulation, yet AF can be terminated by administration of bretylium or amiodarone. Because the porcine heart is sensitive to manipulation and rapid pacing, the inability to induce AF after creating a lesion set can be considered as a successful intervention. This model allows for the evaluation of new technologies, lesion patterns, and interventions for the treatment and prevention of AF.
Acknowledgments
The research was supported in part by National Institutes of Health grants 5R01 HL32257, F32 HL078136-01, and R44 HL67535.
Ralph J. Damiano, Jr., MD, is a consultant for AtriCure, Inc., West Chester, OH USA, and has received research equipment and funding.
Footnotes
Disclosures: Anson M. Lee, MD Jacob R. Miller, MD Rochus K. Voeller, MD Andreas Zierer, MD Shelly C. Lall, MD, Richard B. Schuessler, PhD, and Spencer J. Melby, MD, declare no conflicts of interest.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 2.Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74:236–41. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 3.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–8. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 4.Damiano RJ., Jr Alternative energy sources for atrial ablation: judging the new technology. Ann Thorac Surg. 2003;75:329–30. doi: 10.1016/s0003-4975(02)04844-0. [DOI] [PubMed] [Google Scholar]
- 5.Gillinov AM, Smedira NG, Cosgrove DM., III Microwave ablation of atrial fibrillation during mitral valve operations. Ann Thorac Surg. 2002;74:1259–61. doi: 10.1016/s0003-4975(02)03760-8. [DOI] [PubMed] [Google Scholar]
- 6.Knaut M, Spitzer SG, Karolyi L, et al. Intraoperative microwave ablation for curative treatment of atrial fibrillation in open heart surgery--the MICRO-STAF and MICRO-PASS pilot trial. MICROwave Application in Surgical treatment of Atrial Fibrillation. MICROwave Application for the Treatment of Atrial Fibrillation in Bypass-Surgery. Thorac Cardiovasc Surg. 1999;47(Suppl 3):379–84. doi: 10.1055/s-2007-1013205. [DOI] [PubMed] [Google Scholar]
- 7.Venturini A, Polesel E, Cutaia V, et al. Intraoperative microwave ablation in patients undergoing valvular surgery: midterm results. Heart Surg Forum. 2003;6:409–11. [PubMed] [Google Scholar]
- 8.Knaut M, Tugtekin SM, Spitzer S, et al. Combined atrial fibrillation and mitral valve surgery using microwave technology. Semin Thorac Cardiovasc Surg. 2002;14:226–31. doi: 10.1053/stcs.2002.33754. [DOI] [PubMed] [Google Scholar]
- 9.Mokadam NA, McCarthy PM, Gillinov AM, et al. A prospective multicenter trial of bipolar radiofrequency ablation for atrial fibrillation: early results. Ann Thorac Surg. 2005;78:1665–70. doi: 10.1016/j.athoracsur.2004.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee A, Singh S, Tempe DK. Intraoperative endocardial ablation of chronic atrial fibrillation along with mitral valve surgery using high frequency ultrasound with a ball-tipped harmonic scalpel probe. Indian Heart J. 2004;56:178–80. [PubMed] [Google Scholar]
- 11.Reddy VY, Houghtaling C, Fallon J, et al. Use of a diode laser balloon ablation catheter to generate circumferential pulmonary venous lesions in an open-thoracotomy caprine model. Pacing Clin Electrophysiol. 2004;27:52–7. doi: 10.1111/j.1540-8159.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Natale A, Padanilam BJ, et al. Why Atrial Fibrillation Ablation Should Be Considered First-Line Therapy for Some Patients. 2005;112:1214–1222. doi: 10.1161/CIRCULATIONAHA.104.478263. [DOI] [PubMed] [Google Scholar]
- 13.Smith PK, Holman WL, Cox JL. Surgical treatment of supraventricular tachyarrhythmias. Surg Clin North Am. 1985;65:553–70. doi: 10.1016/s0039-6109(16)43637-6. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi S, Sato S, Schuessler RB. Induced atrial arrhythmias in a canine model of left atrial enlargement. Pacing Clin Electrophysiol. 1990;13:556. (abstract) [Google Scholar]
- 15.Schuessler RB, Grayson TM, Bromberg BI, et al. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circulation Research. 1992;71:1254–67. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 16.Geddes LA, Hinds M, Babbs CF, et al. Maintenance of atrial fibrillation in anesthetized and unanesthetized sheep using cholinergic drive. Pacing Clin Electrophysiol. 1996;19:165–75. doi: 10.1111/j.1540-8159.1996.tb03308.x. [DOI] [PubMed] [Google Scholar]
- 17.Gaynor SL, Ishii Y, Diodato MD, et al. Successful performance of Cox-Maze procedure on beating heart using bipolar radiofrequency ablation: a feasibility study in animals. Ann Thorac Surg. 2004;78:1671–7. doi: 10.1016/j.athoracsur.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 18.Schuessler RB, Lee AM, Melby SJ, et al. Animal studies of epicardial atrial ablation. Heart Rhythm. 2009;6:S41–5. doi: 10.1016/j.hrthm.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]