Abstract
Extracellular matrix modulus plays an important role in regulating cell morphology, proliferation and differentiation during regular and diseased states. Although the effects of substrate topography and modulus on MSC differentiation are well known with respect to osteogenesis and adipogenesis, there has been relatively little investigation on the effects of this phenomenon on tenogenesis. Furthermore, relative roles of topographical factors (matrix alignment vs. matrix modulus) in inducing tenogenic differentiation is not well understood. In this study we investigated the effects of modulus and topographical alignment of type I collagen substrate on tendon differentiation. Type I collagen sheet substrates with random topographical alignment were fabricated with their moduli tuned in the range of 0.1, 1, 10 and 100 MPa by using electrocompaction and controlled crosslinking. In one of the groups, topographical alignment was introduced at 10 MPa stiffness, by controlled unidirectional stretching of the sheet. RT-PCR, immunohistochemistry and immunofluorescence results showed that mimicking the tendon topography, i.e. increasing the substrate modulus as well as alignment increased the tenogenic differentiation. Higher substrate modulus increased the expression of COLI, COLIII, COMP and TSP-4 about 2–3 fold and increased the production of COLI, COLIII and TSP-4 about 2–4 fold. Substrate alignment up regulated COLIII and COMP expression by 2 fold. Therefore, the tenoinductive collagen material model developed in this study can be used in the research and development of tissue engineering tendon repair constructs in future.
Graphical abstract
1. Introduction
Modulus of the extracellular matrix plays an important role in regulating cell morphology, proliferation, differentiation during regular and diseased states. For example, 1 kPa stiffness favors differentiation of MSCs into neuronal-like cells, 10 kPa elasticity promotes myogenic differentiation, and a 100 kPa matrix stiffness stimulates osteogenic differentiation1. The effects of substrate topography and modulus on MSC differentiation are well known with respect to osteogenesis and adipogenesis1–6. However, there has been relatively little investigation of this phenomenon with respect to tenogenesis. Sharma et al. studied the effect of tenogenesis on collagen coated polyacrylamide substrate with modulus gradient in a modulus range less than 0.1 MPa5, 7. On the other hand, it is known that the native modulus of the tendon tissue to be as high as 1200 MPa8, 9. Therefore, studies to date do not cover a modulus range that is more compatible with the tendon tissue environment.
The literature also reports that unidirectionally aligned fibrous topography as another tenogenic differentiation cue. Tong et al. reported that tendon cell differentiation of hMSCs occurs on collagen coated PDMS substrates which replicate native tendon surface10. Yin found that aligned substrate promotes tenogenesis in tendon/stem progenitor cells (TPSC) but did not evaluate MSCs11. Furthermore, aligned electrochemically compacted collagen fiber, electrospun polyurethane (PU), silk fibroin, poly (l-lactic acid) and two-dimensional (2D) microgrooved surface showed elongated cell morphology and tendon related ECM formation12–19.
To date, the effects of matrix alignment and matrix modulus on tenogenesis have been investigated separately and the synergy between the two variables in terms of inducing tenogenic differentiation is not studied. Our research group created a collagen based material platform to investigate the effect of modulus in a broad range of 0.1 MPa to 100 MPa while controlling the topographical alignment random or unidirectional. In this method, collagen solutions are transformed to highly dense randomly oriented collagen sheets by electrochemical compaction induced by planar electrodes20–22. Matrix alignment is introduced in these sheets by controlled unidirectional stretch of the sheets as molecular alignment lacks when planar electrodes are used to fabricate sheets21. The aim of the current study is to study the effects of matrix modulus and alignment on tenogenic differentiation of human MSCs. Importantly, a broad modulus range of 0.1 MPa to 100 MPa is covered logarithmically for the first time in the literature. Elucidation of the mechanism of topographically induced tenogenesis in human MSCs as such will assist in optimizing materials to produce scaffolds for tendon repair and may provide insight into the differentiation mechanisms of MSCs and other stem cells in vivo.
2. Materials and Methods
2.1 Collagen Sheet Fabrication by Electrochemical Compaction [Figure 1]
Figure 1.
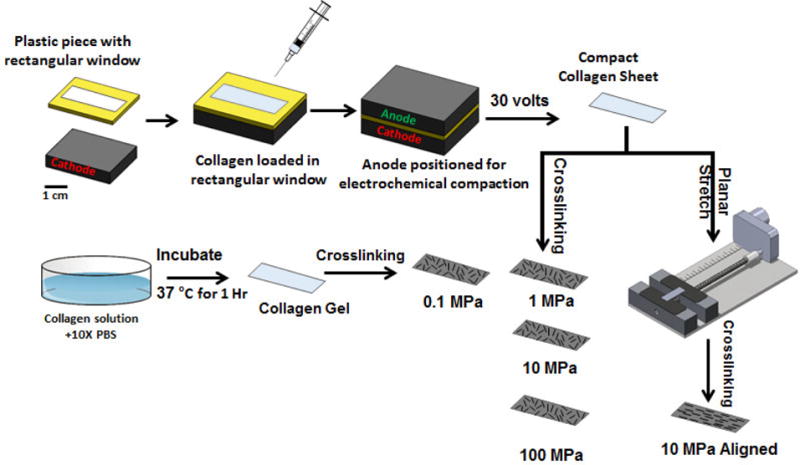
Overview of fabricating collagen sheets with different modulus levels.
Electrocompacted collagen sheet was fabricated by the method as described before21. Briefly, two-fold diluted (with RNAase/DNAase free water) acid soluble monomeric Type-I collagen solution (Collagen Solutions, San Jose, CA; 6 mg/ml) was adjusted for pH 8–10 using 1 N NaOH and dialized using ultrapure water for 18 hours. The collagen solution was then used for electrocompaction.
Collagen sheet was fabricated in sheet form by electrocompaction method described before20, 23. Briefly, a 30×10×1.5 mm rectangular window was cut in a plastic piece. The plastic piece was then sandwiched between two planar carbon electrodes by filling the rectangular window with the collagen solution. A 30 VDC electrical potential was applied across the electrodes for 2 min. Collagen molecules are electrophoretically mobilized and compacted due to pH gradients established between the two electrodes under the mechanisms detailed in an earlier publication24. 100–200 microns thick collagen sheets of 30×10 mm dimensions were generated by the electrocompaction. Collagen molecules are randomly oriented within the plane of the sheet. In one of the experimental groups, collagen molecules were unidirectionally aligned by stretching the sheet using a motorized mechanical device as described earlier21 (Figure 1 & 2). Collagen sheet samples were incubated in phosphate buffered saline (PBS) for six hours at 37 °C to induce fibril formation and treated with 2-propanol solution for 12 hours. Different levels of modulus were attained by crosslinking the sheets as will be described.
Figure 2.
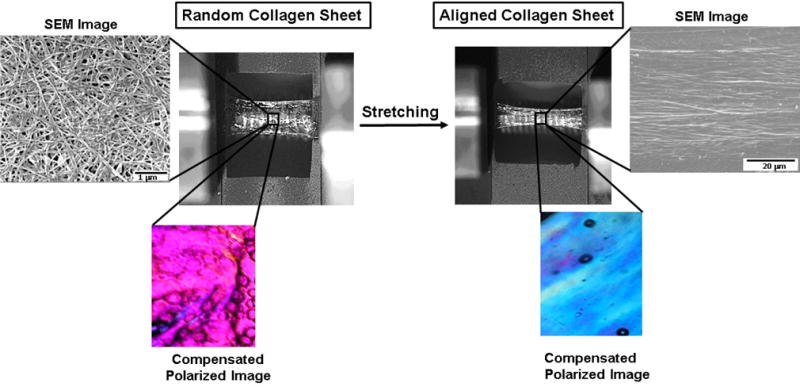
Manufacturing aligned sheet with mechanical stretching. Molecular alignment is evident by CPI image (Emergence of blue color after stretching indicates molecular alignment). Collagen fibril alignment is evident by SEM images.
2.2 Synthesis of Collagen Gel
Elastically most compliant group was the standard collagen gel which was not subjected to electrocompaction. Acid soluble monomeric collagen solution was mixed with 10× PBS at 8:1 ratio. pH was adjusted to 7.4 using 0.1 N NaOH. The collagen solution was then placed in at 37 °C for 1h by pouring in a petri dish to form gel. The gel was then crosslinked with genipin in 95% ethanol for 3-days as detailed in Table 1.
Table 1.
Experimental groups for different level of modulus range and unidirectionally aligned substrate
| No | Groups | Target Modulus (MPa) | Experimental Modulus (MPa) |
|---|---|---|---|
| 1 | Randomly oriented gel (3day, 0.6% genipin crosslinked) | 0.1 | 0.1 ± 0.04 |
| 2 | Randomly oriented compact sheet (1hr, 0.1% genipin crosslinked) | 1 | 1.1 ± 0.18 |
| 3 | Randomly oriented compact sheet (6hr, 0.6% genipin crosslinked) | 10 | 10.4 ± 2.63 |
| 4 | Highly anisotropic and aligned compact sheet (1hr, 0.3% genipin crosslinked) | 10 | 11.8 ± 2.44 |
| 5 | Randomly oriented compact sheet (3 day, 2% genipin crosslinked) | 100 | 92.9 ± 15.24 |
2.3 Experimental Groups
Four levels of modulus were targeted (0.1, 1, 10 and 100 MPa) for randomly aligned collagen sheets to investigate the effect of modulus on tenoinduction. These modulus levels were attained by changing the crosslinking protocols [Table 1]. Crosslinking was carried out by genipin (Wako Chemical, Japan) in 90% v/v ethanol solution at 37 °C. The fifth group was a unidirectionally aligned collagen sheet group at 10 MPa along the axis of alignment to assess the effects of anisotropy. Samples were treated with per acetic acid (Sigma Aldrich, USA) ethanol solution (2% Acetic acid+96% Ethanol) after the cross linking to bleach out extra genipin which may keep crosslinking the samples.
2.4 Imaging of 10 MPa Compacted Collagen Sheets for Molecular Alignment
Polarized optical microscope (Olympus BX51, Melville, NY, USA) was used to assess the alignment of collagen molecules at 10 MPa aligned group as described before21. Briefly, as collagen is a positive birefringent material, the aligned molecules shows blue interference color along the slower axis of first order wavelength gypsum plate25. Therefore, molecular alignment is indicated by blue in the Compensated Polarized Imaging (CPI) and lack of alignment is indicated by Magenta color. Alignment was also confirmed by scanning electron microscopy (SEM-FEI Nova Nanolab 200, Hillsboro, Oregon).
2.5 Assessment of Mechanical Properties
Collagen sheet samples described in Table 1 were tested at a strain rate of 10 mm/min under monotonic tension (Rheometrics Inc., NJ) (n = 6–8 samples per group) to assess the attainment of targeted modulus values. Samples were cut into 20 × 2 mm strips and hydrated in deionized water for 30 minutes before testing. A custom-made micrometer was used to measure the thickness of the sheet samples where the micrometer closes an electrical circuit upon contact with the surface of the sample. Stress-strain curves were constructed using the load-displacement data and sample geometry. Modulus was calculated from the slopes of the linear regions of stress strain curves.
2.6 Effect of Matrix Modulus and Alignment on Tenogenic Differentiation of Human MSCs
Samples were sterilized in 70% ethanol for 4 hours, washed in 1× PBS and placed into ultralow attachment 24 well plates (Corning) (n = 3 wells/group). Human mesenchymal stem cells (MSCs) (Lonza, Walkersville, MD) at passage 5 were seeded at a density of 20,000 cells/cm2. The culture medium composed of alpha MEM (Invitrogen) supplemented with 10% MSC-Qualified FBS (Invitrogen), 1% penicillin/streptomycin and 50 μg/mL ascorbic acid. Cells were cultured for 21 days with medium changes every 3 days. Tenogenic differentiation was assessed by RT-PCR at day 3, 14 and 21. At time points 3, 14 and 21 days total RNA was extracted by lysing the cells using TRIZOL reagent (Invitrogen) following manufacturer’s protocol and as described before21. cDNA was synthesized from 2 μg of total RNA by reverse transcription using the cDNA Reverse Transcription Kit (Applied Biosystems). Taqman real time PCR mastermix (Applied Biosystems) and Taqman gene expression assays (Applied Biosystems) were used to evaluate the expression of the genes by quantitative real time PCR (Applied Biosystems 7500 Real Time PCR System). For tendon related markers (Collagen I, Collagen III, and COMP) and tendon specific markers (Scleraxis, Thrombospondin) were used. To assess MSC differentiation to chondrogenic, osteogenic adipogenic lineages expressions of COL2, RUNX2 and PPARγ were studied. 2 deltadeltaCt method was used to quantify relative fold change in the target gene expression by normalizing the target gene expression to RPLP022 as a housekeeping gene and relative to the expression on 0.1 MPa group at day 3.
2.7 Effect of Matrix Modulus and Alignment on Tendon Related Matrix Synthesis
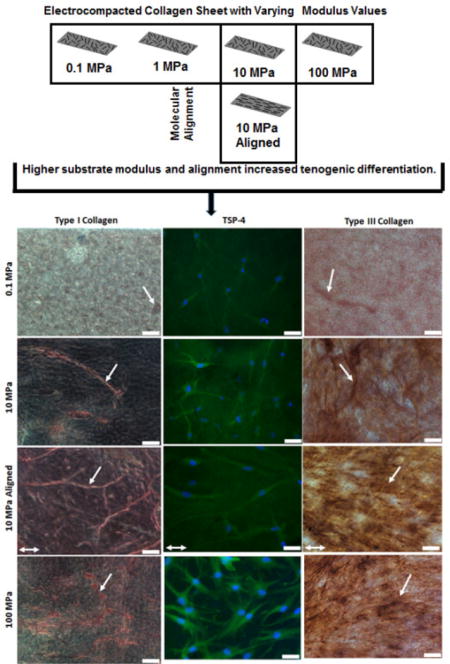
Based on the results of RT-PCR, 1 MPa group was not included in matrix synthesis study. MSCs were seeded on 0.1 MPa, 10 MPa, 10 MPa aligned and 100 MPa groups as described above. MSCs were cultured on the specified groups for 21 days as described in section 2.6. Tendon-related extracellular matrix molecules-i) type-I collagen, ii) type-III collagen and iii) Thrombospondin-4 (TSP-4) were investigated. Immunohistochemistry was performed for type-I and type-III collagen, and for TSP-4 green immunofluorescence was performed.
Samples were fixed and permeabilized in 10% neutral buffered formalin and 0.25% Triton X-100 (in PBS) respectively as described before. Samples were blocked in Phosphate Buffered Saline with Tween (PBST-5% BSA, 22.52 mg/ml glycine and 0.1% Tween 20) for 30 min and then incubated overnight at 4°C with primary antibody. Mouse Anti-Col1A1 (Abcam), rabbit anti-Col III (Abcam), Thrombospondin 4 (Santa-Cruz) were used as primary antibody. Negative control for the secondary antibody was samples incubated with blocking solution without primary antibody. Background negative control was collagen sheet without cells. Alkaline phosphate substrate-chromogen staining was performed using StayRed/AP kit (Abcam) according to manufacturer’s recommendations and as described before. After staining, samples were rinsed in PBS and an Olympus IX83 digital microscope (Olympus Life Science) was used to image the samples using Cellsens Dimension software (Olympus Life Science). Anti-Rabbit Alexa-488 conjugated specific secondary antibody (Pierce Protein Biology, Thermo fisher Scientific) was used for immunofluorescence. The samples were then visualized under the same Olympus IX83 digital microscope.
ImageJ (NIH, Maryland, USA) was used to quantify protein staining. ImageJ colour thresholding26 plug in was employed to calculate the amount of type-I and type-III collagen staining. Corrected total cell fluorescence (CCTF) technique was used to measure the amount of TSP-4 of each cell seeded on different group27–30. Briefly, the CCTF of a cell was measured by deducting the average fluorescence of the background around the cell from the average fluorescence of the cell area. For all the quantifications the areas of interest were picked randomly across the samples.
2.8 Statistical Analysis
One-way analysis of variance (ANOVA) was performed for RT-PCR data and Tuckey’s post hoc analysis was performed for pairwise comparison. Significance was set at p<0.05.
For COL I and COL III quantification Mann-Whitney U test was conducted to compare difference between groups (p<0.05). In case of CCTF quantification of TSP-4, as cell data are involved, a Bonferroni correction was applied and statistical significance was set at p<0.025. Minitab Statistical package (Minitab Inc., State College, PA, USA) was used to perform the statistical analysis.
3. Results
The mechanical test results [Table 1] showed that the targeted modulus levels were attained over 4 order of magnitudes.
Planar stretching introduced molecular as well as fibrillar alignment evident by CPI and SEM image respectively [Figure 2].
SCX expression at day 3 was greater for 100 MPa than for 0.1 MPa (p = 0.02) [Figure 3A]. There was no significant difference between 10 MPa aligned and 10 MPa random groups indicating that alignment doesn’t have any effect at 10 MPa.
Figure 3.
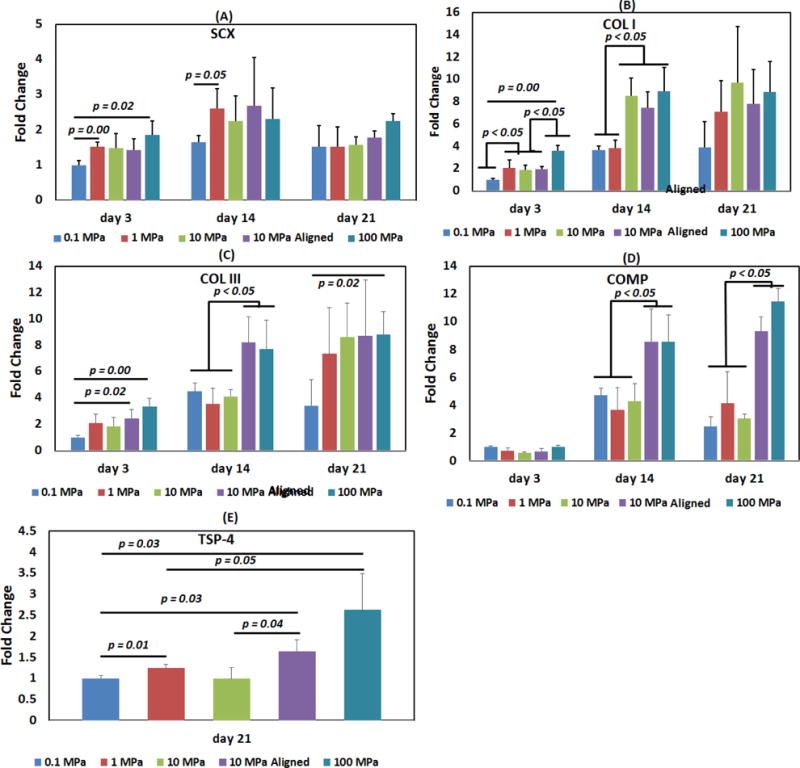
Effect of collagen matrix modulus and anisotropy on tenogenic differentiation of human MSCs. (A) Scleraxis, (B) Collagen-I, (C) Collagen-III, (D) COMP, (E) Thrombospondin-4. Anisotropy and increasing modulus improved tenoinduction. Statistical differences are highlighted by horizontal lines with the corresponding p values. Statistical significance was set at p <0.05.
COL I expression at day 3 was the lowest for 0.1 MPa level and the highest for 100 MPa [Figure 3B]. Expression at 1–10 MPa range modulus values were positioned intermediately. Between each modulus level, there was about 2-fold upregulation in COL I expression. At day 14, the expression was grouped over two levels, 0.1–1 and 10–100 MPa and there was about 2-fold upregulation between these two modulus levels. By day 21, there was no significant difference between any of the groups. Alignment did not have any effect on COL I expression at 10 MPa groups.
COL III expression was the highest for 100 MPa modulus and it showed more than 2-fold upregulation than 0.1 MPa at different time points [Figure 3C]. Alignment favored COL III expression such that the expression for aligned 10 MPa group at day 14 was two-fold greater than that for random 10 MPa group.
COMP expression for 100 MPa group showed 2 and 3-fold greater upregulation than that of random 0.1–10 MPa groups at day 14 and 21 respectively [Figure 3D]. Alignment increased COMP expression such that 10 MPa aligned group’s expression was greater than 10 MPa random group’s expression. Furthermore, aligned 10 MPa group’s COMP expression did not differ from that of 100 MPa group.
There was a gradual increase in TSP-4 expression with increasing modulus [Figure 3E]. Alignment also significantly favored TSP-4 expression (p = 0.04).
None of the groups showed COL2 expression at any time point. At day 21, RUNX2 expression for 100 MPa group was significantly greater than that of the other groups. PPARγ expression was detectable at day 3 only and there were no significant differences between the expressions of experimental groups [Figure 4].
Figure 4.
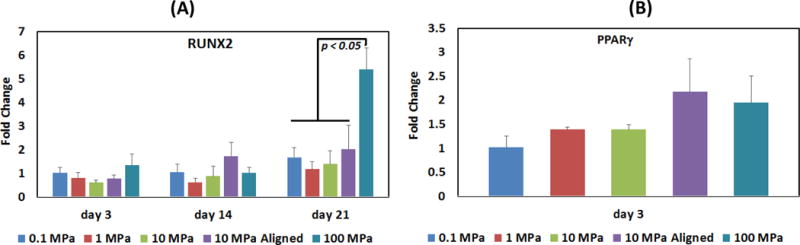
Differentiation of human MSCs seeded on collagen matrix towards lineages other than tenocytic differentiation (A) RUNX2, (B) PPARγ. Statistical differences are highlighted by horizontal lines with the corresponding p values. Statistical significance was set at p <0.05.
After 21 days of culture, Type-I collagen production increased by 2-fold in 10 MPa and 3-fold in 100 MPa group in comparison to the production by the 0.1 MPa group [Figure 5&6]. Alignment increased semi-quantitative COL I staining measurement significantly (p = .031). In case of COL III, there was a gradual increase in semi-quantitative measure of collagen staining with increasing modulus with a 2-fold increase from 0.1 to 100 MPa group [Figure 5&6]. In this case also, alignment increased semi-quantitative COL III staining measurement (p = .034). In case of TSP-4, there was no significant difference between 0.1–10 MPa while 100 MPa showed 4-fold greater TSP-4 production than that of the0.1 MPa group [Figure 5&6]. There was no significant difference in TSP-4 immunofluorescence level of cells between aligned and random group.
Figure 5.
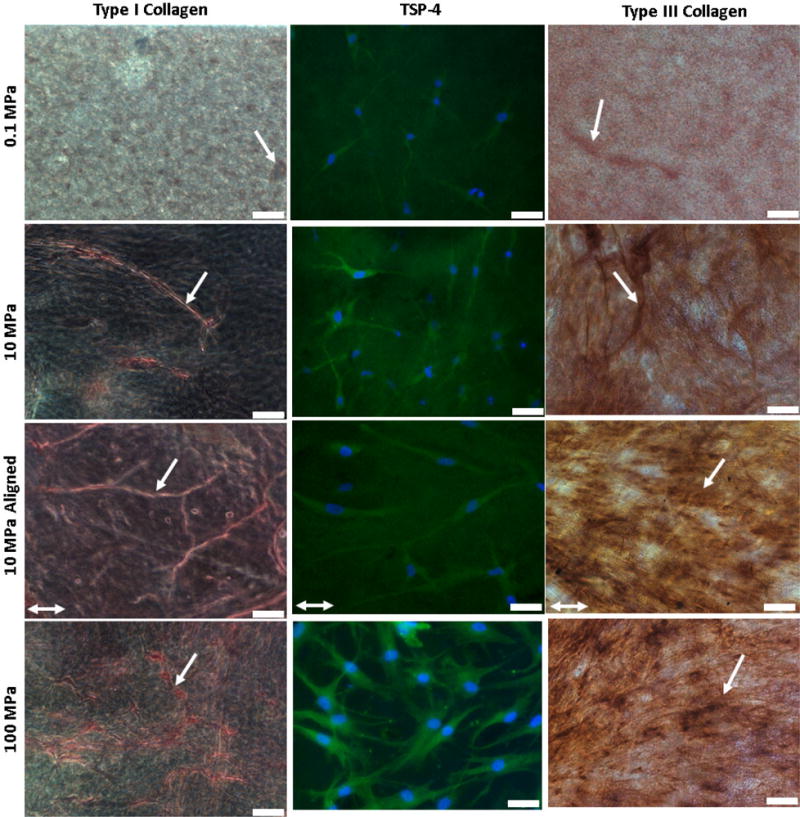
Effect of modulus on long term matrix synthesis. After day 21 days of hMSc culture, higher modulus favored matrix synthesis. For collagen I, stiffer groups showed thick collagen fiber formation. For TSP-4, 100 MPA group showed more production. For Collagen III also 100 MPa showed maximum synthesis and alignment (10MPa aligned) increase matrix synthesis. Scale bar 50 μm. Single headed white arrow indicates collagen synthesis; for type-I and type-III collagen. Alignment direction of 10 MPa aligned group is shown by white arrow.
Figure 6.
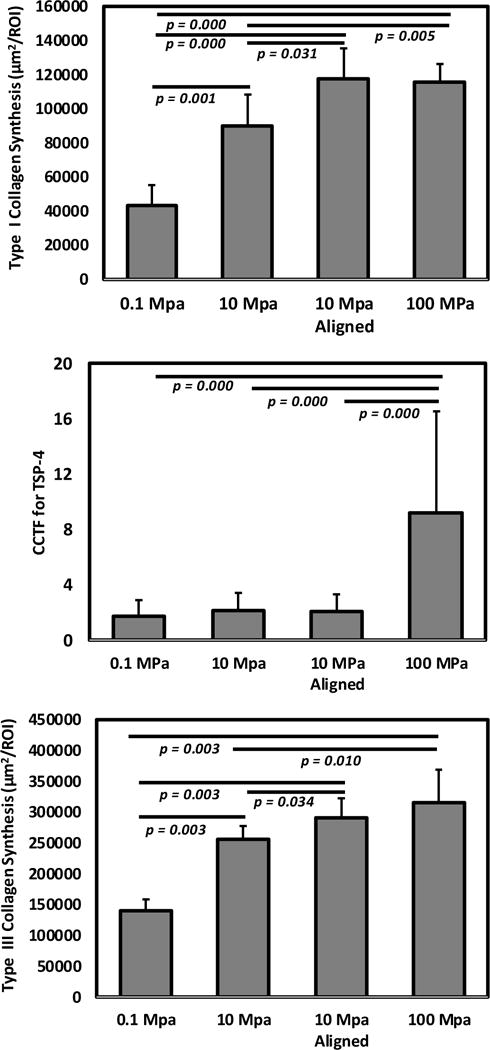
After 21 days of hMSc culture, 100 MPa and 10 MPa aligned groups showed 3-fold increase in semi-quantitative measurement of staining of Type-I collagen than 0.1 MPa group; cells on 100 MPa group showed 4-fold increase in TSP-4 production than 0.1 MPa group indicated by corrected total cell fluorescence (CCTF); and type-III collagen showed 2-fold upregulation from 0.1 MPa to 100 MPa with a slightly increasing trend in aligned group compared to unaligned group.
4. Discussion
Natural tendon is composed of highly stiff and aligned type I collagen fibrils. In this study we investigated both the effect of modulus and alignment of type I collagen substrate for tendon differentiation. This study showed that mimicking the tendon topography, i.e. increasing the substrate modulus as well as alignment increased the differentiation of human MSCs to a tenogenic lineage.
Past literature reported the effect of modulus on differentiation (e.g. muscle, neuron) in the kPa range which was then followed by glass which has a modulus around 90 GPa1, 31. Therefore, there is a gap in the literature on substrate modulus effects in the range of 100 kPa to 100 MPa range in terms of cellular response. This study not only fills the gap of the modulus ranges that had been studied to date, but also incorporates matrix alignment to investigatetendon differentiation5, 7.
Previous studies demonstrated that increasing modulus generally increases cell adhesion, proliferation and multilineage differentiation1, 5, 7, 32–42. Very few of these studies investigated the effect of modulus on tenogenic differentiation on collagen substrate which is the major tendon ECM5, 7. Moreover, the studies that investigated tenogenic differentiation used substrate modulus (10–80 kPa) levels which are orders of magnitude softer than tendon and did not consider synergistic effect of alignment5, 7. This is the first study to our knowledge, which considers modulus of collagen substrate to MPa level as well as investigates effect of substrate alignment on tendon differentiation.
The current study fabricated type I collagen substrates with modulus values encompassing three orders of magnitudes of change beginning fomr hundred kPa, to hundred MPa. Moreover, by introducing planar stretch, we were able to generate aligned substrates. Therefore, the collagen material model developed in this study allowed for systematic investigation of the effects of matrix modulus and anisotropy tenogenic differentiation.
Scleraxis is tendon progenitor and important for tendon development43–45,46,47. Sharma et al. showed increased SCX expression with increasing collagen coated substrate modulus in their studies related to tendon differentiation5, 7. Recently, Chen at al showed arterial stiffening via SCX upregulation48. This indicates that, substrate modulus is associated with SCX expression. This study showed early increase in the scleraxis expression as at day 3 stiffer groups showed higher expression. Similarly, MSCs seeded on highly anisotropic collagen fibers12, 22, knitted silk collagen scaffold49 and on culture plates50 also induced early increase in scleraxis expression. However, the modulus was not varied systematically in these studies. This study also showed that scleraxis expression levels between groups by day 21 (Figure 3A). Therefore, stiffer substrates seem to benefit tenogenesis by expediting the inception of MSC to tendon differentiation cascade.
Tendon is mostly composed of type-I collagen. Studies showed that matrix stiffening increases collagen I synthesis51 and with aging collagen turnover decreases in tendon with decreasing modulus52. The current study showed increased COL I expression with increasing modulus such as 100 > 1–10> 0.1 MPa at day 3 and 100 > 10> 0.1–1 MPa at day 14. This indicates that stiffer matrices induce earlier and greater levels of COL I expression than compliant matrices [Figure 3B]. From the protein production data, at day 21, there was a gradual increase in semi-quantitative measure of COL I staining from 0.1 MPa to 100 MPa. At the production level, alignment showed upregulation of semi-quantitative measure of COL I staining and semi-quantitative staining measurement in 10 MPa aligned group was similar to 100 MPa group. This increase in COL I synthesis may be due to the alignment of the matrix as indicated by previous studies which showed that aligned matrix increased tenogenic differentiation12, 22, 53.
Type-III collagen plays a crucial role for type-I collagen fibrilogenesis54, 55 and thus another major tendon-associated collagen along with type-I collagen. During tendon injury type-III collagen is produced to quickly repair the damage56, 57 and after long periods it is remodeled to type-I collagen56, 58 In the current study, the trend in COL III expression as well as production was similar to that of COL I. Moreover, in the expression level, 10 MPa aligned group showed similar expression as 100 MPa group both at day 3 and 14. In the production level, 10 MPa aligned group showed higher expression than 10 MPa unaligned at day 21. This indicates that alignment also helps in tenogenesis. Previous studies also showed similar outcome as stiffer matrix5 and aligned matrix12 increased COL III expression. It can be noticed that the amount of COL III synthesis is higher than COL I. Whether or not COL III is remodeled to COL I as is the case in tendon injury repair sites would require longer duration studies.
TSP-4 is one of the main tendon-related genes59 as it shows highest expression in tendon ECM than other tissue types59–61. Cells seeded on decellularized tendon slices, engineered scaffold-free tendon tissue and collagen matrix showed upregulation of TSP-462,63, 64. The current study also showed TSP-4 upregulation both with modulus and alignment after day 21 [Figure 3E]. In matrix synthesis study, after day 21, only 100 MPa group showed higher level of TSP-4 immunofluorescence (Figure 5&6) than the other groups. In tendon, TSP-4 binds to collagen and form complexes with COMP61, 65. Smith et. al suggested that COMP has an organizational role in tendon formation as well as COMP is necessary for tendon to resist load66, 67. The current study shows, upregulation of COMP expression with stiffer (100 MPa) and aligned (10 MPa aligned) matrix [Figure 3D]. This indicates that, higher modulus and alignment mimicked tendon topography to an extent and had an influence on tenogenic differentiation.
The level of differentiation induced by alignment was similar to that induced by stiffer but unaligned substrates. Our group and other groups have reported on tenogenic effect of substrate driven cytoskeletal alignment on MSCs previously5, 11–16, 21, 68, 69. Collagen molecules are packed densely when they are registered in parallel (i.e. aligned) in comparison to random. Thus, it is likely that aligned matrix is able to present integrin binding sites more densely than unaligned random configuration. Denser presentation of adhesion sites would result in stronger focal adhesions which in turn is reported to be conducive to actin polymerization35, 70, 71. On stiff substrates, RhoA and Rho-associated kinase (ROCK) activation promotes actomyosin stress fiber assembly and demonstrates long parallel actin stress fiber with elongated cellular shape4, 39, 72. RhoA/ROCK is major molecular pathway that promotes matured focal adhesion, organized stress fibers and elongated cells and this pathway is involved in topography-mediated MSC differentiation4, 39, 73–75. Future studies will test the hypothesis that alignment, independent of stiffness, can activate RhoA/ROCK pathways by presenting integrin adhesion sites more densely due to ordered packing of collagen molecules.
Expressions of chondrogenic, osteogenic and adipogenic markers suggested that the MSCs used in this study did not seem to differentiate towards cartilage, bone or fat formation on the collagen matrices presented to them at various modulus levels. Type II collagen is one of the major markers for chondrogenic differentiation. Differentiating MSCs to chondrogenic lineage requires chondrogenic differentiation media supplemented with growth factors and high density cell seeding 76–81. Lack of type-II collagen expression is not surprising given that no chondrogenic growth factors were used and that cell seeding density was low. Only at one time point, RUNX2 was upregulated for 100 MPa group suggesting that such elevated stiffness level my favor osteogenic differentiation. Rigid substrates were reported to provide higher force feedback to cells which in turn increase cell centrality, cell spreading and bone morphogenetic protein (BMP) expression which finally activates RUNX211, 74, 82 It is interesting to notice that although both 100 MPa and 10 MPa aligned group showed similar level of tenogenesis, only 100 MPa group showed upregulation of osteogenesis. Therefore, it can be speculated that while some level of stiffness is necessary for tenogenesis whereas matrix alignment may be more crucial for tenogenesis than stiffness. Suppression of osteogenesis due to cellular alignment is implied by findings of previous in vitro studies where bone markers were down regulated on aligned collagen threads which have comparable or higher modulus than 100 MPa12. Furthermore, in vivo studies utilizing aligned collagen threads with 100+ MPa stiffness did not result in ectopic mineralization83. PPARγ is an adipogenic transcription factor84, 85 which was expressed only at the earliest time point of day 3. This outcome suggests that MSCs have some adipogenic propensity at the baseline; however, the material model developed in this study does not promote adipogenic differentiation over time.
One limitation of this study was that it did not capture the 3D nature of tendon matrix, which is a common limitation to most biomaterials, except for hydrogel-cell mixtures. On the other hand, hydrogel systems are compliant and it is challenging to attain modulus levels greater than 1 MPa with most known hydrogel formulations. In future studies, this limitation can be addressed by sandwiching cells between two collagen sheet layers. It may be possible that the stiffness-related outcomes we identified in the current 2D settings may be different from the presentation of stiffness in 3D. Another limitation of the study is that the experimental design of the study included aligned group only for 10 MPa group. The absence of aligned groups at other modulus levels prevents us from reaching any conclusions on synergistic effects alignment and modulus.
Aligned electrocompacted collagen is being developed as a growth-factor free platform to induce tenogenesis topographically. Aligned collagen threads have been implanted in the tendon environment in braided form in vivo83. In such orthotopic environment excellent biocompatibility and tissue integration was observed. In environments where endogenous tendon cells or MSCs are absent, the material can be used for priming patient-derived MSCs prior to implantation to treat challenging tendon defects, such as irreparable defects of the rotator cuff. Therefore, aligned and electrocompacted collagen based biomaterial developed in this study has a potential to be use in tendon tissue engineering applications both as a cell-seeded carrier matrix or acellular repair matrix to promote tenogenesis.
5. Conclusions
The current study demonstrates tenogenic differentiation is influenced by both modulus and alignment of the substrate modulus. This material platform developed in this study mimicked both the modulus anisotropy as well as the modulus of the natural tendon and investigated the synergistic effect of these two key factors of tenogenic differentiation. Therefore, the tenoinductive collagen material model developed in this study can be used in the research and development of tissue engineering tendon repair constructs in future.
Statement of Significance.
Although the effects of substrate topography and modulus on MSC differentiation are well known with respect to osteogenesis and adipogenesis, there has been relatively little investigation on the effects of this phenomenon on tenogenesis. Furthermore, a relative role of topographical factors (matrix alignment vs. matrix modulus) in inducing tenogenic differentiation is not well understood. We investigated the effects of modulus and topographical alignment of type I collagen substrate on tendon differentiation. This study showed mimicking the tendon topography, i.e. increasing the substrate modulus as well as alignment increased the tenogenic differentiation. Therefore, the tenoinductive collagen material model developed in this study can be used in the research and development of tissue engineering tendon repair constructs in future.
Acknowledgments
This study was funded in part by grants from the National Science Foundation (Grant Number DMR-1306665) and National Institute of Health (Grant Number R01 AR063701).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochemical and Biophysical Research Communications. 2003;307:355–61. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 3.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4872–7. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 5.Sharma RI, Snedeker JG. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials. 2010;31:7695–704. doi: 10.1016/j.biomaterials.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Song G, Ju Y, Li X, Song Y, Watanabe S. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. Journal of cellular physiology. 2012;227:2722–9. doi: 10.1002/jcp.23016. [DOI] [PubMed] [Google Scholar]
- 7.Sharma RI, Snedeker JG. Paracrine interactions between mesenchymal stem cells affect substrate driven differentiation toward tendon and bone phenotypes. PloS one. 2012;7:e31504. doi: 10.1371/journal.pone.0031504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David L, Grood ES, Noyes FR, Zernicke RE. Biomechanics of ligaments and tendons. Exercise and sport sciences reviews. 1978;6:125–82. [PubMed] [Google Scholar]
- 9.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, L-Y Woo S. Tensile and viscoelastic properties of human patellar tendon. Journal of Orthopaedic Research. 1994;12:796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- 10.Tong WY, Shen W, Yeung CW, Zhao Y, Cheng SH, Chu PK, Chan D, Chan GC, Cheung KM, Yeung KW, Lam YW. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials. 2012;33:7686–98. doi: 10.1016/j.biomaterials.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–75. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 12.Kishore V, Bullock W, Sun X, Van Dyke WS, Akkus O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials. 2012;33:2137–44. doi: 10.1016/j.biomaterials.2011.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261–70. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 14.Ma PX. Biomimetic materials for tissue engineering. Advanced drug delivery reviews. 2008;60:184–98. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Z, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue engineering. 2005;11:101–9. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 16.Mata A, Boehm C, Fleischman AJ, Muschler G, Roy S. Analysis of connective tissue progenitor cell behavior on polydimethylsiloxane smooth and channel micro-textures. Biomedical microdevices. 2002;4:267–75. doi: 10.1023/a:1020950022074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuzaka K, Walboomers XF, Yoshinari M, Inoue T, Jansen JA. The attachment and growth behavior of osteoblast-like cells on microtextured surfaces. Biomaterials. 2003;24:2711–9. doi: 10.1016/s0142-9612(03)00085-1. [DOI] [PubMed] [Google Scholar]
- 18.Walboomers XF, Jansen JA. Cell and tissue behavior on micro-grooved surfaces. Odontology. 2001;89:0002–11. doi: 10.1007/s10266-001-8178-z. [DOI] [PubMed] [Google Scholar]
- 19.Yin Z, Chen X, Chen JL, Shen WL, Hieu Nguyen TM, Gao L, Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 31:2163–75. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 20.Islam A, Chapin K, Younesi M, Akkus O. Computer aided biomanufacturing of mechanically robust pure collagen meshes with controlled macroporosity. Biofabrication. 2015;7:035005. doi: 10.1088/1758-5090/7/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam A, Younesi M, Mbimba T, Akkus O. Collagen Substrate Stiffness Anisotropy Affects Cellular Elongation, Nuclear Shape, and Stem Cell Fate toward Anisotropic Tissue Lineage. Advanced Healthcare Materials. 2016 doi: 10.1002/adhm.201600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younesi M, Islam A, Kishore V, Anderson JM, Akkus O. Tenogenic Induction of Human MSCs by Anisotropically Aligned Collagen Biotextiles. Advanced Functional Materials. 2014;24:5762–70. doi: 10.1002/adfm.201400828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Younesi M, Islam A, Kishore V, Panit S, Akkus O. Fabrication of compositionally and topographically complex robust tissue forms by 3D-electrochemical compaction of collagen. Biofabrication. 2015;7:035001. doi: 10.1088/1758-5090/7/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uquillas JA, Akkus O. Modeling the electromobility of type-I collagen molecules in the electrochemical fabrication of dense and aligned tissue constructs. Annals of biomedical engineering. 2012;40:1641–53. doi: 10.1007/s10439-012-0528-1. [DOI] [PubMed] [Google Scholar]
- 25.Martin R, Farjanel J, Eichenberger D, Colige A, Kessler E, Hulmes DJ, Giraud-Guille MM. Liquid crystalline ordering of procollagen as a determinant of three-dimensional extracellular matrix architecture. Journal of molecular biology. 2000;301:11–7. doi: 10.1006/jmbi.2000.3855. [DOI] [PubMed] [Google Scholar]
- 26.Papadopulos F, Spinelli M, Valente S, Foroni L, Orrico C, Alviano F, Pasquinelli G. Common tasks in microscopic and ultrastructural image analysis using ImageJ. Ultrastructural pathology. 2007;31:401–7. doi: 10.1080/01913120701719189. [DOI] [PubMed] [Google Scholar]
- 27.Burgess A, Vigneron S, Brioudes E, Labbe JC, Lorca T, Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12564–9. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–43. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell cycle (Georgetown, Tex) 2014;13:1400–12. doi: 10.4161/cc.28401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potapova TA, Sivakumar S, Flynn JN, Li R, Gorbsky GJ. Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited. Molecular biology of the cell. 2011;22:1191–206. doi: 10.1091/mbc.E10-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Discher DE, Janmey P, Wang Y-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 32.Floren M, Bonani W, Dharmarajan A, Motta A, Migliaresi C, Tan W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta biomaterialia. 2016;31:156–66. doi: 10.1016/j.actbio.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Her GJ, Wu H-C, Chen M-H, Chen M-Y, Chang S-C, Wang T-W. Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomaterialia. 2013;9:5170–80. doi: 10.1016/j.actbio.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Abdeen AA, Zhang D, Kilian KA. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials. 2013;34:8140–8. doi: 10.1016/j.biomaterials.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 35.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature materials. 2014;13:979–87. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophysical journal. 2004;86:617–28. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness pathological implications for soft or stiff microenvironments. The Journal of cell biology. 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nature methods. 2011;8:949–55. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 39.Park JS, Chu JS, Tsou AD, Diop R, Tang Z, Wang A, Li S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-β. Biomaterials. 2011;32:3921–30. doi: 10.1016/j.biomaterials.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pek YS, Wan AC, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials. 2010;31:385–91. doi: 10.1016/j.biomaterials.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 41.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. American Journal of Physiology-Cell Physiology. 2008;295:C1037–C44. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 42.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 43.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development (Cambridge, England) 2001;128:3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 44.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–48. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 45.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development (Cambridge, England) 1995;121:1099–110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 46.Perez AV, Perrine M, Brainard N, Vogel KG. Scleraxis (Scx) directs lacZ expression in tendon of transgenic mice. Mechanisms of development. 2003;120:1153–63. doi: 10.1016/j.mod.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Chen X, Yin Z, Chen J-l, Shen W-l, Liu H-h, Tang Q-m, Fang Z, Lu L-r, Ji J, Ouyang H-w. Force and scleraxis synergistically promote the commitment of human ES cells derived MSCs to tenocytes. Sci Rep. 2012;2 doi: 10.1038/srep00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen K, Zhou X, Sun Z. Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension. 2015;66:1006–13. doi: 10.1161/HYPERTENSIONAHA.115.06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Yin Z, Chen JL, Liu HH, Shen WL, Fang Z, Zhu T, Ji J, Ouyang HW, Zou XH. Scleraxis-overexpressed human embryonic stem cell-derived mesenchymal stem cells for tendon tissue engineering with knitted silk-collagen scaffold. Tissue engineering Part A. 2014;20:1583–92. doi: 10.1089/ten.TEA.2012.0656. [DOI] [PubMed] [Google Scholar]
- 50.Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue engineering Part A. 2010;16:2941–51. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. The Journal of cell biology. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bank RA, TeKoppele JM, Oostingh G, Hazleman BL, Riley GP. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: changes with age and in chronic rotator cuff tendinitis. Annals of the Rheumatic Diseases. 1999;58:35–41. doi: 10.1136/ard.58.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yin Z, Chen X, Chen JL, Shen WL, Nguyen TMH, Gao L, Ouyang HW. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–75. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 54.Lejard V, Blais F, Guerquin MJ, Bonnet A, Bonnin MA, Havis E, Malbouyres M, Bidaud CB, Maro G, Gilardi-Hebenstreit P, Rossert J, Ruggiero F, Duprez D. EGR1 and EGR2 involvement in vertebrate tendon differentiation. The Journal of biological chemistry. 2011;286:5855–67. doi: 10.1074/jbc.M110.153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proceedings of the National Academy of Sciences. 1997;94:1852–6. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. The Journal of Bone & Joint Surgery. 2005;87:187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 57.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. Journal of Musculoskeletal and Neuronal Interactions. 2006;6:181. [PubMed] [Google Scholar]
- 58.Millar L, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, Murrell GA, Liew FY, Kurowska-Stolarska M, McInnes IB. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nature communications. 2015:6. doi: 10.1038/ncomms7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. Journal of Orthopaedic Research. 2010;28:289–97. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- 60.Hauser N, Paulsson M, Kale AA, DiCesare PE. Tendon extracellular matrix contains pentameric thrombospondin-4 (TSP-4) FEBS letters. 1995;368:307–10. doi: 10.1016/0014-5793(95)00675-y. [DOI] [PubMed] [Google Scholar]
- 61.Södersten F, Ekman S, Schmitz M, Paulsson M, Zaucke F. Thrombospondin-4 and cartilage oligomeric matrix protein form heterooligomers in equine tendon. Connective tissue research. 2006;47:85–91. doi: 10.1080/03008200600584124. [DOI] [PubMed] [Google Scholar]
- 62.Ning L-J, Zhang Y-J, Zhang Y, Qing Q, Jiang Y-L, Yang J-L, Luo J-C, Qin T-W. The utilization of decellularized tendon slices to provide an inductive microenvironment for the proliferation and tenogenic differentiation of stem cells. Biomaterials. 2015;52:539–50. doi: 10.1016/j.biomaterials.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 63.Barsby T, Bavin EP, Guest DJ. Three-dimensional culture and transforming growth factor beta3 synergistically promote tenogenic differentiation of equine embryo-derived stem cells. Tissue Engineering Part A. 2014;20:2604–13. doi: 10.1089/ten.tea.2013.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ni M, Rui YF, Tan Q, Liu Y, Xu LL, Chan KM, Wang Y, Li G. Engineered scaffold-free tendon tissue produced by tendon-derived stem cells. Biomaterials. 2013;34:2024–37. doi: 10.1016/j.biomaterials.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 65.Narouz-Ott L, Maurer P, Nitsche DP, Smyth N, Paulsson M. Thrombospondin-4 binds specifically to both collagenous and non-collagenous extracellular matrix proteins via its C-terminal domains. Journal of Biological Chemistry. 2000;275:37110–7. doi: 10.1074/jbc.M007223200. [DOI] [PubMed] [Google Scholar]
- 66.Smith R, Gerard M, Dowling B, Dart A, Birch H, Goodship A. Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: A proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Veterinary Journal. 2002;34:241–4. doi: 10.1111/j.2042-3306.2002.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 67.Smith R, Zunino L, Webbon P, Heinegård D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix biology. 1997;16:255–71. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- 68.Popielarczyk TL, Nain AS, Barrett JG. Aligned Nanofiber Topography Directs the Tenogenic Differentiation of Mesenchymal Stem Cells. Applied Sciences. 2017;7:59. [Google Scholar]
- 69.Yin Z, Chen X, Song H-x, Hu J-j, Tang Q-m, Zhu T, Shen W-l, Chen J-l, Liu H, Heng BC. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials. 2015;44:173–85. doi: 10.1016/j.biomaterials.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 70.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–27. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nature reviews Molecular cell biology. 2013;14:467–73. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 72.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers–assembly, dynamics and biological roles. J Cell Sci. 2012;125:1855–64. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 73.Keung AJ, de Juan-Pardo EM, Schaffer DV, Kumar S. Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem cells. 2011;29:1886–97. doi: 10.1002/stem.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y-K, Yu X, Cohen DM, Wozniak MA, Yang MT, Gao L, Eyckmans J, Chen CS. Bone morphogenetic protein-2-induced signaling and osteogenesis is regulated by cell shape, RhoA/ROCK, and cytoskeletal tension. Stem cells and development. 2011;21:1176–86. doi: 10.1089/scd.2011.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woods A, Wang G, Beier F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. Journal of Biological Chemistry. 2005;280:11626–34. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- 76.Awad HA, Halvorsen Y-DC, Gimble JM, Guilak F. Effects of transforming growth factor β 1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue engineering. 2003;9:1301–12. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 77.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Experimental cell research. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 78.Bean AC, Tuan RS. Fiber diameter and seeding density influence chondrogenic differentiation of mesenchymal stem cells seeded on electrospun poly (ε-caprolactone) scaffolds. Biomedical Materials. 2015;10:015018. doi: 10.1088/1748-6041/10/1/015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Correa D, Somoza RA, Lin P, Greenberg S, Rom E, Duesler L, Welter JF, Yayon A, Caplan AI. Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation. Osteoarthritis and Cartilage. 2015;23:443–53. doi: 10.1016/j.joca.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagmann S, Moradi B, Frank S, Dreher T, Kämmerer PW, Richter W, Gotterbarm T. Different culture media affect growth characteristics, surface marker distribution and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. BMC musculoskeletal disorders. 2013;14:223. doi: 10.1186/1471-2474-14-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Mesenchymal Stem Cell Assays and Applications. 2011:253–78. doi: 10.1007/978-1-60761-999-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. Journal of cell science. 2009;122:546–53. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kishore V, Uquillas JA, Dubikovsky A, Alshehabat MA, Snyder PW, Breur GJ, Akkus O. In vivo response to electrochemically aligned collagen bioscaffolds. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:400–8. doi: 10.1002/jbm.b.31962. [DOI] [PubMed] [Google Scholar]
- 84.Janani C, Kumari BR. PPAR gamma gene–a review, Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 85.Spiegelman B. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–14. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]


