Abstract
There is growing concern among US-based clinicians, patients, policy makers, and in the media about the personal and community health risks associated with opioids. Perceptions about the efficacy and appropriateness of opioids for the management of chronic non-cancer pain (CNCP) have dramatically transformed in recent decades. Yet, there is very little social scientific research identifying the factors that have informed this transformation from the perspectives of prescribing clinicians. As part of an on-going ethnographic study of CNCP management among clinicians and their patients with co-occurring substance use, we interviewed 23 primary care clinicians who practice in safety-net clinical settings. In this paper, we describe the clinical and social influences informing three historic periods: (1) the escalation of opioid prescriptions for CNCP; (2) an interim period in which the efficacy of and risks associated with opioids were re-assessed; and (3) the current period of “opioid pharmacovigilance,” characterized by the increased surveillance of opioid prescriptions. Clinicians reported that interpretations of the evidence-base in favor of and opposing opioid prescribing for CNCP evolved within a larger clinical-social context. Historically, pharmaceutical marketing efforts and clinicians’ concerns about racialized healthcare disparities in pain treatment influenced opioid prescription decision-making. Clinicians emphasized how patients’ medical complexity (e.g. multiple chronic health conditions) and structural vulnerability (e.g. poverty, community violence) impacted access to opioids within resource-limited healthcare settings. This clinical-social history of opioid prescribing practices helps to elucidate the ongoing challenges of CNCP treatment in the US healthcare safety net and lends needed specificity to the broader, nationwide conversation about opioids.
Keywords: United States, opioids, chronic non-cancer pain, pharmacovigilance, primary care safety net, poverty, social medicine
Introduction
Concerns about increases in the prescription, use, and misuse of opioid analgesics have garnered national attention in scientific, political, and media domains (Dowell, et al. 2016; The White House, 2016; Newkirk, 2016). The number of opioid prescriptions increased dramatically between the late-1990s and mid-2000s (Manchikanti and Singh, 2008). The Centers for Disease Control (CDC) reported that by 2006, more unintentional overdoses were attributed to prescription opioids than to heroin and cocaine combined (CDC, 2011). Scientific evidence of a link between the increase in prescription opioids and a wide-spread overdose ”epidemic” influenced a call for reform in the medical community, governmental regulatory bodies, and in the larger public domain (American Medical Association (AMA), 2016).
Chronic non-cancer pain (CNCP), defined as pain that persists for greater than three months not caused by a malignancy or associated with pain at the end of life (Trescot, et al., 2008), affects approximately 25% of the United States (US) adult population and causes significant decrements in quality of life (Chou et al., 2009). CNCP interferes with a person's ability to perform activities of daily living, family life, and employment, and is associated with significant psychological stress (Gureje et al., 1998). The efficacy and appropriateness of prescription opioids for acute pain management are well documented (Carr & Goudas, 1999). However, the efficacy of chronic opioid therapy for CNCP is being re-evaluated due to a lack of evidence demonstrating functional improvements, significant side effects (e.g., endocrine and sleep disruption, opioid dependence, overdose, constipation, mental status changes) and community harm resulting from non-medical use of prescribed opioids (e.g., overdoses, violence, increased policing associated opioid misuse) (Ballantyne and Shin, 2008; Noble, et al., 2010; Schrager, et al., 2014).
The majority of Americans receive treatment for CNCP in primary care settings, not in pain specialty care clinics (Institute of Medicine (IOM), 2011). CNCP is a common condition among persons with co-occurring substance use disorders (Morasco, 2011). Safety net healthcare settings, defined by the Institute of Medicine (IOM) as those settings that “offer care to patients regardless of their ability to pay for services and [for which] a substantial share of their patients are uninsured, Medicaid, or other vulnerable patients,” (Dunn et al, 2010) serve a disproportionate number of patients with co-occurring CNCP and substance use disorder, making them important clinical settings to study changes in opioid prescribing (Sullivan et al., 2008). Patients may be initiated on opioids in safety net emergency departments (ED), or use this venue to obtain opioids, leading to an active debate is about the impact of ED opioid guidelines on safety net primary care (Barnett et al, 2017). CNCP patients with a history of substance use are more likely to be prescribed opioids, than patients without a history of substance use (Ives, et al., 2006; Fishbain, et al., 2008). Opioid analgesic misuse, defined as “the use of any drug in a manner other than how it is indicated or prescribed”, and aberrant behaviors including diverting prescriptions for non-medical use, altering the route of administration, or forging prescriptions, are more common among individuals with a history of substance use and mental health disorders (Turk, Swanson & Gatchel, 2008).
This paper explores the phenomenon of opioid prescribing for CNCP from the perspective of primary care clinicians (clinicians) who treat CNCP patients with a history of substance use (past or current). Although many studies have described the social and health consequences of opioid-associated overdose morbidity and mortality, little research has offered contextual information about the opioid prescribing of clinicians. An in-depth understanding of the educational, clinical, and social factors that contribute to opioid prescribing can improve responses to the unintended consequences of current opioid prescription practices. We describe the recent clinical-social history of opioid prescribing by examining factors that influence clinicians’ opioid treatment decision-making processes. We elaborate on the potential unintended consequences of “opioid pharmacovigilance,” the emergent climate of increased restriction on opioid prescriptions.
Theoretical Orientation and Historical Context
Historically informed, social medicine studies of diabetes, sickle cell anemia, schizophrenia, cancer, and HIV/AIDS reveal how scientific, cultural, and social influences coalesce to inform clinical decision-making (Montoya, 2011; Rouse, 2009; Metzl, 2009; Lochlain Jain, 2013; Epstein, 1998; Farmer, 1992). Clinicians’ responses to a given disease are embedded within a larger social milieu (Stonington and Holmes, 2006). The need to “do something” - to respond to suffering - is felt by both clinicians and patients. Multiple factors influence how these responses might be enacted in clinical settings. In day-to-day decision-making about appropriate treatment, the expertise of an individual clinician, current scientific evidence, and social and political forces play important roles (Knight, 2015; Holmes, 2013).
Medical anthropologists have long been engaged in a critical examination of the phenomena of pain. Much of this early work addressed the impact of structural factors (e.g., disability claims, insurance status) on a chronic pain diagnosis, described the phenomenological experience of pain from the patients’ perspectives, and explored miscommunication between patients and clinicians about chronic pain’s etiology and validity (Good et al. 1992). More recent anthropological investigations (Greenlaugh, 2001; Buchbinder, 2015, Crowley-Matoka & True, 2012) are focused on the diagnosis and treatment of pain within diverse clinical specialties and settings, and describe cultural norms and expectations about opioid prescriptions and clinicians’ “anxiously ambivalent responses to pain and pain medications.” (Crowley-Matoka and True, 2012: 689) Historical examinations of the emergence of pain medicine (Baszanger, 1998) and the rise in the number of specific opioid prescriptions (Wailoo, 2014) underscore the importance of social and political context.
Drawing from this more recent turn toward the experiences of clinicians and an examination of the construction of a “clinical culture” (Boutin-Foster, Foster & Konopasek, 2008), we investigated clinicians’ perceptions about opioid prescribing for CNCP. Our analysis offers perspectives that were not well studied, including how clinicians reflected on the social and historical context of their education about pain management; grappled with a lack of scientific evidence for opioid efficacy; and assessed the potential positive and negative consequences of increased surveillance of opioid prescribing.
We triangulated our qualitative interview data with epidemiological findings about national opioids prescriptions, overdose morbidity and mortality, and substance abuse treatment enrollment data, and historical analyses of the US opioid epidemic to identify three historical periods in which clinical understandings about opioids for pain management and practices of opioid prescribing experienced significant transitions. The three recent historical periods in which this analysis is situated are:
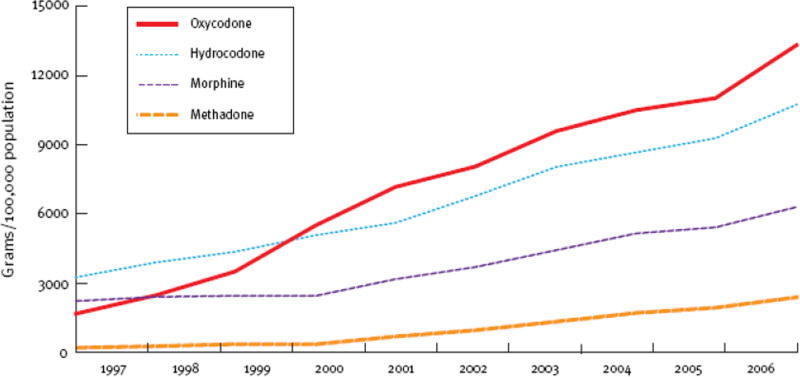
A period of increases in opioid prescriptions (1990s through mid/late 2000s). See Figure 1.
A “pendulum swing” toward increased scrutiny about the safety and the efficacy of chronic opioid therapy (mid-2000s to approximately 2011).
Increased opioid prescription surveillance (opioid pharmacovigilance) in which national, state, and clinical-level polices are implemented to curtail opioid prescriptions for CNCP (2011-present).
Figure 1.
Increase in therapeutic opioid use in the United States --- 1997 – 2006. Manchikanti and Singh, “Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse and nonmedical use of opioids. “ Pain Physician 2008 Mar;11(2 Suppl):S63–88.
“Pharmacovigilance” is “defined as a set of practices aimed at the detection, understanding, and assessment of the risks related to the use of drugs in a population” (Langlitz, 2009:395). According to World Health Organization (WHO), “the aims of [p]harmacovigiliance are to enhance patient care and patient safety in relation to the use of medicines, and to support public health programs by providing reliable, balanced information for the effective assessment of the risk-benefit profile of medicines.” (WHO, 2006:8) Pharmacovigilance is considered essential to the modern governance of pharmaceutical medications and modern medicine. We use the term “opioid pharmacovigilance” to describe the current focus on patient and public safety in relationship to opioid prescribing. We document clinicians’ experiences with changing patterns of opioid prescribing to explore how medical education, clinical experiences, scientific evidence, concerns about individual versus community health, and prescription guidelines coalesce to affect clinicians’ management of CNCP.
Methods
We recruited twenty-three clinicians from six safety net primary care settings across four counties in the San Francisco Bay Area. Interested clinicians responded to an informational email about the study, or enrolled after research staff presented information about the study at a clinical staff meeting. Approximately 20% of eligible clinicians declined to participate, often citing lack of interest in research or time constraints. We defined “clinician” as a physician, nurse practitioner, or physician assistant who provided longitudinal primary care to a panel of patients. Eligible clinicians had patients with both CNCP and a substance use disorder (past or current). We interviewed clinicians using a semi-structured interview guide that focused on CNCP management in safety net clinical settings. After obtaining written informed consent, researchers conducted 1–2 hour, audio-recorded interviews. This research was approved by the University of California – San Francisco’s Committee on Human Research (IRB #13-11217).
All audio-recorded material was transcribed verbatim. Transcripts were uploaded into the qualitative data analysis software ATLAS.ti. The coding scheme was developed through an iterative process. Deductive codes were initially derived from the topic areas of the interview guide (e.g., “clinic pain policies”, “clinical decision-making”). We developed inductive codes by assessing the frequency of broad themes during interviews (e.g. “clinical uncertainties” and “clinician expectations”). Through successive iterations of independent coding of transcripts and meetings to discuss our evolving understanding of the various codes, a set of 40 thematic codes were finalized. To ensure interpretative consensus between the three data coders, each interview was coded by at least two researchers and discrepancies were resolved prior to final data entry into ATLAS.ti.
Results
We interviewed 23 clinicians, of whom 18 were physicians, four were nurse practitioners, and one was a physician assistant. Sixteen were women. Clinicians worked in various settings: nine worked in clinics based in public hospitals; nine in county-funded community-based clinics, and five in federally qualified health centers.
The following sections of the paper describe three historic phases of opioid prescribing from the perspectives of clinicians who treated patients with co-occurring CNCP and substance use in the health care safety net in the United States. Clinicians indicated that a myriad of factors influenced trends in opioid prescribing, including: their medical education; scarcity of services in safety net settings; patient poverty; and questionable evidence of benefit from prescription opioids for the management of CNCP.
I. The recent past: medical education, “clinical justice”, and opioid overprescription
When asked about her medical training on prescription opioids, one clinician described the messages about pain assessment and treatment during her residency:
When I went through residency, so in the mid-90s, the mantra that you would hear around pain was, “Believe the patient’s pain, pain is the fifth vital sign, treat, keep escalating narcotics until a patient is pain-free, you [a clinician] can’t tell [assess] pain, you just have to hear [listen to the patient describing it].” There was a lot of teaching around trying to maximize pain control in sort of a non-judgmental way.
Many of the clinicians echoed the above sentiment, indicating that, in the mid-1990s, they were encouraged to inquire about and measure pain during clinical training, as well as to prescribe opioids with the goal of near-complete alleviation of pain symptoms. In 1995, assessment of pain as “the fifth vital sign” was first introduced to underscore the importance of assessing patient’s experiences of pain, just as clinical staff measured and documented other vital signs (e.g., temperature, blood pressure) (Campbell, 1996). The material representation of this shift became the ubiquitous “FACES pain scale,” in which staff used pictorials of faces and asked patients, “On a scale of 0 to 10, with 0 being no pain at all,10 being the worst pain imaginable, tell me about your pain today?” (Wong-Baker FACES Foundation, 2016). This shift in the clinical response to patients’ pain was not the only factor that contributed to increased prescription of opioids for CNCP. However, many clinicians underscored its importance in creating a clinical culture in which pain management practices, including opioid prescriptions, were perceived as patient-specific and not systematically reviewed at the clinic level.
One clinician described the perception that opioid prescribing was “the good thing to do,” even without a strong evidence-base to support the efficacy of chronic opioid therapy for CNCP. An increased focus in medical education on pain assessment was a factor that contributed to increased opioid prescribing because those assessments led to the need to treat pain biomedically. One clinician described this sequence from assessment to treatment, indicating that “opiates” became the default treatment choice:
It was just believed that [treating pain] was what we were supposed to be doing. I don’t even know if it was pressure, it was like that was the good thing to do, that was the right thing to do, ‘If people have pain, treat their pain.” At the time it wasn’t really clear, like “Do we have evidence to show that these opiates are going to work in chronic non-malignant pain? You know “no I don’t think so.” [laughter] But there was nothing else [to treat pain with at the time].
According to safety net clinicians, during this period of increased pain assessment and treatment within clinical settings, larger structural forces related to pharmaceutical marketing and a recognition of racialized healthcare disparities in pain treatment influenced opioid prescribing practices. Pharmaceutical drug marketing for chronic opioid therapy escalated significantly in the form of sponsorships for research and research meetings, and aggressive marketing to prescribing clinicians (Quinones, 2015). One clinician, when asked about increased prescribing during this period said, “I’m sure the [pharmaceutical] drug companies probably had some effect on that. I mean, Oxycontin is a wonderful example of something that came out of nowhere and became huge and now is just almost entirely gone”. Another clinician, with many decades of clinical experience, recalled the influence of pharmaceutical clinic-based marketing:
The [pharmaceutical] drug reps coming around [the clinic], pushing their particular brand, their [own] opioid analgesic. We’ve seen a lot more of them in the last ten years [2003–2013] than we ever did before. We never saw them before.
While clinicians were experiencing greater availability of opioids as a result of pharmaceutical investments in development and marketing, research findings were reported that suggested under treatment of acute pain in women and members of racial minority groups (Todd, Samaroo, & Hoffman, 1993; Todd, et al., 2000). Several studies published during this period influenced clinicians’ pain management practices, even though these studies drew conclusions based on very small sample sizes. First, two widely cited studies reported that the use of chronic opioid therapies rarely led to adverse consequences [e.g. opioid dependence] in patients (Porter and Jick, 1980; Portenoy & Foley, 1986). Second, several studies found that African-Americans, Latinos, and women were less likely to receive pain medications for acute pain (Green, et al., 2003). Awareness of this emergent research may have led to increases in opioid prescribing, within a broader clinical culture that was focused on the assessment and amelioration of pain (Quinones, 2015). One clinician described the combined role of research on the under treatment of pain, a clinical culture of being responsive to patients’ subjective experiences, and opioid availability:
CLINICIAN: I remember going to lectures on pain and those things [the research on racial disparities in opioid prescribing] would always be emphasized.
INTERVIEWER: They would talk about racial bias and prescribing?
CLINICIAN: Yeah, and just under-prescribing. But what was driving that, I’m not sure. Maybe there was more sort of appreciation I guess of the patient[’s] point of view and less paternalism, maybe that had some role in it. And then probably just availability [of opioids], too. There was [a] pretty strong educational push on how to use these meds [opioids].
Clinicians reported a strong commitment toward understanding racialized health disparities. The clinicians in this study worked in safety net clinics, where patients were low income and over 70% were from minority racial/ethnic groups. One clinician extended the conversation from one on health disparities based on race to a larger conversation about clinicians’ alignment with a notion of socially responsible medicine. Her education had linked responsiveness to her patients’ requests for pain relief and/or opioid medications with clinical “justice”.
Providers just really, really, really want to do the right thing and it’s very interesting for me, looking at that arc of time. In my training, doing what people [patients] said they wanted in terms of opiates for their pain was framed in terms of justice.
The larger structural forces of patient poverty and scarce health care resources played a role in increased prescription of opioids. In these safety net clinical settings, access to equitable and appropriate primary care for patients was considered a right, and clinicians felt that ensuring this access was part of their responsibility as clinicians. Clinicians described their education as one that sought to be responsive, attentive, and “non-judgmental” toward a patient’s pain. This approach reflected a desire that medical care provided in safety net settings helped ameliorate the social and structural vulnerabilities that many patients experienced, such as racialized bias in treatment access, poverty, and underemployment. One clinician linked his training, the race of his patients, and their impoverishment when he discussed his past approach toward opioid prescribing:
Many of us got trained in that model, on the inpatient service with patients with sickle cell disease who have an extreme degree of pain and an extremely vulnerable setup of being poor and African American. A lot of things are at play that lead to the teaching of, “Believe your patient, treat their pain no matter how many kilograms of morphine they’re requiring.”
In addition, clinicians described the majority of their patients as “medically complex,” with serious, acute and chronic health conditions, and significant social and economic barriers to accessing healthcare. The combination of racialization, poverty, medical complexity, and opioid availability contributed to increases in opioid prescribingClinicians acknowledged that prescription opioids were a motivation for many of their patients to engage with health care. This impression, in turn, influenced their prescribing practices. One clinician described this phenomenon from the perspective of educating residents to manage CNCP:
The resident sees a person with lots of chronic disease and they want that person to be fixed, they want that person to get better in terms of their health and they think that [patients will] keep coming to appointments if they’re given opioids because that’s [what the patient is] talking about…And I think in the past a lot of those patients that basically were probably too high risk and should have never been started on opioids were started on them in the past. [They were given opioids] probably just to help with engagement [into care], you know? And let’s be clear, nobody’s lying about pain, I think they’re all in pain.
For this clinician, the well intentioned desire of residents to “fix” very sick patients may have led to the prescription of opioids when they were contraindicated. This clinician reinterprets past practices using current risk assessment criteria by saying that patients who were “probably too high risk and should never have been started were started on them in the past.” While this example draws on a clinician’s recollections of residency education, the impression that patients’ engagement in care and care continuity were linked to the receipt of opioids was shared by most clinicians in our study. Clinicians reported a common belief at the time was that meeting the opioid requests of medically complex patients facilitated adherence with clinical recommendations and medications.
Another clinician described that her safety net clinic had limited resources to manage CNCP with anything other than opioids. While other modalities (e.g., physical therapy, chiropractic, acupuncture) might be beneficial, opioids were the only option.
[Opioids] just got totally overprescribed because that was the only thing that there was. [The patient] can’t go to physical therapy. I think the community has tried to have acupuncture and chiropractic and things like that but like they were [outside of the clinic and not funded through primary care]…I don’t think it was like the best way to do it…So I don’t think it was widely…there was not any other thing for it so I think a lot of patients just got thrown opiates.”
In sum, the historic increase in opioid prescriptions was influenced by trends in clinicians’ experiences in which they were encouraged to be responsive to patients’ descriptions of their pain and to patients’ requests for opioid medications. This orientation toward clinician responsiveness was enacted in light of a heightened awareness of patients’ poverty and of the limited clinical resources allocated toward non-pharmacologic treatments. Clinicians’ perceived that opioid prescriptions incentivized patients to engage in health care and/or adhere to other medications. In addition, the larger structural context of aggressive pharmaceutical marketing, reports about racial discrimination, and lack of alternate pain management options contributed to a culture of increased opioid prescribing.
II. The Pendulum Swing: The efficacy and safety of opioids comes into question
Clinicians described their participation in a “pendulum swing” in the clinical culture of opioid prescriptions for CNCP that began in the mid-2000s. In contrast to the era of increased opioid prescribing, recent concerns about prescription opioid-associated overdoses repositioned opioids as dangerous medications. Two related problems shaped clinicians’ concerns about opioid prescribing, namely the problem of assessing opioid efficacy and the problem of applying aggregate data to individual patient care. The problem of efficacy asks: “What is the evidence for the effectiveness of opioids for CNCP and do the potential benefits outweigh the risks?” Coupled with concerns about opioid medication misuse, clinicians described limited evidence for the efficacy of opioids CNCP management. One clinician described how questions about patient safety and of medication efficacy changed the clinical landscape.
Many years ago there was very little pain medications prescribed and then there was a big movement of “pain, it’s the next, another vital sign, it’s totally subjective.” Somebody says they have pain, you treat it, and so that was pushed very much [in my clinical training]. And then I think now…with sort of all the overdose and, and diversion kind of information that’s come out and also sort of lack of efficacy of long term opiates, in treating these problems, the pendulum has kind of moved the other way. We’re trying to be more thoughtful in prescribing. So I think that’ll mean less prescriptions, stopping meds that are not effective or dangerous. Safety has become kind of an issue, a bigger issue as well.
The problems presented by the analysis of aggregate data led clinicians to question how to provide appropriate pain management for individual patients who have co-occurring CNCP and substance use conditions in light of growing concerns about opioid safety. Clinicians asked themselves: where does opioid prescribing fit in to the mandate of treating an individual’s pain without subjecting patients or the community to iatrogenic harm? One clinician described the tension between observing an individual patient’s positive response to opioids and a burgeoning scientific literature that questions opioid efficacy:
The evidence coming out about the spread of the drugs and the deaths and the public health problem leading us to revisit how we take care of individuals. Now often, there’s often a risky step there, from public health problem to [what] we’re going to do with individuals. The pendulum may be swinging a little bit too far but right now [toward too severe opioid restrictions]. We’ve got a fairly balanced approach that feels comfortable to me, which is, we give these drugs if we believe that they’re indicated but under very tight conditions. Whether or not they work is the part in my mind that is the least answered. There’s all this stuff in the literature about—none of the trials show that opiates for this kind of pain [CNCP] work, [but] it’s just not been my experience. My experience has been that actually it does work. It doesn’t mean it doesn’t come with problems but it actually can help people.
The clinicians in our study viewed themselves as stewards of community health. As one clinician said, “We see ourselves as the doctors for this community”. As “doctors for the community,” they tried not to contribute to the harms, at the community-level, associated with the widespread circulation and potential misuse of prescription opioids. One clinician outlined the distinction between her legal concerns about liability for diversion of prescription opioids and her greater concern that her actions might cause individual patient and community harm:
[What are the harms?] Patients could overdose, or [I might be] contributing to a culture of violence by [patients] taking these drugs and then selling them, or selling the drugs [opioids] for crack. So the danger of contributing to the culture of violence in the community and a danger of physical harm to them [patients]. And then of course me feeling vulnerable to malpractice. [But, when I discovered my patient was selling medications], that wasn’t a feeling about malpractice. That wasn’t a feeling like I’m going to get in trouble for it, it was a feeling of like, “I am a drug dealer, I’ve been a drug dealer for the community for three years,” and that’s a shitty, shitty feeling. That is not why I got into medicine, and that’s something I would never want to be. It wasn’t like a fear that I was going to get sued over it, it was the actual reality that like I was contributing to that [drug use] because I wasn’t thinking it through. I wasn’t being firm enough in my prescription use.
In this example, the clinician is concerned about a number of harms, including the potential for overdose, contributing to violence, legal harms (e.g., loss of medical license, investigation by the US Drug Enforcement Agency), and personal/emotional harms, as a clinician in a conflicted relationship with her patient about opioid prescription.
Another clinician attributed changes in his opioid prescribing behavior to his experience observing that chronic opioid therapy was not treating CNCP effectively, while at the same time potentially harming the broader community through medication misuse.
I’ve definitely shifted over the last few years to not really believing that any controlled opiates help much in chronic non-cancer pain. There’s no evidence that I get that people who use it seem to be better off as opposed to people that don’t. And the harm that it causes is so significant.
While clinicians are changing their opioid prescribing practices to reduce harm, scientific uncertainty still plagues clinical decision-making in the management of CNCP. The IOM concluded that a dearth of scientific research exists to guide clinicians in pain management, particularly with patients who have a history of substance use (Chou, et al, 2009). The confluence of concerns about the safety of opioids for patients and communities and the lack of evidence about the efficacy of chronic opioid therapy formed the conceptual basis for the current era of opioid pharmacovigilance.
III. Opioid pharmacovigilance: strategic approaches to manage opioid-associated risks
In the context of questionable treatment efficacy and high potential for individual misuse and community harms, the Centers for Disease Control and Prevention (CDC), the American Pain Society, and the American Medical Association suggested strategies to promote reduced prescription of opioids, as well as increased surveillance for aberrant behaviors and side effects (Dowell, et al., 2016; AMA, 2016). Suggested strategies included the use of pain agreements that outline specific terms under which the prescription of opioids can take place; the use of urine toxicology screens (UTS) to confirm the presence of prescribed opioids and the absence of illicit substances or unprescribed medications; requirements to attend substance use treatment or pain groups if illicit substances are detected in the UTS; limited opioid access through insurance regulatory mechanisms and pharmacy restrictions; and clinicians’ use of the state prescription drug monitoring programs (PDMPs) to assess whether patients are accessing opioid prescriptions from more than one clinician.
The new era of increased opioid pharmacovigilance represents a sea change in the perceptions of the benefits of chronic opioid therapy among clinicians. The emergence of opioid pharmacovigilance resulted in several benefits, according to clinicians. First, the adoption of these strategies helped reduce differential prescribing practices between clinicians within clinics and within healthcare systems. Second, it helped to depersonalize and legitimatize individual clinician’s decisions to terminate opioids. Third, it helped to create an opportunity to link clinicians’ concerns about the potential harms of opioids in the wider community with an individual patient’s CNCP treatment. Aspects of all of these benefits are described by one clinician who compared past experiences managing opioid prescription safety with the current clinical practices of opioid pharmacovigilance.
[I used to say] “You have cocaine in your urine again, I just can’t do this [prescribe opioids],” and they’d be like, “Well, forget it, I’m firing you as a doctor, I’m going to go to this other doctor, Dr. X will give me narcotics,” …. in an effort to stop that, we’ve all gotten better at saying, “It doesn’t have to do with me, this is the policy of the clinic,” which, for those of us like myself who find it a little hard to say no, gives me a little stronger backing when I feel like I do need to cut someone off [discontinue opioids]. I don’t have to own that decision alone, and stand up to the patient and say, “No, you know, you’re using cocaine, you really can’t do this anymore. It’s my decision and I say no.” It more becomes, “Look, this is the policy at our clinic, we’re very concerned about drugs in the community, this is the policy, it’s not just me deciding.” I mean, maybe it’s wimpy but, [I can say] “I’m powerless in this decision, it’s the clinic policy, charts are being reviewed, there’s nothing I can do.” That makes it easier for me.
Most clinicians expressed concerns about opioid-associated overdose and diversion and misuse of prescription opioids. They expressed relief resulting from the introduction of increased opioid pharmacovigilance measures (e.g., UTS, use of statewide PDMPs, pain agreements). Opioid pharmacovigilance shifted the goals of pain management. Whereas in the past, opioid prescribing fit into a framework of pain relief and social justice within a resource limited healthcare delivery context, now the goals of risk management for the patient and the community are weighed equally in pain management. While in the past, clinicians felt compelled to assess and manage pain with opioids, now most described a strong reluctance to prescribe opioids. One clinician described how she had always felt reluctant to prescribe opioids. In the past, she felt fear about her decision not to prescribe. Today, she is more emboldened:
There was that kind of fear [in the past], that opioids were the only way to address pain in the beginning of my career, but now I’m feeling more comfortable just to say no. I feel now that the research is there…That’s what’s changed, I think I’ve always felt that but I wouldn’t practice that as much. Now, I’m more comfortable saying, “There’s lots of choices to fix your pain, and the opiate choice for me is not the most comfortable.”
While many clinicians identified benefits from the implementation of strategies to increase surveillance of opioid prescribing, they expressed concerns about the unintended consequences of these shifts in the culture of CNCP management. First, clinicians described concerns about the increased regulatory environment, which included signed pain agreements, clinicians checking statewide PDMP databases, and routine UTS, and created a climate of mistrust that might threaten the clinician-patient therapeutic alliance. One clinician emphasized that this mistrust was particularly damaging to patients who were vulnerable to poor health care experiences because of their race/ethnicity, history of substance use, and/or lower socio-economic status (Chang, et al., 2016). Revisiting the theme of clinical justice that formed one rationale for widespread opioid prescribing in the past, this clinician argued that some of her patients were very distrustful of health care systems, and that her role as a clinician was to rebuild that trust. This clinician felt that the mechanisms of clinical surveillance and their potential consequences (e.g., rapid tapering of high doses of opioids or complete discontinuation of opioids) might worsen patients’ health because patients would see healthcare as a site of mistrust and abuse, and not return to the clinic for ongoing health care.
Even though I have a very close relationship with many of my patients, once you stop their opiates, you lose a lot of the relationship you’ve built. It may be fixable and it just may not. I think we sugarcoat that a lot because for many of these patients, we were the place that put them on opiates when nobody else believed they were in pain. Then something happens -- they refuse to give a UTOX, or they don’t show up for a certain visit or they leave a cocaine positive UTOX … Maybe I’m being naïve but at some level, with some patients I think this is very true, that we really believed them at a time when nobody else would believe them, nobody else would give them these drugs [opioids]. They got better with these drugs and then we stop [the opioids] and we’re like every other provider who has been treating them like shit for their entire lives.
One clinician offered a frank account of the conflict she saw between the demands on the patients and the demands on her as a clinician. This clinician felt that the responsibility for the over-prescription of opioids was being transferred from clinicians (where the clinician felt it belonged) to patients who will suffer further negative consequences through the newly initiated restrictions on opioid prescribing.
There’s a lot of irony here because for many, many, many years I was the one who kept arguing, I refused to write the medication doses that [the pain clinic] wanted us to write and I was a big stickler … The doses were too high and I didn’t want my name on [the prescription] because I didn’t think there was any evidence that it [medication at high doses] helped. But there was this big backlash that “you’re not trusting the patients.” So, I’m feeling very sensitive to the fact that people thought I was not respecting our patients because I really didn’t want to write these [high] doses. I don’t think it’s fair for us all of the sudden to say [to patients], “Sorry, policy has changed.” Because [patients] can’t just walk over to [another hospital] and say, “Hey, I used to be on [a high dose of opioids], will you start giving it to me?” We have to take responsibility for the doses that we put people on.
Most clinicians expressed agreement that the medical establishment, and specifically clinicians, bore some responsibility for the current problem of opioid over-prescription and its consequences. They identified both the large numbers of patients now dependent on opioids and high numbers of unintentional overdoses associated with opioid misuse, as stemming from iatrogenic prescribing practices. In the safety net clinical environments in which clinicians expressed a strong commitment to humanistic and compassionate healthcare, the lack of objective measures – for pain treatment efficacy, for appropriate dosing levels of opioids, for assessing functional states – was problematic. Most clinicians expressed frustration at being caught in the trap of simultaneously worrying about the health effects of withdrawing opioids and the health effects of continuing to prescribe them.
Discussion
We documented how clinical education, clinicians’ perceptions about patients’ socioeconomic status, resource scarcity, and a dearth of scientific evidence about opioid efficacy played significant roles in shaping the current climate of restricted opioid prescriptions. The risk of presentism, in which viewpoints about the past are influenced by current understandings, is a limitation of any socio-historical study in which participants reflect on previous practices. While opioid prescribing now carries a degree of clinical stigma and is situated in a publicly-mediated politics of regret, clinicians in our study demonstrated candor and self-reflection about their past understandings and practices. Very little research has contextualized the clinical and social factors that influenced the historic increases in opioid prescriptions and that now influence dramatic decreases in the prescription of these analgesics from the perspectives of clinicians.
Clinicians described how the use of research findings about pain management gleaned from studies conducted in one population or in a specific clinical setting were applied to populations to which the results were not generalizable. This lack of generalizability may be due to the heterogeneity in the diagnosis and treatment of CNCP. A CNCP diagnosis can reference a wide range of clinical conditions to which individual patients have a wide range of responses. Biological markers do not exist to verify pain symptoms, and other objective tools (e.g., x-rays and MRI scans) sometimes fail to confirm patients’ reports about loss of function and pain intensity. The efficacy of chronic opioid therapy for CNCP is still an unresolved issue. The ways in which clinicians in our study used evidence, or its lack, relative to CNCP treatment decision-making reflected ongoing clinical uncertainty that is not resolved by the strategies of opioid pharmacovigilance (Ceaser, et al., 2016). Over treatment and under treatment of CNCP among safety net patients remain on-going challenges for clinicians.
Anthropologists and medical sociologists have documented how a clinical culture can be created over time in which iatrogenic practices, and clinician biases, are reinforced in a manner that can be largely invisible. Our analysis highlights the inseparability of clinical and social forces and reveals how these intersections impact everyday clinical practice. Robin Higashi and colleagues (2013) demonstrated how a hidden curriculum which marked patients from vulnerable social groups as “time consuming” affected patient care, while masking a structurally-imposed pressure to finish appointments quickly. Clinicians in our study reported that their prescribing practices were influenced by their peers and superiors in the period of increased opioid prescribing and in the current period of opioid pharmacovigilance, underscoring the role of the hidden curriculum. Changes in opioid prescribing that are currently occurring reflect an evaluation of the available evidence. Many clinicians view reductions in opioid prescriptions as a necessary and appropriate corrective action to counteract previous prescribing practices. Yet, clinicians in our study raised questions about whether individual patients will suffer, unnecessarily, in efforts to protect the community’s health. In settings of increasing opioid pharmacovigilance, clinicians’ concerns about the potential harms associated with opioid prescribing (e.g. misuse; diversion; overdose) can diverge from patients’ concerns (e.g. opioid dependence; stigmatization in clinical settings) (Hurstak et al, In Press). Limited, available data suggest that increased monitoring of patients may lead them to limit substance use disclosures to their primary care clinicians (Ceasar et al, 2016); to leave primary care (Coffin & Banta-Green, 2014); and, to increase their use of non-pharmaceutical opioids such as heroin (Harocopos et al, 2016).
During the period when opioid prescriptions escalated, safety net clinicians were influenced by extra-clinical factors. Clinicians reported being committed to providing pain relief to a population who were structurally vulnerable as a result of poverty; violence in their communities, including gun violence; and forms of low wage, dangerous employment in which injuries were commonplace. Medical complexity among impoverished patients played a role in opioid prescription decision-making. Acquiescence to patients’ opioid prescription requests was perceived to facilitate healthcare engagement and medical adherence for commonplace and serious chronic conditions (e.g. HIV, hypertension, diabetes). Clinicians indicated that the resources available to treat CNCP were limited within the healthcare system designed to serve the very poor. The manner by which concerns about the under treatment of pain were racialized and understood is the context of poverty among the study clinicians is likely influenced by the fact that the majority reported training in safety net clinical settings in the San Francisco Bay Area, a clinical setting with an embedded social justice focus in medical education in a socially liberal US city.
In the last 25 years, opioid prescribing has undergone a rapid rise, leading it to become a widely used treatment for CNCP, followed by a precipitous decline, as opioids are now perceived to be problematic. Using social scientific methods and theory, we delineated the clinical and social factors that influenced opioid prescribing practices, at different historical periods. The prescription of opioids for the management of CNCP is a complex issue that raises serious questions of both treatment efficacy and patient and community safety. Implications of our findings for clinical care and medical education include the need present opioid prescribing in socio-historical context as a component of mandatory pain management education; address the challenges of clinical uncertainty in opioid prescribing; and provide students and clinicians with opportunities to problem solve opioid-related challenges common in clinician-patient interactions. Models for these types of education are currently being implemented in elective and continuing education formats (SCOPE, 2017). In California, where this study took place, health departments and non-profit organizations have responded to opioid pharmacovigilance by advocating for public opioid education; naloxone distribution in clinical and community settings; expansion of medically-assisted treatment for opioid-dependent patients; and insurance reimbursement for non-pharmacologic pain treatment modalities (CHCF 2015; CHCF 2016). To inform policy moving forward, prospective, contextual, social scientific research is needed that documents the consequences of opioid pharmacovigilance for CNCP patients, their clinicians, and community health.
Highlights.
Opioid prescribing practices of US primary care clinicians are under-researched.
Safety net healthcare settings serve the majority of patients receiving opioids.
The evidence-base in favor of and opposing opioid prescribing has evolved.
Poverty, race, and limited healthcare resources influence opioid prescribing.
This analysis elucidates current consequences of opioid pharmacovigilance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Medical Association (AMA) Preventing Opioid Abuse: Be Part of the Solution. [Accessed, Spetember 29, 2016];2016 http://www.ama-assn.org/ama/pub/advocacy/topics/preventing-opioid-abuse.page?
- Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24(6):469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- Barnett ML, Olenski AR, Jena AB. Opioid-Prescribing Patterns of Emergency Physicians and Risk of Long-Term Use. N Engl J Med. 2017;376:663–73. doi: 10.1056/NEJMsa1610524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baszanger I. Inventing Pain Medicine: From the Laboratory to the Clinic. New Brunswick, N.J.: Rutgers University Press; 1998. [Google Scholar]
- Buchbinder M. All in Your Head: Making Sense of Pediatric Pain. Oakland: University of California Press; 2015. p. 233. [Google Scholar]
- California Heath Care Foundation (CHCF) Pain Care on a New Track: Complimentary Therapies in the Safety Net. 2016 Jul; [Google Scholar]
- California Heath Care Foundation (CHCF) Striking A Balance: Safety Net Leaders Explore Solutions to the Prsecription Pain Killer Epidemic. 2015 Mar; [Google Scholar]
- Ceasar R, Chang J, Zamora K, et al. Primary care providers' experiences with urine toxicology tests to manage prescription opioid misuse and substance use among chronic noncancer pain patients in safety net health care settings. Subst Abus. 2016;37(1):154–160. doi: 10.1080/08897077.2015.1132293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. American Pain Society 1995 Presidential Address. Journal of Pain. 1996 Spring;5(1):85–88. [Google Scholar]
- Carr DB, Goudas LC. Acute pain. The Lancet. 1999;353(9169(12)):2051–2058. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers---United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492. [PubMed] [Google Scholar]
- Chang JS, Kushel M, Miaskowski C, et al. Provider experiences with the identification, management, and treatment of co-occurring chronic non-cancer pain and substance use in the safety net. Substance Use and Misuse. doi: 10.1080/10826084.2016.1223138. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009 Feb;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin P, Banta-Green C. The dueling obligations of opioid stewardship. Ann Intern Med. 2014;160(3):207. doi: 10.7326/M13-2781. [DOI] [PubMed] [Google Scholar]
- Crowley Matoka M, True G. No One Wants to be the Candy Man: Ambivalent Medicalization and Clinician Subjectivity in Pain Management. Cultural Anthropology. 2012;27(4):689–712. [Google Scholar]
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain-- United States, 2016. Jama. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010 Jan 19;152(2):85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S. Impure Science: AIDS, Activism, and the Politics of Knowledge. Berkeley: University of California Press; 1998. p. 480. [PubMed] [Google Scholar]
- Fact Sheet: Obama Administration Announces Additional Actions to Address the Prescription Opioid Abuse and Heroin Epidemic [press release] [Accessed September 19, 2016];The White House. 2016 https://www.whitehouse.gov/the-press-office/2016/03/29/fact-sheet-obama-administration-announces-additional-actions-address.
- Farmer P. Aids and Accusation: Haiti and the Geography of Blame. Berkeley: University of California Press; 1992. p. 352. [Google Scholar]
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008 May-Jun;9(4):444–59. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Green GR, Anderson KO, Baker TA, et al. Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, et al. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain medicine. 2003;4(3):277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- Greenhalgh S. Under the Medical Gaze: Facts and Fictions of Chronic Pain. Berkeley: University of California Press; 2001. p. 383. [Google Scholar]
- Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization study in primary care. JAMA. 1998;280:147–51. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- Harocopos A, Allen B, Paone D. Circumstances and contexts of heroin initiation following non-medical opioid analgesic use in New York City. Int J Drug Policy. 2016;28:106–11. doi: 10.1016/j.drugpo.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Higashi RT, Tillack A, Steinman MA, Johnston CB, Harper GM. The 'worthy' patient: rethinking the 'hidden curriculum' in medical education. Anthropol Med. 2013 Apr;20(1):13–23. doi: 10.1080/13648470.2012.747595. [DOI] [PubMed] [Google Scholar]
- Holmes, Seth M. Fresh Fruit, Broken Bodies: Migrant Farmworkers in the United States. Berkeley: University of California Press; 2013. p. 234. [Google Scholar]
- Hurstak E, Kushel M, Miaskowski C, Chang JS, Ceasar R, Zamora K, Knight KR. The risks of opioid treatment: Perspectives of primary care practitioners and patients from safety-net clinics. Subst Abus. doi: 10.1080/08897077.2017.1296524. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, Shilliday BB, DeWalt DA, Pignone MP. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006 Apr 4;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman A. Pain and Resistance: The Delegitimation and Relegitimation of Local Worlds. In: DelVecchio Good Mary-Jo, Brodwin Paul E, Good Byron, Kleinman Arthur., editors. Pain as Human Experience. Berkeley: University of California Press; 1992. [Google Scholar]
- Knight KR. addicted. pregnant. poor. Durham, NC: Duke University Press; 2015. p. 328. [Google Scholar]
- Langlitz N. Pharmacovigilance and post-black market surveillance. Social Studies of Science. 2009 Jun;39(3):395–420. doi: 10.1177/0306312708101977. [DOI] [PubMed] [Google Scholar]
- Lochlann Jain S. Malignant: How Cancer Becomes Us. Berkeley: University of California Press; 2013. p. 304. [Google Scholar]
- Manchikanti L, Singh A. Therapeutic opioids: a ten-year perspective on the complexities and complications of the escalating use, abuse and nonmedical use of opioids. Pain Physician. 2008 Mar;11(2 Suppl):S63–88. [PubMed] [Google Scholar]
- Metzl JM. The Protest Psychosis: How Schizophrenia Became a Black Disease. Boston: Beacon Press; 2009. p. 288. [Google Scholar]
- Morasco BJ, Gritzner S, Lewis L, Oldham R, Turk DC, Dobscha SK. Systematic review of prevalence, correlates, and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorder. Pain. 2011;152(3):488–497. doi: 10.1016/j.pain.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya MJ. Making the Mexican Diabetic: Race, Science, and the Genetics of Inequality. Berkeley: University of California Press; 2011. p. 282. [Google Scholar]
- Newkirk VR. The Pain Points of Opioid Policy. [accessed, September 29, 2016];The Atlantic. 2016 Apr 4; http://www.theatlantic.com/politics/archive/2016/04/the-pain-points-of-opioid-policy/476754/
- Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. The Cochrane database of systematic reviews. 2010 doi: 10.1002/14651858.CD006605.pub2. Cd006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: report of 38 cases. Pain. 1986 May;25(2):171–86. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1980 Jan 10;302(2):123. doi: 10.1056/nejm198001103020221. [DOI] [PubMed] [Google Scholar]
- Quinones S. Dreamland: The True Tale of America’s Opiate Epidemic. New York and London: Bloomsbury Press; 2015. p. 368. [Google Scholar]
- Rouse C. Uncertain Suffering: Racial Health Care Disparities and Sickle Cell Disease. Berkeley: University of California Press; 2009. p. 328. [Google Scholar]
- Safe and Confident Opioid Prescribing Education (SCOPE) [Accessed February 23, 2017]; https://www.scopeofpain.com/
- Schrager SM, Kecojevic A, Silva K, Jackson Bloom J, Iverson E, Lankenau SE. Correlates and Consequences of Opioid Misuse among High-Risk Young Adults. Journal of Addiction. 2014;2014:156954. doi: 10.1155/2014/156954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonington S, Holmes SM. The PLoS Medicine Editors Social Medicine in the Twenty-First Century. PLoS Med. 2006;3(10):e445. doi: 10.1371/journal.pmed.0030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd KH, Deaton C, D’Adamo AP, Goe L. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11–6. doi: 10.1016/s0196-0644(00)70099-0. [DOI] [PubMed] [Google Scholar]
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537–9. [PubMed] [Google Scholar]
- Trescot AM, Helm S, Hansen H, et al. Opioids in the management of chronic non-cancer pain: an update of American Society of Interventional Pain Physicians' (ASIPP) guidelines. Pain Physician. 2008;11:S5–S62. [PubMed] [Google Scholar]
- Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008 Jul-Aug;24(6):497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- Wailoo K. Pain: A Political History. Baltimore, MD: John Hopkins University Press; 2015. [Google Scholar]
- Wong-Baker FACES Foundation. Wong-Baker FACES® Pain Rating Scale. [September 29, 2016];2016 Retrieved with permission from http://www.WongBakerFACES.org.
- World Health Organization. The Safety of Medicines in Public Health Programmes: Pharmacovigilance an Essential Tool. [Accessed September 29, 2016];2006 http://www.who.int/medicines/areas/quality_safety/safety_efficacy/Pharmacovigilance_B.pdf?ua=1.