Abstract
Objective
In people without prior stroke, covert findings on serial MRI of incident brain infarcts and worsening leukoaraiosis are associated with increased risk for ischemic stroke and dementia. We evaluated whether various measures of blood pressure and heart rate are associated with these MRI findings.
Approach and Results
In the Cardiovascular Health Study, a longitudinal cohort study of older adults, we used relative risk (RR) regression to assess the associations of mean, variability, and trend in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR) measured at four annual clinic visits between two brain MRIs with incident covert brain infarction (CBI) and worsening white matter grade (WMG, using a 10 point scale to characterize leukoaraiosis). We included participants who had both brain MRIs, no stroke before the follow-up MRI, and no change in antihypertensive medication status during follow-up. Among 878 eligible participants, incident CBI occurred in 15% and worsening WMG in 27%. Mean SBP was associated with increased risk for incident CBI (RR per 10 mmHg 1.28; 95% confidence interval (CI) 1.12–1.47), and mean DBP was associated with increased risk for worsening WMG (RR per 10 mmHg 1.45; 95% CI 1.24–1.69). These findings persisted in secondary and sensitivity analyses.
Conclusions
Elevated mean SBP is associated with increased risk for CBI, and elevated mean DBP is associated with increased risk for worsening leukoaraiosis. These findings reinforce the importance of hypertension in the development of silent cerebrovascular diseases, but the pathophysiologic relationships to blood pressure for each may differ.
Keywords: silent brain infarction, covert brain infarction, white matter disease, leukoaraiosis, hypertension, blood pressure variability, risk factors
Subject codes: epidemiology, primary prevention, cerebrovascular disease/stroke
INTRODUCTION
In people without prior stroke, covert brain infarction (CBI) and leukoaraiosis are described as “silent” cerebrovascular diseases, but they have clinically important consequences. CBI is associated with a two-to-four fold increased risk of clinically defined ischemic stroke, independent of vascular risk factors, and a two-to-three fold increased risk of dementia.1–4 They may also directly disrupt functional networks, leading to deficits affecting cognition, gait, and other functions.5 Similarly, leukoaraiosis is associated with an increased risk for ischemic stroke, worse outcomes after stroke, dementia, and mortality.6–11 Leukoaraiosis prevalence in older adults exceeds 95%, and CBI is also common: the estimated prevalence of CBI in adults over age 50 is approximately 20% compared to 2–14% for overt ischemic stroke in the U.S.2,11 While the American Heart Association/American Stroke Association recently published a scientific statement highlighting the importance of these conditions and the need for further studies to guide their management, optimal prevention strategies after detection of these conditions have not been established, in part due to uncertainties about their pathogenesis that may resemble or differ from ischemic stroke.12 Furthermore, strategies for the prevention of incident CBI and worsening of leukoaraiosis have not been established. Given their high prevalence and clinical sequelae, improving understanding of the pathogenesis of CBI and leukoaraiosis may help guide strategies for prevention of cerebrovascular diseases.
Hypertension is associated with CBI and leukoaraiosis as well as clinically defined ischemic stroke.2,13–15 Both primary and secondary stroke prevention guidelines focus on absolute reductions in systolic and diastolic blood pressure, but some studies suggest that very aggressive blood pressure reductions may lead to an increased risk of recurrent stroke.16 Accordingly, investigators have sought to improve current understanding of the role of cardiovascular physiology in brain infarction by studying other measures including variability in blood pressure and heart rate. Prior studies suggest associations of blood pressure and heart rate variability with ischemic stroke, but these associations are inconsistent and depend on the time interval of measurement (beat-to-beat, daily, weekly, or visit-to-visit).17–19 Despite these potential associations with ischemic stroke, it is not known if visit-to-visit blood pressure and heart rate measures are associated with an increased risk of incident CBI or progression of leukoaraiosis.
In this study, we used data from two brain MRIs performed approximately five years apart in the Cardiovascular Health Study (CHS) to assess associations of visit-to-visit cardiovascular measurements, including mean, variability, and trend in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR), with incident CBI and progression of leukoaraiosis, building upon findings from prior work based on these scans.20–21
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Study Population
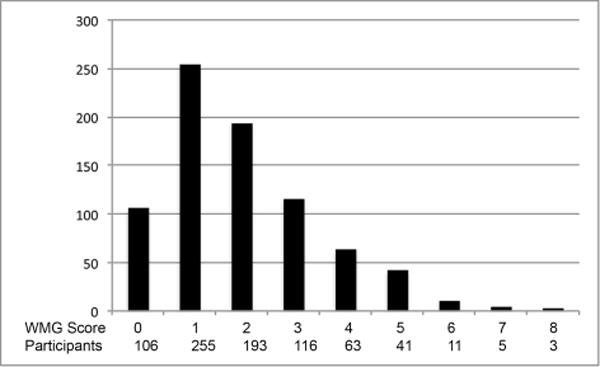
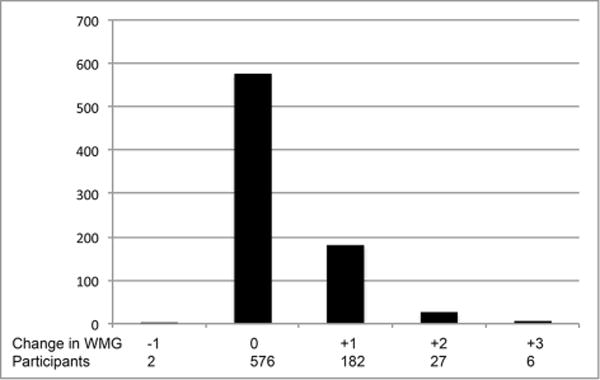
As detailed in Figure 1, 878 participants were eligible for the primary analysis and 1844 participants for the sensitivity analysis. Baseline characteristics and mean (SD) for the exposure variables in the primary and sensitivity analysis samples are presented in Table 1 (additional descriptive statistics for each individual analysis and a comparison to all CHS participants undergoing the first MRI are provided in the Online Supplement Table I). Among 683 participants who completed both brain MRIs and demonstrated no infarcts on the initial MRI, 101 individuals (15%) had incident CBI. Among 793 participants with white matter grade measurements (WMG, a 10 point scale characterizing the extent of leukoaraiosis) on both MRIs and a grade of 8 or less on the initial MRI, 215 (27%) demonstrated worsening WMG. The distributions of number of CBI, WMG, and changes in WMG are shown in Figure 2. Evaluation of the exposure variables indicated minimal correlation for most pairs and at most modest correlations for a few pairs (Supplemental Table II).
Figure 1. Flow chart of participants.
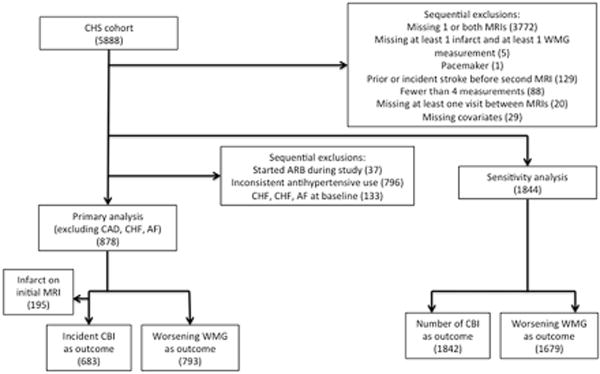
CHS = Cardiovascular Health Study, CAD = coronary artery disease, CHF = congestive heart failure, AF = atrial fibrillation, MRI = magnetic resonance imaging, WMG = white matter grade, ARB = angiotensin receptor blocker.
Table 1.
Baseline characteristics (primary analyses and sensitivity analyses).
| Characteristic | Primary analysis, mean SD or n (%) | Sensitivity analysis, mean SD or n (%) |
|---|---|---|
| Total number of participants | 878 | 1844 |
| Age (years, at follow-up MRI) | 73.5 (4.2) | 74.0 (4.4) |
| Sex (male) | 315 (36.0%) | 734 (39.8%) |
| Race (black) | 111 (12.6%) | 274 (14.9%) |
| Body mass index | 26.1 (4.1) | 26.7 (4.3) |
| Smoking status | ||
| Never smoker | 429 (48.9%) | 867 (47.0%) |
| Former smoker | 365 (41.6%) | 823 (44.6%) |
| Current smoker | 84 (9.6%) | 154 (8.4%) |
| Diabetes | 69 (7.9%) | 223 (12.1%) |
| CHS Clinic | ||
| North Carolina | 197 (22.4%) | 435 (23.6%) |
| California | 281 (32.0%) | 547 (29.7%) |
| Maryland | 165 (18.8%) | 362 (19.6%) |
| Pennsylvania | 235 (26.8%) | 500 (27.1%) |
| Antihypertensive medications | 236 (26.9%) | 711 (38.6%) |
| Interval between MRI scans (days) | 1808.9 ± 215 | 1827.2 ± 217 |
| MRI findings | ||
| Incident covert brain infarct | 101/683 (14.8%) | N/A |
| Worsening white matter grade | 215/793 (27.1%) | 472 (28.1%) |
| Systolic blood pressure | ||
| Mean (mm Hg) | 129.8 (15.0) | 133.1 (16.2) |
| Variability | 7.4 (4.5) | 8.3 (5.4) |
| Trend | 0.6 (3.4) | −0.1 (4.2) |
| Diastolic blood pressure | ||
| Mean (mm Hg) | 68.8 (7.9) | 68.9 (8.7) |
| Variability | 4.5 (3.2) | 4.9 (3.5) |
| Trend | −0.39 (2.2) | −0.8 (2.5) |
| Heart rate | ||
| Mean (beat per minute) | 63.7 (8.2) | 63.4 (8.7) |
| Variability | 3.8 (2.6) | 4.1 (3.2) |
| Trend | 0.1 (2.5) | 0.1 (2.6) |
MRI = magnetic resonance imaging.
Figure 2. Incident covert brain infarcts (CBI) and white matter grade (WMG) on the follow-up MRI.
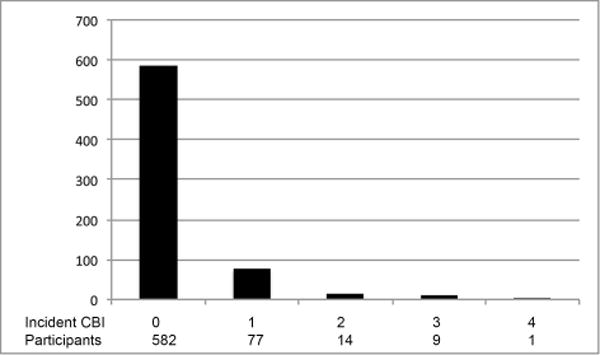
A. Incident CBI
B. WMG
C. Change in WMG
(A) Distribution of number of incident CBI on the follow-up MRI. (B) Distribution of WMG on the follow-up MRI. (C) Distribution of changes in WMG on the follow-up MRI.
Primary, secondary, and exploratory analyses: association with incident CBI and worsening WMG
In primary analyses, mean SBP was associated with increased risk for incident CBI (RR 1.28, 1.12–1.47, p<0.001), and mean DBP was associated with increased risk for worsening WMG (RR 1.45, 1.24–1.69, p<0.001) (Table 2). An association between positive HR trend and increased risk for incident CBI was nominally significant (RR 2.03, 1.11–3.72, p=0.02, using a conservative Bonferroni correction of p=0.003 to account for multiple comparisons). In exploratory analyses, mean PP was nominally associated with increased risk for incident CBI (RR 1.22, 1.03–1.44, p=0.02), and mean MAP was nominally associated with increased risk for incident CBI (RR 1.42, 1.12–1.79, p=0.004) and associated with worsening WMG (RR 1.29, 1.12–1.48, p<0.001) (Table 3). In secondary analyses, we observed associations between mean SBP and increased number of incident CBIs (beta 0.06, 0.02–0.10, p=0.002) and between mean DBP and higher WMG (beta 0.13, 0.07–0.19, p<0.001) (Online Supplement, Table III). An association between positive HR trend and increased number of incident CBIs was again nominally significant (beta 0.18, 0.01–0.34, p=0.03). The findings of models incorporating both SBP and DBP and both PP and MAP demonstrated similar findings except that the nominal associations between mean PP and incident CBI and between mean MAP and incident CBI were no longer significant (Online Supplement, Tables IV–V).
Table 2.
Relationships of SBP, DBP, and HR to dichotomous outcomes: incident covert brain infarcts (CBI) and worsening white matter grade (WMG).
| Incident CBI (n=683) | SBP (10 points mm Hg) | DBP (10 points mm Hg) | HR (10 bpm) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Mean | 1.28 (1.12, 1.47) | <0.001 | 1.30 (0.99, 1.71) | 0.06 | 1.17 (0.93, 1.47) | 0.19 |
| Variability | 0.89 (0.60, 1.31) | 0.54 | 1.56 (0.95, 2.56) | 0.08 | 0.81 (0.42, 1.56) | 0.54 |
| Trend | 0.65 (0.39, 1.07) | 0.09 | 0.94 (0.34, 2.62) | 0.90 | 2.03 (1.11, 3.72) | 0.02 |
|
| ||||||
| Worsening WMG (n=793) | SBP (10 points mm Hg) | DBP (10 points mm Hg) | HR (10 bpm) | |||
|
| ||||||
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Mean | 1.09 (1.00, 1.20) | 0.06 | 1.45 (1.24, 1.69) | <0.001 | 1.10 (0.95, 1.28) | 0.19 |
| Variability | 0.97 (0.72, 1.31) | 0.83 | 1.26 (0.85, 1.86) | 0.25 | 1.19 (0.78, 1.82) | 0.43 |
| Trend | 1.10 (0.77, 1.57) | 0.62 | 0.98 (0.56, 1.72) | 0.95 | 1.13 (0.73, 1.74) | 0.58 |
Visit-to-visit mean was calculated from four annual measurements. Visit-to-visit trend was calculated using linear regression (slope). Visit-to-visit variability was calculated from the standard deviation of the residuals of the linear regression. Relative risks (RR) and 95% confidence intervals (CI) estimated from RR regression model adjusted for age, sex, race, clinic location, smoking status, BMI, diabetes, time between MRI scans, and antihypertensive medication status. RRs are presented for mean, variability, and trend per 10 points of mm Hg for systolic and diastolic blood pressure and 10 beats per minute of heart rate. The Bonferroni corrected p-value is 0.003.
SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate, bpm = beats per minute.
Table 3.
Relationships of PP and MAP to dichotomous outcomes: incident covert brain infarcts (CBI) and worsening white matter grade (WMG).
| Incident CBI (n=683) | PP (10 points mm Hg) | MAP (10 points mm Hg) | ||
|---|---|---|---|---|
|
| ||||
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Mean | 1.22 (1.03, 1.44) | 0.02 | 1.42 (1.12, 1.79) | 0.004 |
| Variability | 1.13 (0.75, 1.71) | 0.55 | 1.15 (0.67, 1.97) | 0.61 |
| Trend | 0.63 (0.34, 1.14) | 0.13 | 0.63 (0.26, 1.53) | 0.31 |
|
| ||||
| Worsening WMG (n=793) | PP (10 points mm Hg) | MAP (10 points mm Hg) | ||
|
| ||||
| RR (95% CI) | P value | RR (95% CI) | P value | |
| Mean | 0.98 (0.88, 1.10) | 0.74 | 1.29 (1.12, 1.48) | <0.001 |
| Variability | 1.04 (0.77, 1.41) | 0.81 | 1.12 (0.74, 1.69) | 0.60 |
| Trend | 1.15 (0.77, 1.71) | 0.51 | 1.07 (0.62, 1.86) | 0.80 |
Visit-to-visit mean was calculated from four annual measurements. Visit-to-visit trend was calculated using linear regression (slope). Visit-to-visit variability was calculated from the standard deviation of the residuals of the linear regression. Relative risks (RR) and 95% confidence intervals (CI) estimated from RR regression model adjusted for age, sex, race, clinic location, smoking status, BMI, diabetes, time between MRI scans, and antihypertensive medication status. RRs are presented for mean, variability, and trend per 10 points of mm Hg for pulse pressure and mean arterial pressure. The Bonferroni corrected p-value is 0.003.
PP = pulse pressure, MAP = mean arterial pressure.
Sensitivity analysis
An inclusive sensitivity analysis corroborated results of the primary analyses (Table 4). Similar to the primary and secondary analyses, associations between mean SBP and increased number of CBI (beta 0.07, 0.04–0.09, p<0.001) and between mean DBP and worsening WMG (RR 1.32, 1.20–1.45, p<0.001) remained significant. Mean DBP was also associated with increased number of CBI (beta 0.13, 0.08–0.19, p<0.001). Several associations were nominally significant including mean HR and increasing number of CBI, mean SBP and worsening WMG, DBP variability and worsening WMG, and mean HR and worsening WMG.
Table 4.
Sensitivity analysis: Relationships of SBP, DBP, and HR to number of covert brain infarcts (CBI) and worsening white matter grade (WMG) without exclusions for prior CBI, changing antihypertensive status, or cardiovascular comorbidities.
| Number of CBI (n=1842) | SBP (10 points mm Hg) | DBP (10 points mm Hg) | HR (10 bpm) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Beta | P value | Beta | P value | Beta | P value | |
| Mean | 0.07 (0.04, 0.09) | <0.001 | 0.13 (0.08, 0.19) | <0.001 | 0.06 (0.009, 0.10) | 0.02 |
| Variability | 0.05 (−0.04, 0.13) | 0.28 | 0.12 (0.001, 0.24) | 0.05 | −0.04 (−0.16, 0.07) | 0.47 |
| Trend | −0.001 (−0.10, 0.10) | 0.98 | 0.03 (−0.13, 0.20) | 0.69 | 0.13 (−0.02, 0.27) | 0.09 |
|
| ||||||
| Worsening WMG (n=1679) | SBP (10 points mm Hg) | DBP (10 points mm Hg) | HR (10 bpm) | |||
|
| ||||||
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| Mean | 1.06 (1.00, 1.12) | 0.04 | 1.32 (1.20, 1.45) | <0.001 | 1.10 (1.00, 1.20) | 0.04 |
| Variability | 1.03 (0.88, 1.20) | 0.72 | 1.30 (1.05, 1.62) | 0.02 | 1.09 (0.89, 1.32) | 0.41 |
| Trend | 1.07 (0.88, 1.32) | 0.49 | 1.06 (0.76, 1.47) | 0.66 | 1.01 (0.76, 1.34) | 0.96 |
Visit-to-visit mean was calculated from four annual measurements. Visit-to-visit trend was calculated using linear regression (slope). Visit-to-visit variability was calculated from the standard deviation of the residuals of the linear regression. CBI analysis: beta coefficients and 95% confidence intervals (CI) estimated from linear regression model adjusted for age, sex, race, clinic location, smoking status, BMI, diabetes, time between MRI scans, and antihypertensive medication status (always, never, variable). WMG analysis: relative risks (RR) and 95% confidence intervals (CI) estimated from RR regression model adjusted for age, sex, race, clinic location, smoking status, BMI, diabetes, time between MRI scans, and antihypertensive medication status (always, never, variable). RRs are presented for mean, variability, and trend per 10 points of mm Hg for systolic and diastolic blood pressure and 10 beats per minute of heart rate. The Bonferroni corrected p-value is 0.003.
SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate, bpm = beats per minute.
Stratified analysis: antihypertensive medications
Stratification by antihypertensive medication use status corroborated the association between mean DBP and worsening WMG in non-users (RR 1.49, 1.23–1.79, p<0.001) (Supplemental Table VI). The association between mean SBP and increased risk for incident CBI in non-users was still present but slightly less robust (RR 1.24, 1.06–1.44, p=0.007). There were nominally significant associations between mean DBP and increased risk for incident CBI, DBP variability and CBI, HR trend and CBI, and mean SBP and worsening WMG. All associations were attenuated in participants taking antihypertensive medications. When testing for interactions with antihypertensive medication use, the interactions were not statistically significant.
DISCUSSION
Mean BP is associated with incident CBI and worsening leukoaraiosis
In this prospective cohort study of older adults without prior stroke and cardiovascular disease, elevated mean SBP was associated with increased risk for incident CBI, and elevated mean DBP was associated with increased risk for worsening leukoaraiosis, extending prior findings from the CHS.20–21 These associations remained robust across several secondary and sensitivity analyses incorporating adjustment for vascular risk factors and antihypertensive medication status, inclusion or exclusion of participants with prior imaging-defined infarcts (in the incident CBI analyses), and classification of the outcome as dichotomous or as counts. These findings are particularly important in the setting of potentially conflicting recommendations: the Joint National Committee advises less stringent control of hypertension in elderly adults, whereas a recent scientific statement from the American Heart Association/American Stroke Association highlights the clinical relevance of imaging-defined vascular brain injury and urges, at a minimum, initiation of stroke primary prevention measures in affected individuals.12,22 In patients without a history of stroke, this study suggests that incident CBI and worsening leukoaraiosis could be treatment targets for control of systolic and diastolic hypertension.
Differential associations between measures of pulsatile and steady flow with CBI and leukoaraiosis
Across several analyses, measures of steady blood flow (DBP and MAP) were associated with worsening leukoaraiosis. There was also a suggestion that measures of pulsatile blood flow (SBP and PP) were associated with incident CBI, although MAP was also associated with incident CBI in contrast to DBP and to a greater degree than PP. While prior studies have reported variable associations of pulsatile and steady blood flow with different forms of imaging-defined vascular brain injury, this study provides evidence suggesting the roles of different types of hypertension in the pathogenesis of CBI and leukoaraiosis.23–28 The more consistent finding is the relationship of measures of steady blood flow with leukoaraiosis: multiple pathophysiologic mechanisms for the development of leukoaraiosis have been postulated, but this study emphasizes that diastolic hypertension likely has a critical role. One theory is that increased peripheral arterial stiffness (represented by increased brachial DBP as a proxy for carotid DBP) in conjunction with increased aortic pulsatility may augment transmission of the effects of aortic pulsatility to the cerebral small vessels, resulting in increased endothelial shear stress and dysfunction.24 Alternatively, diastolic hypertension may correspond with increased peripheral arterial stiffness and reduced carotid flow velocity, resulting in reduced blood flow to cerebral small vessels and local hypoperfusion, independent of atherosclerosis.25 By contrast, regarding peripheral measures of pulsatile blood flow, it is hypothesized that the late-life development of atherosclerosis in intracranial arteries may arise in conjunction with increased peripheral pulse pressure.29 This study may support that hypothesis and may implicate atherosclerotic pathologies in the development of CBI. In light of these differential associations and the clinical significance of these conditions, future studies in the treatment of hypertension in adults (such as those in the CHS) should include incident CBI and worsening leukoaraiosis as distinct outcomes from one another and from clinically defined ischemic stroke.
HR trend and increased risk for CBI
We did find an association between positive HR trend and incident CBI, though its risk estimate has a moderately wide confidence interval and its p-value did not reach significance with a conservative Bonferroni correction. This association persisted through almost all analyses, including the stratified analyses. To our knowledge, an association between visit-to-visit HR trend and cerebrovascular outcomes has not been previously described. While mechanisms of vascular injury due to hypertension have been rigorously studied, it is less certain how changes in HR would lead to vascular brain injury. It is possible that this association may reflect a downstream or compensatory response to other changes in vascular biology that more directly lead to increased risk for CBI.
Other BP and HR measures are not consistently associated with incident CBI or worsening leukoaraiosis
In this study, variability and trend of SBP and DBP and mean and variability of HR were not consistently associated with CBI or worsening WMG: some demonstrated nominal statistical significance in the most inclusive sensitivity analysis, but these findings were unstable across analyses with exclusions aimed at reducing confounding. While HR mean and visit-to-visit variability were associated with mortality in a prior CHS analysis, we did not find a consistent relationship between these measures and covert imaging-defined vascular brain injury.30 The lack of association between SBP or DBP visit-to-visit variability and CBI is notable as it may suggest that CBI and clinically defined ischemic stroke have different relationships with blood pressure variability.17–19
The role of antihypertensive medication use
We assessed the potential for effect modification by antihypertensive medication use by stratifying study participants into users and non-users of a stable medication regimen. While the interactions in the full primary analysis sample were not statistically significant, we found that the associations between our primary exposure variables and incident CBI and worsening leukoaraiosis appeared to persist in the stratum of non-users. Interpretation of the lack of association in the stratum of medication users is less certain due to a low sample size and an absence of medication dosage and administration timing data in this study, both of which would tend to increase the variability of exposure among medication users. Future clinical trials may be useful to determine whether there is a beneficial effect of antihypertensives on imaging-defined vascular brain injury.
Strengths and limitations
Our study has several strengths including standardized BP and HR measurements at structured annual study visits, central adjudication of clinical events, centralized analysis of MRI scans with characterization of infarcts and WMG, and high-quality data on vascular risk factors collected prospectively. Additionally, this study population of healthy older adults offers an opportunity to establish these associations with relatively minimal confounding.
Our study had several limitations. First, it was limited to participants who underwent two brain MRIs according to the CHS protocol, an exclusion that reduced our study sample size and likely introduced a selection bias. Having no MRI or only a single MRI was the most frequent reason for exclusion from this analysis, which resulted from loss to follow-up, death, and various other reasons. Prior studies of the CHS demonstrated that participants who underwent brain MRI scans were healthier than those who did not, and those who underwent both brain MRIs were healthier than those that only underwent a single scan.20–21,31 Accordingly, our study may underestimate the frequency of incident CBI and worsening WMG.
Second, the sample size for the primary analyses was also limited due to the exclusions of participants with baseline CAD, CHF, and AF. We were concerned about the relationships between these cardiovascular conditions and our primary exposures of interest: all could be confounders or mediators of the association of these exposures on incident CBI and worsening WMG. We chose to exclude these individuals from the primary analyses and include them in a sensitivity analysis. The beta coefficients and risk estimates in the sensitivity analysis did not change substantially, suggesting that these conditions may be minor confounders or mediators. Nonetheless, the main findings of this study, the associations between mean SBP and incident CBI and between mean DBP and worsening leukoaraiosis, were not affected substantially by these conditions.
Third, our study focused on annual measurements and cannot detect potential associations between shorter intervals of BP and HR measurements and the outcomes.
Finally, our analysis involved multiple comparisons with potentially non-independent exposure variables and outcomes; if these were all independent, we would expect one significant finding by chance alone among 18 tests of interest. Since a Bonferroni correction may inadvertently obscure meaningful associations if the exposures or outcomes are not independent, we elected to present both uncorrected p-values and a formal Bonferroni threshold for comparison.
CONCLUSIONS
In summary, this study provides evidence that systolic hypertension is associated with increased risk for CBI, and diastolic hypertension is associated with increased risk for leukoaraiosis. Combining these findings with prior studies, these differential associations could suggest differences in pathophysiologic mechanisms underlying CBI, leukoaraiosis, and clinically defined ischemic stroke.17–19 Given the clinical significance of silent cerebrovascular diseases, these findings suggest a need to reevaluate the balance of benefits and risks of controlling systolic and diastolic hypertension in elderly adults. These findings need to be confirmed in other populations, including younger adults, and clinical trials may better establish the potential effect of antihypertensive medications on the risk of incident CBI and worsening leukoaraiosis.
Supplementary Material
HIGHLIGHTS.
Persistently elevated SBP is associated with incident CBI.
Persistently elevated DBP is associated with worsening white matter disease.
This suggests that hypertension is a major risk factor in the development of both forms of silent cerebrovascular diseases.
The exact relationship of blood pressure levels and variability to cerebrovascular diseases may differ between CBI, white matter disease, and stroke, suggesting potential differences in pathophysiology.
Acknowledgments
None.
SOURCES OF FUNDING
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, N01HC15103, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Abbreviations
- CBI
covert brain infarction
- CHS
Cardiovascular Health Study
- WMG
white matter grade
Footnotes
DISCLOSURES
Dr. Psaty serves on the DSMB of a clinical trial funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson.
The remaining authors have no conflicts of interests or relevant financial disclosures.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Fanning JP, Wesley AJ, Wong AA, Fraser JF. Emerging spectra of silent brain infarction. Stroke. 2014;45:3461–3471. doi: 10.1161/STROKEAHA.114.005919. [DOI] [PubMed] [Google Scholar]
- 2.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 2014;12:119. doi: 10.1186/s12916-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, Wright C, Beiser AS, Seshadri S, Pandya A, Kamel H. Silent brain infarction and risk of future stroke – a systematic review and meta-analysis. Stroke. 2016;47:719–725. doi: 10.1161/STROKEAHA.115.011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price TR, Manolio TA, Kronmal RA, Kittner SJ, Yue NC, Robbins J, Anton-Culver H, O’Leary DH. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. The Cardiovascular Health Study. Stroke. 1997;28:1158–1164. doi: 10.1161/01.str.28.6.1158. [DOI] [PubMed] [Google Scholar]
- 6.Arba F, Palumbo V, Boulanger JM, Pracucci G, Inzitari D, Buchan AM, Hill MD. Leukoaraiosis and lacunes are associated with poor clinical outcomes in ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke. 2016;11:62–67. doi: 10.1177/1747493015607517. [DOI] [PubMed] [Google Scholar]
- 7.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;34:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 8.Moghekar A, Kraut M, Elkins W, Troncoso J, Zonderman AB, Resnick SM, O’Brien RJ. Cerebral white matter disease is associated with Alzheimer pathology in a prospective cohort. Alzheimers Dement. 2012;8:S71–7. doi: 10.1016/j.jalz.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr, Weiner M, DeCarli C. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuller LH, Arnold AM, Longstreth WT, Jr, Manolio TA, O’Leary DH, Burke GL, Fried LP, Newman AB. White matter grade and ventricular volume on brain MRI as markers of longevity in the Cardiovascular Health Study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ., Jr White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 12.Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, Kasner SE, Seshadri S. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. doi: 10.1161/STR.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 13.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB. Prevalence and risk factors of silent brain infarcts in the population based Rotterdam Scan study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 14.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, Shibata DK, Knopman DS, Jack CR, Mosley TH., Jr Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernick C, Kuller L, Dulberg C, Longstreth WT, Jr, Manolio T, Beauchamp N, Price T. Silent MRI infarcts and the risk of future stroke: The Cardiovascular Health Study. Neurology. 2001;57:1222–1229. doi: 10.1212/wnl.57.7.1222. [DOI] [PubMed] [Google Scholar]
- 16.Obviagele B, Diener HC, Yusuf S, Martin RH, Cotton D, Vinisko R, Donna GA, Bath PM. Level of systolic blood pressure within the normal range and risk of recurrent stroke. JAMA. 2011;306:2137–2144. doi: 10.1001/jama.2011.1650. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. The Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 18.Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, Manson JE, Wassertheil-Smoller S. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women’s Health Initiative. Hypertension. 2012;60:625–630. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, Davis BR, Oparil S. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longstreth WT, Jr, Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr, O’Leary D, Carr J, Furberg CD. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 21.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 22.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: the Northern Manhattan Study. J Hypertens. 2015;33:2115–2122. doi: 10.1097/HJH.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–2636. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- 25.Turk M, Zaletel M, Pretnar-Oblak J. Ratio between carotid artery stiffness and blood flow – a new ultrasound index of ischemic leukoaraiosis. Clin Interv Aging. 2016;11:65–71. doi: 10.2147/CIA.S94163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus J, Gardener H, Rundek T, Elkind MS, Sacco RL, Decarli C, Wright CB. Baseline and longitudinal increases in diastolic blood pressure are associated with greater white matter hyperintensity volume: the northern Manhattan study. Stroke. 2011;42:2639–2641. doi: 10.1161/STROKEAHA.111.617571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Wang Y, Zhao X, et al. Factors associated with severity of leukoaraiosis in first-ever lacunar stroke and atherosclerotic ischemic stroke patients. J Stroke Cerebrovasc Dis. 2014;23:2862–2868. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, Kawanami T, Kato T. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community-based elderly japanese. Am J Hypertens. 2014;27:1257–1267. doi: 10.1093/ajh/hpu045. [DOI] [PubMed] [Google Scholar]
- 29.Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130:1407–1414. doi: 10.1161/CIRCULATIONAHA.114.011147. [DOI] [PubMed] [Google Scholar]
- 30.Floyd JS, Sitlani CM, Wiggins KL, Wallace E, Suchy-Dicey A, Abbasi SA, Carnethon MR, Siscovick DS, Sotoodehnia N, Heckbert SR, McKnight B, Rice KM, Psaty BM. Variation in resting heart rate over 4 years and the risks of myocardial infarction and death among older adults. Heart. 2015;101:132–138. doi: 10.1136/heartjnl-2014-306046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


