Abstract
Stroke is the leading cause of disability in the United States. Sex differences, including smaller infarcts in females and greater involvement of immune-mediated inflammation in males may affect the efficacy of immune-modulating interventions. To address these differences, we sought to identify distinct stroke-modifying mechanisms in female vs. male mice. The current study demonstrated smaller infarcts and increased levels of regulatory CD19+CD5+CD1dhi B10 cells as well as anti-inflammatory CD11b+CD206+ microglia/macrophages in the ipsilateral vs. contralateral hemisphere of female but not male mice undergoing 60 min middle cerebral artery occlusion followed by 96 h of reperfusion. Moreover, female mice with MCAO had increased total spleen cell numbers but lower B10 levels in spleens. These results elucidate differing sex-dependent regulatory mechanisms that account for diminished stroke severity in females and underscore the need to test immune-modulating therapies for stroke in both males and females.
Keywords: middle cerebral artery occlusion, sex-specific, lymphocyte, regulatory B cells, anti-inflammatory macrophages, regulatory T cells
1. Introduction
Stroke is the 5th leading cause of death and the leading cause of disability in the United States. An estimated 795,000 people will have a stroke each year resulting in nearly $33 billion each year in health care costs and missed work days [1]. It has become clear there are differences in stroke outcomes in men and women. Men have a significantly higher mortality from stroke compared to women in the age group of 45–74 years old. However, after age 85 the mortality from stroke significantly increases in women compared to men [2]. In addition to differences in stroke severity between the sexes it has also become apparent the immune system plays a pivotal role in increasing neural injury following stroke. Differences have also been observed in the immune responses in male and female rodents following experimental stroke induced by middle cerebral artery occlusion (MCAO).
One clear immunologic difference between male and female mice is that splenectomy two weeks prior to experimental stroke significantly decreases infarct volumes in male but not in female mice [3]. All prior studies used only male animals but consistently demonstrated significant neural protection for male animals that underwent splenectomy two weeks prior to permanent [4, 5] or transient ischemia [6] and intracerebral hemorrhage (ICH) [7] and even traumatic brain injury (TBI) [8]. Inclusion of female mice in the same study showed that splenectomy reduced the number of circulating activated T cells and monocytes/macrophages in male but not in female mice. There was also a significant decrease in circulating regulatory T cells (Treg) in female mice that underwent splenectomy but not in male mice [3]. Additionally, splenectomy in male mice decreased the frequency of activated microglia/macrophages to levels not significantly different from spleen-intact female mice [3, 9]. Splenectomy in male rodents decreased the levels of interferon gamma (IFNγ) in the brain after MCAO [5, 6].
One immune cell type that has consistently been shown to be important in reducing infarct size in males is B cells, particularly interleukin 10 (IL-10) producing B cells. Male B cell knock-out mice, μMT−/−, had increased infarct volumes compared to wild type male mice [10]. Treating μMT−/− male mice with IL-10 secreting B cells reduced infarct size [11] in contrast to IL-10−/− B cells that had no effect [12]. IL-10 secreting B cells (Breg) also reduced infarct size in wild type male mice [13]. While these studies were all done using only male mice several studies in experimental autoimmune encephalomyelitis (EAE) showed that IL-10 producing Breg cells were important, with estrogen treatment, in protecting female mice from disease progression. Regulatory B cells (Breg) are important immune modulators in neurological diseases like experimental autoimmune encephalomyelitis [14] through the secretion of IL-10. These cells provide protection that is partially mediated by estrogen. Female mice are protected from disease progression in EAE when treated with estrogen [15]. Female B cell knock-out mice, μMT−/−, are not protected from EAE progression even when treated with estrogen, suggesting B cells are important in protecting the CNS from EAE induced neural damage [16]. Restoring B cells in female μMT−/− mice with IL-10 producing B cells restored the protection from EAE with estrogen treatment to levels seen in wild type female mice [15]. B10 cells, a subset of Breg (CD19+CD5+CD1dhi) are known to secrete IL-10 and modulate the function of T cells and monocytes [17–19].
This study sets out to address gaps in the current knowledge base on how the female immune system responds to ischemic stroke and how this response differs from the male immune response. The immune response to stroke has been documented in male animals with few studies comparing male and female animals. Females have smaller infarcts than males and this protection is thought to be largely mediated by sex hormones, but some of the protection seen in females could be due to an altered immune response to stroke. Some previous work has shown immune modulating therapies that are protective in male animals that are not protective in female animals. This study looks at the role of regulatory lymphocyte populations in the spleen and brain in males and females after experimental ischemic stroke, and how these regulatory populations could be modulating other immune cell functions in the spleen and brain after stroke.
2. Materials and Methods
2.1 Ethics Statement
The study was conducted in accordance with National Institutes of Health guidelines for the use of experimental animals, and the protocols were approved by the Portland Veteran Affairs Medical Center Institutional Animal Care and Use Committee, protocol #2840–12, and the Oregon Health and Science University Animal Care and Use Committee, protocol #IS00003885.
2.2 Animals
Male and female C57BL/6J mice, age 8–10 weeks, were purchased from The Jackson Laboratory (Sacramento, CA). Mice were given food and water ad libitum and kept on a 12 h light/dark cycle in climate controlled housing. Animals were cared for according to institutional guidelines in the animal resource facility at the Oregon Health and Science University, Portland, OR.
2.3 Middle Cerebral Artery Occlusion Model
Transient focal ischemia was induced in male and female mice for 60 min by reversible middle cerebral artery occlusion (MCAO) under isoflurane anesthesia followed by 96 h of reperfusion, as previously described [3, 13], Briefly, mice were anesthetized with isoflurane, 5% induction and 2% maintenance, then the right common carotid artery (CCA) and external carotid artery (ECA) were exposed and the ECA was ligated. A 6–0 nylon monofilament (ETHICON, Inc., Somerville, NJ) was advanced into the ECA and up the internal carotid artery (ICA) to the base of the middle cerebral artery (MCA) occluding blood flow to the MCA territory. The filament was left in place for 60 min and then withdrawn to allow reperfusion of the MCA. After reperfusion the mice were survived for 96 h. To ensure adequate drops in blood flow during occlusion and increases in flow during reperfusion, cerebral blood flow was monitored by Laser Doppler Flowmetry (LDF) (Model DRT4, Moor Instruments Ltd., Wilmington, DE) with a probe fixed to the skull during the surgery. Mice were excluded from the study if the mean intra-ischemic LDF was greater than 30% of the pre-ischemic baseline LDF. No mice were excluded from the study. During the surgery mouse body and head temperature was maintained at 37±1.0°C with a warm water blanket and a heating lamp.
2.4 Infarct Volume Quantification
Brains were harvested for infarct volume quantification after 96 h of reperfusion. Brains were sectioned into five 2 mm thick coronal sections and stained with 1.2% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma, St. Louis, MO) for 15 min at 37°C, as described previously (Hurn 2007). The sections were then fixed in 10% formalin for 24 h. Infarct was measured using digital imaging and image analysis software (Systat, Inc., Point Richmond, CA). Infarct volume was calculated for the cortex, striatum, and hemisphere by subtracting the non-infarcted regional volume of the ipsilateral side from the contralateral regional volume. This value was divided by the contralateral regional volume and multiplied by 100 to obtain a regional infarct volume as a percentage of the contralateral region to control for edema.
2.5 Leukocyte Isolation from Brain and Spleen
Spleens were processed into single cell suspensions by passing the spleen through a 100μm nylon mesh (BD Falcon, Bedford, MA) into RPMI 1640. Red blood cells were lysed with 1x red cell lysis buffer (eBioscience, Inc., San Diego, CA). After being washed with RPMI 1640 the cells were counted on a Cellometer Auto T4 cell counter (Nexcelom, Lawrence, MA). Cells were centrifuged and resuspended in staining buffer (PBS with 0.1% NaN3 and 1% bovine serum albumin) for staining. Brains were split into right (ipsilateral) and left (contralateral) hemispheres and placed in a solution of 1mg/ml of Type IV collagenase (Sigma Aldrich, St. Louis, MO) and 50mg/ml of DNase I (Roche Diagnostics, Indianapolis, IN) for 1h at 37°C with intermittent shaking. Cells were then washed in RPMI 1640 and centrifuged. Cells were resuspended in 80% Percoll, overlaid with 40% Percoll then centrifuged at 1600 rpm for 30 min. Cells were removed from the 80/40 interface and resuspended in RPMI 1640 to remove any remaining Percoll. Cells were resuspended in staining buffer for staining and counted on a hemocytometer.
2.6 Flow Cytometry Analysis
For flow cytometric analysis, cells were placed in staining buffer at a concentration of 1×106 cells/ml for splenocytes and 2×105 cell/ml for brain and blood cells. Cells were blocked with rat anti-mouse CD16/CD32 Mouse BD Fc Block™ (BD Bioscience, San Jose, CA) and then incubated, protected from light, with various combinations of fluorescently tagged antibodies. To assess cell survival, 7-aminoactinomycin D (7AAD) viability dye was used in some samples. For intracellular and FoxP3 staining, the samples were fixed with 4% paraformaldehyde and washed. FoxP3 staining was done using the fixation/permeabilization reagents per the manufacturer’s instructions (eBioscience). For intracellular staining, cells were resuspended in 1× permeabilization buffer (BD Bioscience) and incubated with antibodies or isotype controls. The samples were then run on a BD AccuriTM C6 (BD Bioscience) under a four color (FITC, PE, PerCP/PECy5.5, and APC) fluorescence flow cytometry analysis.
The following antibodies were used: CD4 (GK1.5), PDL1 (M1H5), CD11b (M1/70), CD19 (1D3), CD1d (1B1), CD23 (B3B4), TIM1 (RMT1–4), CD138 (281–2), CD25 (PC61), CD86 (GL1), CD21 (7G6), IA/IE (2G9), TNFα (18139A), NOS2 (CXNFT), CD206 (CO68C2) (BD Biosciences), CD44 (1M7), FoxP3 (FJK-16s), IRF4 (3E4), IFNγ (XMG1.2) (eBiosciece), CD9 (MZ3), CD5 (53–7.3) (Biolegend), and PE-ARG1 (R&D Systems, Minneapolis, MN).
2.7 Statistics
All analyses were performed blinded. Data were analyzed using Prism (GraphPad Software, La Jolla, CA) using the Student’s t-test with a value of p≤0.05 considered statistically significant. All data are represented as means ± standard error of the mean (SEM).
3. Results
3.1 Infarct volume and splenocyte counts are lower in female mice
Previous studies have demonstrated that young female rodents have smaller infarcts than their age matched males [20]. Consistent with previously published work, female mice (n=7) had significantly smaller infarct volumes in than male mice (n=6) at 96 h after 60 min MCAO (Fig. 1A, p<0.05). Additionally, female mice had significantly more splenocytes than male mice at 96 h post MCAO (Fig. 1B, p<0.05).
Fig. 1. Females have decreased infarct and increased splenocyte counts following MCAO.
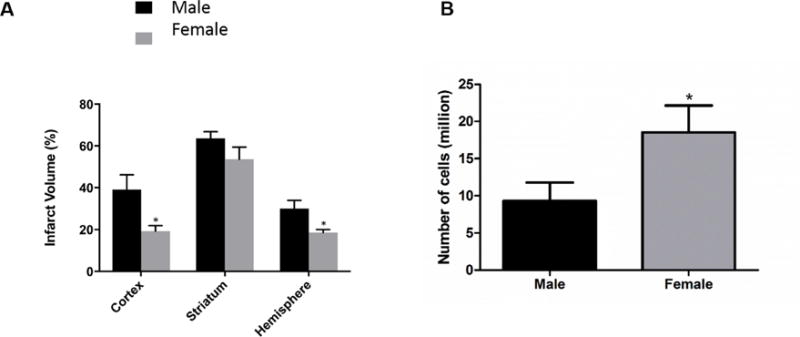
Male and female mice underwent 60 min of transient MCAO followed by 96 h of reperfusion. A) Female mice (n=7) have a significantly decreased infarct volume at 96h compared to male mice (n=6) p<0.05. B) Females have a significantly increased number of splenocytes compared to males 96 h after stroke p<0.05. * denotes p<0.05.
3.2 Regulatory B cells and anti-inflammatory microglia/macrophages are increased after stroke in brain
IL-10 secreting B cells decrease infarct volume in B cell knock-out mice and wild type male mice [11, 13]. Therefore the presence of B10 cells was evaluated in male and female mice following MCAO. Female mice (n=7) had a significantly higher frequency of B10, CD19+CD5+CD1dhi, cells in the ipsilateral hemisphere compared to the contralateral hemisphere (Fig. 2B, p<0.001). In contrast, in male mice (n=4) there was no difference in the frequency of B10, CD19+CD5+CD1dhi, cells in the ipsilateral hemisphere compared to the contralateral hemisphere (Fig. 2A). IL-10 producing B cells and anti-inflammatory microglia/macrophages can have a positive feedback loop that promotes increases in both cell types [21]. Therefore the anti-inflammatory marker CD206 on CD11b cells was investigated in the brain after MCAO. Female mice had an increased frequency of anti-inflammatory microglia/macrophages, CD11b+CD206+, in the ipsilateral hemisphere compared to the contralateral hemisphere 96 h post MCAO (Fig. 3B, p<0.0001). However, there was no difference between the ipsilateral and contralateral hemisphere in the frequency of CD11b+CD206+ cells in male mice (Fig. 3A). However, there was a significant decrease in the frequency of CD11b+Arg-1+ cells, another marker associated with anti-inflammatory microglia/macrophages, in the ipsilateral hemisphere of both male (Fig. 3C, p<0.05) and female (Fig. 3D, p<0.001) mice compared to the contralateral hemisphere.
Fig. 2. Increased regulatory B cells in the ispilateral hemisphere of female mice.
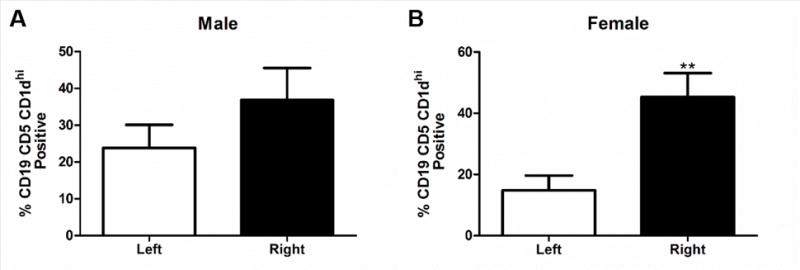
A) B10, CD19+ CD5+ CD1dhi, cells in the brain of males 96 h after MCAO (n=4). B) Significant increase in the frequency of B10, CD19+ CD5+ CD1dhi, cells in the ipsilateral hemisphere of the brain of females 96 h after MCAO p>0.001 (n=7). ** denotes significance of p<0.001.
Fig. 3. Increased frequency of anti-inflammatory microglia/macrophages in the ispilateral hemisphere of female mice.
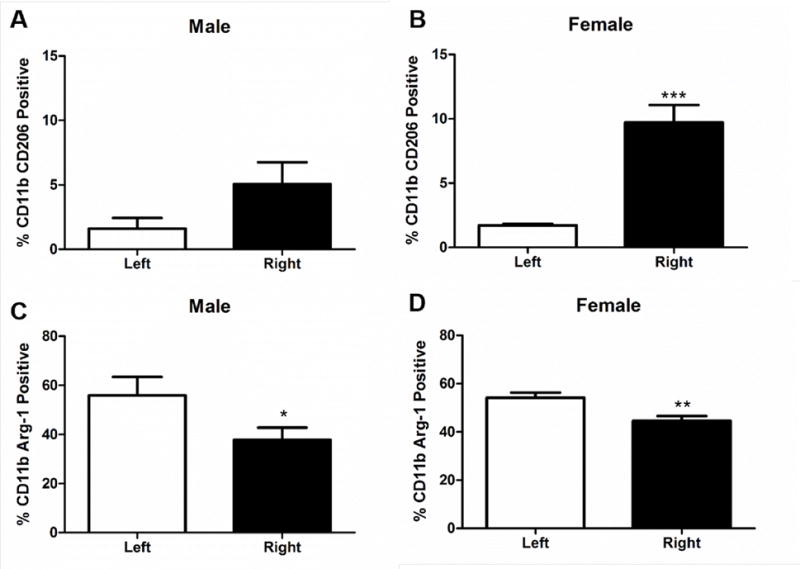
A) CD11b+ CD206+ cells in the brain of males 96h after MCAO (n=4). B) Significant increase in the frequency of CD11b+ CD206+ cells in the brain of females 96 h after MCAO p>0.0001 (n=7). C) Significant decrease in CD11b+ Arg-1+ frequency in the brain of males 96 h after MCAO p<0.05. D) Significant decrease in CD11b+ Arg-1+ decreased frequency in the brain of females 96 h after MCAO p<0.001. * denotes significance of p<0.05. ** denotes significance of p<0.001. *** denotes significance of p<0.0001.
3.3 IFNγ expression and anti-inflammatory macrophages are decreased in the spleens of female but not male mice after stroke
Anti-inflammatory macrophages, CD11b+CD206+, in the spleens of female mice (n=13) were significantly decreased compared to male spleens (n=8) at 96 h following MCAO (Fig. 4A, p<0.0001). The intensity of IFNγ staining on all cells in the spleens was significantly decreased in female mice compared to male mice after MCAO (Fig. 4D, p<0.001). When different cell populations were examined, both macrophages and B cells, CD11b+ and CD19+, had significantly lower intensity of IFNγ staining in female spleens compared to male spleens after MCAO (Fig. 4B, p<0.001 and Fig. 4C, p<0.001), respectively.
Fig. 4. Decreased levels of anti-inflammatory macrophages and IFNγ in the spleen in female mice.
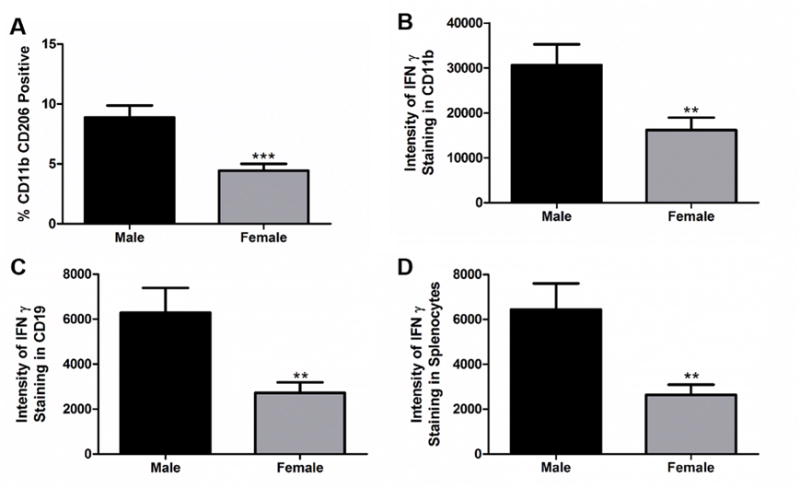
A) CD11b+ CD206+ cells are significantly decreased in females in the spleen 96 h after MCAO p<0.0001 (n=13). B) Female mice have a decrease in the intensity of CD11b+ IFNγ+ cells in the spleen 96 h after MCAO p>0.001. C) Female mice have a decrease in CD19+ IFNγ+ intensity in spleen 96 h after MCAO p<0.001. D) Female mice have a decrease in the overall intensity of IFNγ+ staining in spleen after MCAO p<0.001 compared to males (n=8). ** denotes significance of p<0.001. *** denotes significance of p<0.0001.
3.4 Breg are decreased, while B cells and Treg are increased in the spleens of female mice following MCAO
The number of B cells in the spleens of mice 96 h after MCAO is known to decrease significantly compared to naïve mice [11, 22]. Therefore splenic B cells were examined in both male (n=8) and female (n=13) mice after 96 h following 60 min MCAO. The spleens of female mice had a significantly higher frequency of CD19+ cells than male mice 96 h following MCAO (Fig. 5A, p<0.05). However, female mice had a lower frequency of B10, CD19+CD5+CD1dhi, cells (Fig. 5B, p<0.05) as well as plasmablasts, CD19+CD138+CD44hi, in the spleen (Fig. 5C, p<0.05) compared to male mice 96 h after MCAO. Unlike Bregs, the frequency of CD4+ Tregs CD4+CD25+ cells (Fig. 6A, p<0.0001) and CD4+CD25+FoxP3+ cells (Fig. 6B, p<0.001), were increased in female spleens compared to male spleens 96 h post MCAO.
Fig. 5. Female mice have an increase in B cells but a decrease in Breg cells.
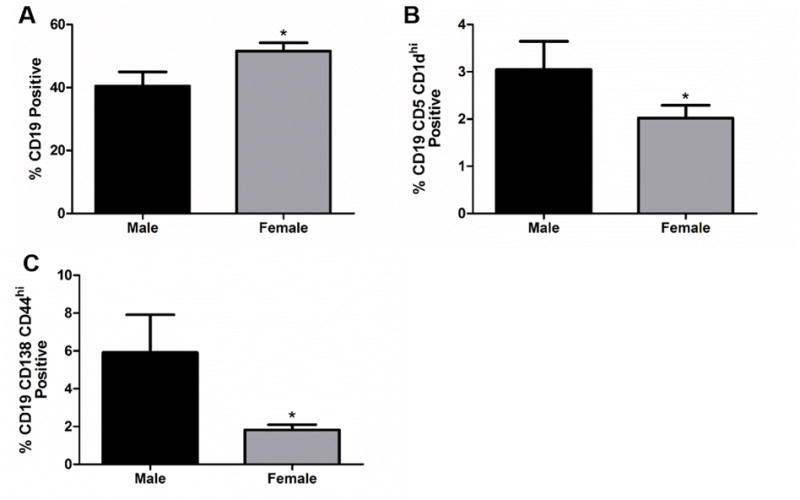
A) The frequency of CD19+ cells in spleen 96 h after MCAO is significantly increased in females (n=13) after MCAO p<0.05. B) Decrease in the frequency of CD19+ CD5+ CD1dhi, B10, cells in the spleen of females 96 h after MCAO p>0.05. C) Decrease in the frequency of CD19+ CD138+ CD44hi, plasmablasts, in the spleen of females 96 h after MCAO, p<0.05 compared to males (n=8). * denotes significance of p<0.05.
Fig. 6. Regulatory T cells are increased in the spleens of female mice.
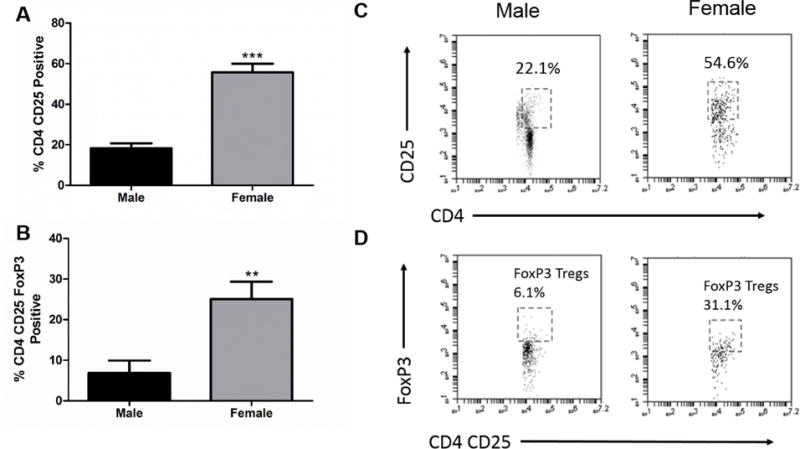
A) CD4+ CD25+ cells in spleen are increased in females 96 h after MCAO p<0.0001. B) Increase in the frequency of CD4+ CD25+ FoxP3+, Treg, cells in the spleen of females 96 h after MCAO p>0.001. C) Representative dot plot of CD4+ CD25+ staining in spleens of males and females. D) Representative dot plot of CD4+CD25+ FoxP3+ staining in spleens of males (n=8) and females (n=13). ** denotes significance of p<0.001. *** denotes significance of p<0.0001.
4. Discussion
There are clear differences in stroke outcomes in males and females. At younger ages, 45–74 years old, women have smaller infarcts than age matched men but as women age, over 85 years old, they lose the protection from stroke seen at younger ages [2]. These differences observed in women are thought to be linked to sex hormones. While the exact roles sex hormones play in providing protection to women is unknown, they are likely to be involved in multiple aspects of how the body responds to stroke. In female mice there is also a decrease in infarct compared to males [3, 9], which was again demonstrated in this study. One area that has been widely studied in male rodent models of stroke is the immune response to stroke, particularly the splenic immune response [5, 22, 23]. In males there is a significant decrease in splenocyte counts after MCAO but females have significantly more splenocytes than males following MCAO. Different ways to modulate the immune system following experimental stroke have been developed in male rodent models. One of the more robust protective effects seen in modulating the immune system after a stroke to decrease neural injury is splenectomy two weeks prior to stroke. Thus, splenectomy prior to permanent [4] or transient ischemia [6], or ICH [7] decreases neural injury. This protection is provided in part by a decrease in inflammatory cells released into the circulation [3, 9] with corresponding decreases in IFNγ [5]. When female mice underwent splenectomy prior to transient ischemia there was not a significant decrease in infarct volume or changes in circulating inflammatory cells seen in male mice. Moreover, regulatory T cells were decreased in the circulation of female mice that underwent splenectomy compared to spleen-intact females. This study demonstrates how a successful approach to change the immune response to stroke in males failed to work in females. The decrease in Treg cells in the circulation could be one reason why splenectomy failed to work in females, as there was a significant increase in Treg cells in the spleen after MCAO compared to males. IFNγ is another component of the immune response that is altered by splenectomy in a positive way in males [5, 6]. Splenectomy is also likely to not affect this response in females as female mice were found to have lower intensities of IFNγ in the spleen compared to male mice after MCAO.
B cells are known to play an important protective role in male mice after MCAO [10] particularly IL-10 producing regulatory B cells [11–13]. Female mice have an increase in total B cells but have a significant decrease in Breg cells, both B10 and plasmablasts, in the spleen compared to male mice. This decrease of Breg cells in the spleen could be explained by their migration to the injured brain that corresponds to the increase in B10 Breg cells seen in the ipsilateral hemisphere in female mice but not male mice, as other splenocyte cell types leave the spleen after MCAO [23]. B10 cells are known to produce IL-10 and create an anti-inflammatory environment [17–19]. These data are consistent with the protective role Breg cells play in EAE in female mice. This protective response in EAE is regulated by estrogen [15]. The presence of B10 cells in the brain could contribute to the increase in anti-inflammatory microglia/macrophages seen in the ipsilateral hemisphere of female mice and not in male mice. As estrogen has been shown to influence Breg cells that positively interact with anti-inflammatory microglia/macrophages to synergistically increase both cell populations [21], another possible explanation for the increase in these anti-inflammatory microglia/macrophages is a decrease in the frequency of CD206 positive cells in the spleens of female mice. Previous studies have shown the spleen releases leukocytes into the circulation that then travel to the injured brain following MCAO [23]. It is likely these anti-inflammatory microglia/macrophages are of a reparative phenotype due to the increased frequency of CD206 and the decreased frequency of Arg-1.
Additionally, Breg cells can interact with T cells to increase Treg cells through the production of IL-10 and transforming growth factor beta (TGFβ) [24]. It is currently unknown when or if Breg cells become elevated in the spleen following stroke. If these cells are present early after a stroke within the spleen this could affect T cells, creating more Tregs, and also creating more anti-inflammatory macrophages. It could also be that Treg cells leave the spleen at a time point after 96 h or that they stay in the spleen to direct immune responses from the spleen. One limitation of this study is looking at just one time point after MCAO. However, the differences seen at just one time point demonstrate how different the immune responses to ischemic stroke are in males and females.
5. Conclusion
Female mice have smaller infarcts than male mice after ischemic stroke and this is due in part to the vastly different immune responses generated by each sex to stroke. Female mice have an overall anti-inflammatory regulatory immune response to stroke compared to male mice that have a more inflammatory immune response to the injured brain following stroke. These findings suggest immune modulating therapies for stroke should be tested in males and females as treatments that work in males might not work in females. This could lead to therapeutic failure at the clinical level and in some cases stroke therapies might need to be tailored to each sex to account for the immune differences.
Highlights.
Female mice with MCAO had smaller infarcts than males
In brain, MCAO females had more Bregs and anti-inflammatory macrophages than males
In spleen, females had higher total cell numbers but fewer B10 Bregs than males
Increased immunosuppressive activity in brain accounts for milder stroke in females
Sex-dependent differences in immune regulation affect stroke treatments
Acknowledgments
Funding:
This work was supported by NIH/National Institute of Neurological Disorders and Stroke 1RO1 NS075887 (H.O.) and 1RO1 NS0076013 (H.O., J.S.) and the American Heart Association 17GRNT33220001 (H.O). This material is the result of work supported, in part, with resources and the use of facilities at the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors have a conflict of interest.
References
- 1.M. Writing Group. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, C. American Heart Association Statistics, S. Stroke Statistics Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dotson AL, Wang J, Saugstad J, Murphy SJ, Offner H. Splenectomy reduces infarct volume and neuroinflammation in male but not female mice in experimental stroke. J Neuroimmunol. 2015;278:289–298. doi: 10.1016/j.jneuroim.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajmo CT, Jr, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, Willing AE, Pennypacker KR. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012;27:131–141. doi: 10.1007/s11011-012-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin R, Zhu X, Liu L, Nanda A, Granger DN, Li G. Simvastatin Attenuates Stroke-induced Splenic Atrophy and Lung Susceptibility to Spontaneous Bacterial Infection in Mice. Stroke. 2013;44:1135–1143. doi: 10.1161/STROKEAHA.111.000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, Hong NH, Kim JH, Ban JJ, Park HK, Kim SU, Park CG, Lee SK, Kim M, Roh JK. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 8.Das M, Leonardo CC, Rangooni S, Mohapatra SS, Mohapatra S, Pennypacker KR. Lateral fluid percussion injury of the brain induces CCL20 inflammatory chemokine expression in rats. J Neuroinflammation. 2011;8:148. doi: 10.1186/1742-2094-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee A, Wang J, Bodhankar S, Vandenbark AA, Murphy SJ, Offner H. Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Transl Stroke Res. 2013;4:554–563. doi: 10.1007/s12975-013-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:8556–8563. doi: 10.1523/JNEUROSCI.1623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. IL-10-producing B-cells limit CNS inflammation and infarct volume in experimental stroke. Metab Brain Dis. 2013;28:375–386. doi: 10.1007/s11011-013-9413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Offner H, Hurn PD. A novel hypothesis: regulatory B lymphocytes shape outcome from experimental stroke. Translational Stroke Research. 2012;3 doi: 10.1007/s12975-012-0187-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metab Brain Dis. 2014;29:59–73. doi: 10.1007/s11011-013-9474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedek G, Zhang J, Bodhankar S, Nguyen H, Kent G, Jordan K, Manning D, Vandenbark AA, Offner H. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. J Neuroimmunol. 2016;293:45–53. doi: 10.1016/j.jneuroim.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Lapato A, Bodhankar S, Vandenbark AA, Offner H. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metab Brain Dis. 2015;30:1117–1127. doi: 10.1007/s11011-015-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodhankar S, Wang C, Vandenbark AA, Offner H. Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol. 2011;41:1165–1175. doi: 10.1002/eji.201040992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, Heise MT, Tedder TF. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol. 2013;190:1158–1168. doi: 10.4049/jimmunol.1201427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Dotson AL, Offner H. Sex differences in the immune response to experimental stroke: Implications for translational research. J Neurosci Res. 2017;95:437–446. doi: 10.1002/jnr.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedek G, Zhang J, Nguyen H, Kent G, Seifert H, Vandenbark AA, Offner H. Novel feedback loop between M2 macrophages/microglia and regulatory B cells in estrogen-protected EAE mice. J Neuroimmunol. 2017;305:59–67. doi: 10.1016/j.jneuroim.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. Journal of immunology. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 23.Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacology. 2012;7:1017–1024. doi: 10.1007/s11481-012-9406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–132. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]


