Abstract
Background
While a recent meta-analysis of observational studies reported a statistically significant association between sarcopenia and both all-cause mortality and functional decline, a recently developed inverse heterogeneity (IVhet) model has been shown to be more valid than the traditional random-effects model used.
Objective
The objective of this short report was to use a previous meta-analysis to compare the two approaches.
Methods
Aggregate data meta-analysis of prospective observational studies conducted in any setting. Men and women 60 years of age and older in which all-cause mortality (12 studies, 14,169 participants) or functional decline (6 studies, 8,561 participants) were assessed. Using the IVhet model, pooling of previous studies regarding the association between sarcopenia and all-cause mortality as well as functional decline. Absolute and relative differences between IVhet and random-effects results were also calculated as well as influence analysis with each study deleted once. Non-overlapping 95% confidence intervals (CI) for odds ratios (OR) were considered statistically significant.
Results
Sarcopenia was associated with an increased risk for all-cause mortality (OR = 3.64, 95% CI = 2.94 to 4.51) and functional decline (OR = 2.58, 95% CI = 1.33 to 4.99). Compared to the random-effects model, the OR was slightly higher (0.04 or 1.1%) but with wider CI (0.16 or 11.3%) for all-cause mortality and 0.45 (14.9%) lower with a CI that was 0.34 (10.2%) wider for functional decline. With each study deleted from the model once, results remained statistically significant for both all-cause mortality and functional decline.
Conclusion
These results provide additional and more accurate evidence in support of an association between sarcopenia and an increased risk for both all-cause mortality and functional decline.
Keywords: sarcopenia, mortality, aging, meta-analysis, IVhet model
INTRODUCTION
Sarcopenia is a major public health problem worldwide. Depending on the definitions applied, the prevalence of sarcopenia has been reported to range between 5% and 13% in adults 60 to 70 years of age and 11% and 50% for those aged 80 and older [1]. In terms of costs, the direct healthcare costs attributed to sarcopenia in the United States in 2000 were estimated at $18.5 billion, $10.8 billion in men and $7.7 billion in women [2]. This was estimated to account for approximately 1.5% of the total healthcare expenditures for that year [2]. Given the aging population worldwide [3], the absolute number of people with sarcopenia is also expected to increase.
Recently, Beaudart et al. [4] conducted a systematic review with meta-analysis on the association between sarcopenia and all-cause mortality as well as functional decline in adults 60 years of age and older. Overall, sarcopenia was reported to be associated with an increased risk, i.e., odds ratio (OR), for both all-cause mortality (OR = 3.60, 95% CI = 2.96 to 4.37) and functional decline (OR = 3.03, 95% CI = 1.80 to 5.12) [4]. While these findings are noteworthy, they were derived using a conventional random-effects model [5]. However, a recently developed and alternative approach, the inverse heterogeneity (IVhet) model [6], has been shown to outperform the traditional random-effects model [4]. This includes greater preservation of coverage probabilities as well as a lower observed variance [6].
Given the current and projected prevalence of sarcopenia and its potential deleterious consequences on both all-cause mortality and functional decline as well as the need to provide accurate overall estimates regarding the magnitude and dispersion of these associations, the purpose of this short report was to use the IVhet model [6] to examine the association between sarcopenia and all-cause mortality, as well as functional decline, and compare these findings with previous meta-analytic results based on the random-effects model [4].
METHODS
Data Source
Data for this short report were derived from a recently published systematic review with meta-analysis on the association between sarcopenia and all-cause mortality as well as functional decline, details of which have been described elsewhere [4]. Briefly, studies were limited to those that defined sarcopenia according to the European Working Group on Sarcopenia in Older People [7]. Twelve prospective studies that included 14,169 participants in which all-cause mortality was assessed and 6 studies representing 8,561 participants in which functional decline was assessed, were pooled. All participants were 60 years of age and older, with the percentage of females ranging from 0% to 60% for all-cause mortality (x̄ ± SD = 52.6 ± 17.6, median = 56) and 0% to 63% for functional decline (x̄ ± SD = 48.4 ± 24.0, median = 56). Follow-up time, in years, ranged from 0.25 to 10 for all-cause mortality (x̄ ± SD = 4.1 ± 3.5, median = 4) and 0.25 to 10 for functional decline (x̄ ± SD = 4.1 ± 3.2, median = 4). The prevalence of sarcopenia for all-cause mortality studies ranged from 4.3% to 73.3% (x̄ ± SD = 26.5 ± 20.4, median = 22) and 4.3% to 58.0% for functional decline (x̄ ± SD = 17.4 ± 20.1, median = 10).
Data Synthesis
Effect size calculations
The effect sizes pooled for the current study were extracted from previously reported OR results for sarcopenia and all-cause mortality as well as functional decline [4].
Effect size pooling
The recently developed IVhet model [6] was used to pool OR results with respect to the presence of sarcopenia and resultant all-cause mortality and functional decline. This quasi-likelihood model is produced by calculating weights that sum to 1 from each study, pooling the effects from all studies, and then calculating the variance of the pooled effect sizes. This is accomplished as follows:
Where, wj above are weights that sum to 1, and vj is the variance, followed by
Where θ̂IVhet above is the estimated pooled effect for changes in all-cause mortality and functional decline, followed by
Where var(θ̂IVhet) above is the estimated variance of pooled effects for all-cause mortality or functional decline and τ2 is the between-study variance.
The IVhet model has been shown to be more valid then the original random-effects, method-of-moments model of Dersimonian and Laird [5], the most commonly used model for pooling aggregate data meta-analytic results [8]. Specifically, simulation studies have demonstrated that the IVhet model maintains correct coverage probabilities and a lower observed variance than the random-effects model, irrespective of heterogeneity [6].
The pooled results for all-cause mortality and functional decline derived from the IVhet model were then compared to those previously calculated using the original random-effects method-of-moments model of Dersimonian and Laird [4]. In addition, Q and I2 statistics for heterogeneity and inconsistency were calculated as well as influence analysis with each study deleted from the model once. For I2, inconsistency was considered to be very low (<25%), low (25% to <50%), moderate (50% to <75%) or large (≥ 75%) [9]. All analyses were conducted using the log transformation and then back transformed to OR for presentation purposes. Analyses were performed using Meta XL, version 5.3 (EpiGear International, Queensland, Australia).
RESULTS
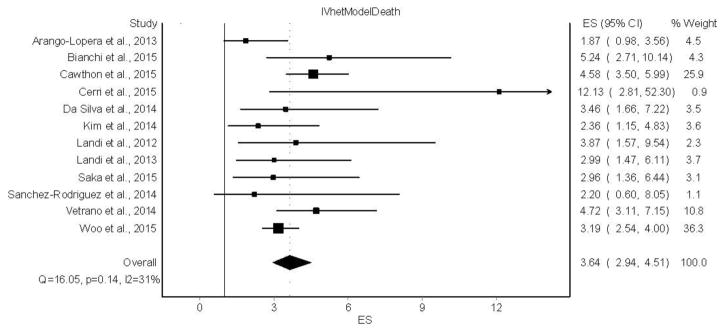
A forest plot of the association between sarcopenia and all-cause mortality is shown in Figure 1. Overall, sarcopenia was associated with an increased risk for all-cause mortality (OR =3.64, 95% CI = 2.94 to 4.51). No statistically significant heterogeneity was observed (Q = 16.1, p = 0.14) and low inconsistency based on the mean for I2 was found (I2 = 31.5%, 95% CI = 0% to 65.4%). The IVhet model yielded an OR that was slightly higher (0.04 or 1.1%) and a 95% confidence interval that was wider (0.16 or 11.3%) than previously reported results using a random-effects model [4]. With each study deleted from the model once, IVhet results remained statistically significant across all deletions, ranging from an OR of 3.36 (95% CI, 2.70 to 4.18) to 3.93 (95% CI, 3.06 to 5.04).
Figure 1.
Forest plot for the association between sarcopenia and all-cause mortality using the IVhet model. The black squares represent the odds ratios (OR) while the left and right extremes of the squares represent the corresponding 95% confidence intervals for the OR. The middle of the black diamond represents the OR while the right and left extremes of the diamond represent the corresponding 95% confidence intervals. References for individual studies listed available from Beaudart et al. [4].
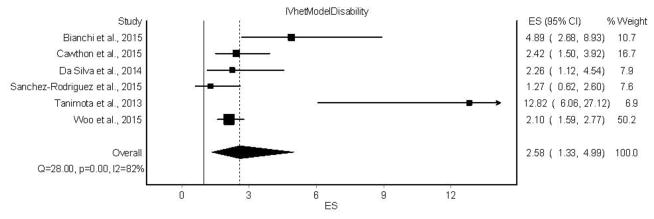
A forest plot of the results for the association between sarcopenia and functional decline is shown in Figure 2.
Figure 2.
Forest plot for the association between sarcopenia and functional decline using the IVhet model. The black squares represent the odds ratios (OR) while the left and right extremes of the squares represent the corresponding 95% confidence intervals for the OR. The middle of the black diamond represents the OR while the right and left extremes of the diamond represent the corresponding 95% confidence intervals. References for individual studies listed available from Beaudart et al. [4].
Overall, sarcopenia was associated with an increased risk for functional decline (OR = 2.58, 95% CI = 1.33 to 4.99). Statistically significant heterogeneity was observed (Q = 28.0, p <0.001) and large inconsistency was found (I2 = 82.1%, 95% CI = 62.1% to 91.6%). IVhet model results yielded an OR that was 0.45 (14.9%) lower and a 95% confidence interval that was 0.34 (10.2%) wider than the random-effects model [4]. With each study deleted from the model once, IVhet results remained statistically significant across all deletions, ranging from an OR of 2.29 (95% CI = 1.54 to 3.40) to 3.18 (95% CI = 1.54 to 6.56).
DISCUSSION
This short report provides additional and more accurate information regarding the increased risk for all-cause mortality and functional decline in adults with sarcopenia, an increasing problem worldwide [1]. These findings are probably important for at least two reasons. First, the current results using the IVhet model provide more accurate information than the random-effects model, yielding wider confidence intervals for both all-cause mortality (11.3%) and functional decline (10.2%) as well a smaller odds ratio for functional decline (14.9%) given the large amount of heterogeneity observed. This is important for determining the true magnitude of the association between sarcopenia, all-cause mortality and functional decline. However, these findings are not surprising given the tendency for overly liberal findings when the random-effects versus IVhet model is used, especially when substantial heterogeneity is present [6]. More specifically, as heterogeneity increases, there is greater dispersion between the IVhet and random-effects model [6]. Thus, while there is little difference between the IVhet and random-effects results for mortality given the small amount of heterogeneity, there is an alternatively greater divergence in results for disability between the two models because of the large amount of heterogeneity [6]. As random effects weights become more equal with greater heterogeneity, the findings reduce to the arithmetic mean and thus, less precise estimates. For example, for the current disability results, the random effects weights differed by only 5.3% while the IVhet weights differed by 43.3%.
Second, the current findings suggest that caution may be warranted when interpreting any meta-analysis when a random-effects model is used to examine the association between sarcopenia and selected variables. This is important because meta-analyses are often considered to be at the hierarchy of the evidence-based pyramid, and thus, are often used to make decisions about the effects of a condition such as sarcopenia on outcomes such as all-cause mortality and functional decline.
While the results of this study are important, they should be viewed with respect to the following potential limitations. First, since the current findings were based on aggregate data, there is the potential for ecological fallacy. Second, there was significant statistical heterogeneity and inconsistency for functional decline, regardless of the model used. As a result, and while beyond the scope of this brief report, there may be some subgroups in which the association between sarcopenia and functional decline, as well as all-cause mortality, may be different [4]. However, because studies are not randomly assigned to subgroups in meta-analysis, they are considered to be observational in nature. Therefore, the results of any subgroup analyses conducted in an aggregate data meta-analysis do not support causal inferences [10]. In addition, multiple subgroup analyses increase the risk for chance findings and are often limited to simple meta-regression because of missing data for different variables from different studies. In conclusion, the results of this short report provide additional and more accurate evidence in support of an association between sarcopenia and an increased risk for both all-cause mortality and functional decline. Future aggregate data meta-analyses should consider using the IVhet versus traditional random-effects model.
Acknowledgments
Conflicts of Interest
The authors have no conflicts of interest to declare.
Author Contributions
GAK was responsible for the conception and design, acquisition of data, analysis and interpretation of data, drafting the initial manuscript and revising it critically for important intellectual content. KSK was responsible for the conception and design, acquisition of data, and reviewing all drafts of the manuscript.
Sponsor’s Role
This study was supported by the National Institute of General Medical Sciences of the National Institutes of Health (grant number U54 GM104942). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 3.United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing. 2015. 2015 [Google Scholar]
- 4.Beaudart C, Zaaria M, Pasleau FEO, Reginster JY, Bruyere O. Health outcomes of sarcopenia: A systematic review and meta-analysis. PLoS One. 2017:12. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 6.Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials. 2015;45:130–138. doi: 10.1016/j.cct.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dersimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Littell JH, Corcoran J, Pillai V. Systematic reviews and meta-analysis. New York: Oxford University Press; 2008. [Google Scholar]