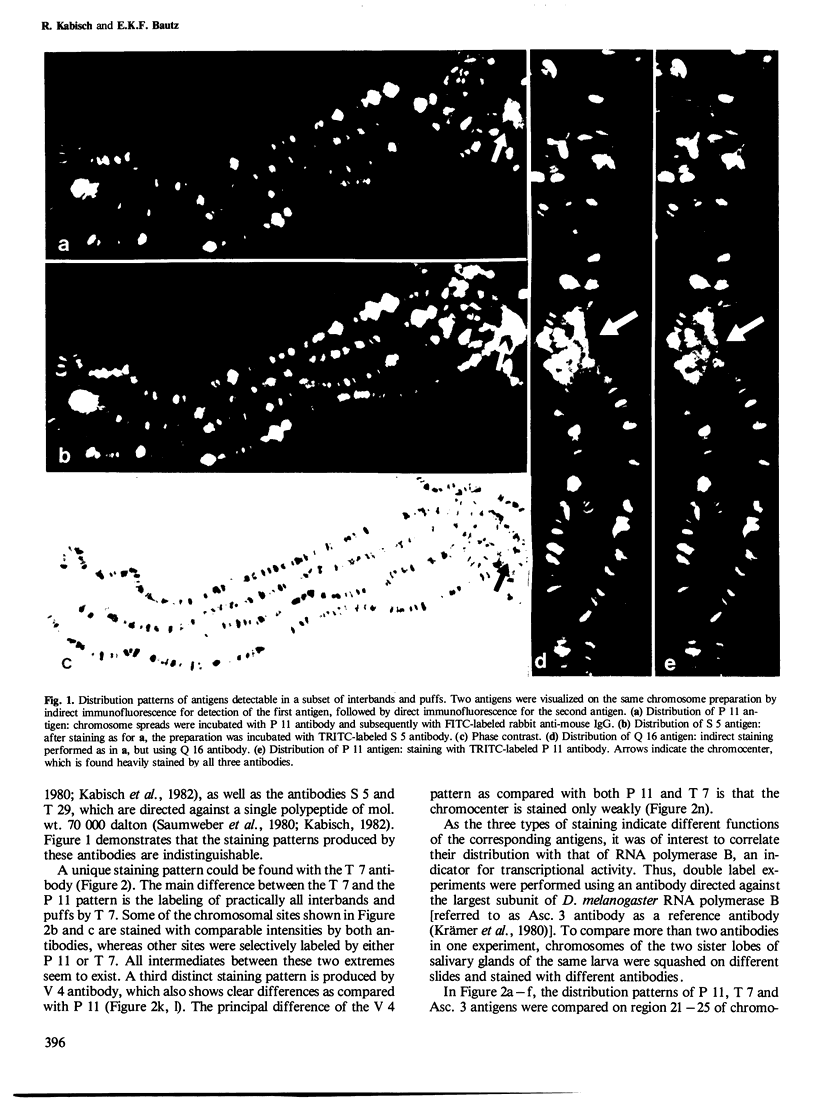
Abstract
The distribution patterns of nonhistone chromosomal proteins (NHCP) associated with pulse-labeled RNA were determined by indirect immunofluorescence on salivary gland chromosomes of Drosophila melanogaster using monoclonal antibodies. By staining for two different antigens simultaneously, using antibodies tagged with different fluorescent probes, it became possible to position RNA-associated antigens as well as RNA polymerase B in relation to each other. Three separate staining patterns could be observed with anti-NHCP antibodies, none of which showed a pattern which was identical with that of RNA polymerase B. Furthermore, no correlation with the synthesis of the primary trancript, as monitored by the RNA polymerase B content of chromosomal sites, could be found by following the fluorescence patterns during inactivation of intermolt puffs or activation of early ecdysone-induced puffs. Finally, no strict correlation was observed between puffing activity and the accumulation of a certain antigen in these selected chromosomal sites.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. E., Roberts D. B., Richards G. P., Ashburner M. Drosophila: the genetics of two major larval proteins. Cell. 1978 Feb;13(2):215–225. doi: 10.1016/0092-8674(78)90190-3. [DOI] [PubMed] [Google Scholar]
- Amante L., Ancona A., Forni L. The conjugation of immunoglobulins with tetramethylrhodamine isothiocyanate. A comparison between the amorphous and the crystalline fluorochrome. J Immunol Methods. 1972 May;1(3):289–301. doi: 10.1016/0022-1759(72)90006-3. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Chihara C., Meltzer P., Richards G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:655–662. doi: 10.1101/sqb.1974.038.01.070. [DOI] [PubMed] [Google Scholar]
- Beckendorf S. K., Kafatos F. C. Differentiation in the salivary glands of Drosophila melanogaster: characterization of the glue proteins and their developmental appearance. Cell. 1976 Nov;9(3):365–373. doi: 10.1016/0092-8674(76)90081-7. [DOI] [PubMed] [Google Scholar]
- Berendes H. D. Factors involved in the expression of gene activity in polytene chromosomes. Chromosoma. 1968;24(4):418–437. doi: 10.1007/BF00285017. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Bouton A. H., Miller O. L., Jr Correlation of hnRNP structure and nascent transcript cleavage. Cell. 1981 Oct;26(2 Pt 2):155–165. doi: 10.1016/0092-8674(81)90299-3. [DOI] [PubMed] [Google Scholar]
- Beyer A. L., Miller O. L., Jr, McKnight S. L. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980 May;20(1):75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. Ecdysone-stimulated RNA synthesis in imaginal discs of Drosophila melanogaster. Assay by in situ hybridization. Chromosoma. 1976 Oct 12;58(1):87–99. doi: 10.1007/BF00293443. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. Ecdysone-stimulated RNA synthesis in salivary glands of drosophila melanogaster: assay by in situ hybridization. Cell. 1977 Sep;12(1):219–225. doi: 10.1016/0092-8674(77)90199-4. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., LeStourgeon W. M., Jamrich M., Howard G. C., Serunian L. A., Silver L. M., Elgin S. C. Distribution studies on polytene chromosomes using antibodies directed against hnRNP. J Cell Biol. 1981 Jul;90(1):18–24. doi: 10.1083/jcb.90.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C., Serunian L. A., Silver L. M. Distribution patterns of Drosophila nonhistone chromosomal proteins. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):839–850. doi: 10.1101/sqb.1978.042.01.085. [DOI] [PubMed] [Google Scholar]
- Howard G. C., Abmayr S. M., Shinefeld L. A., Sato V. L., Elgin S. C. Monoclonal antibodies against a specific nonhistone chromosomal protein of Drosophila associated with active genes. J Cell Biol. 1981 Jan;88(1):219–225. doi: 10.1083/jcb.88.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügle B., Guldner H., Bautz F. A., Alonso A. Cross-reaction of hnRNP-proteins of HeLa cells with nuclear proteins of Drosophila melanogaster demonstrated by a monoclonal antibody. Exp Cell Res. 1982 Nov;142(1):119–126. doi: 10.1016/0014-4827(82)90416-5. [DOI] [PubMed] [Google Scholar]
- Jamrich M., Greenleaf A. L., Bautz F. A., Bautz E. K. Functional organization of polytene chromosomes. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):389–396. doi: 10.1101/sqb.1978.042.01.040. [DOI] [PubMed] [Google Scholar]
- Kabisch R., Krause J., Bautz E. K. Evolutionary changes in non-histone chromosomal proteins within the Drosophila melanogaster group revealed by monoclonal antibodies. Chromosoma. 1982;85(4):531–538. doi: 10.1007/BF00327348. [DOI] [PubMed] [Google Scholar]
- Korge G. Chromosome puff activity and protein synthesis in larval salivary glands of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4550–4554. doi: 10.1073/pnas.72.11.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge G. Genetic analysis of the larval secretion gene Sgs-4 and its regulatory chromosome sites in Drosophila melanogaster. Chromosoma. 1981;84(3):373–390. doi: 10.1007/BF00286027. [DOI] [PubMed] [Google Scholar]
- Korge G. Larval saliva in Drosophila melanogaster: production, composition, and relationship to chromosome puffs. Dev Biol. 1977 Jul 15;58(2):339–355. doi: 10.1016/0012-1606(77)90096-3. [DOI] [PubMed] [Google Scholar]
- Krämer A., Haars R., Kabisch R., Will H., Bautz F. A., Bautz E. K. Monoclonal antibody directed against RNA polymerase II of Drosophila melanogaster. Mol Gen Genet. 1980;180(1):193–199. doi: 10.1007/BF00267369. [DOI] [PubMed] [Google Scholar]
- Mayfield J. E., Serunian L. A., Silver L. M., Elgin S. C. A protein released by DNAase I digestion of drosophila nuclei is preferentially associated with puffs. Cell. 1978 Jul;14(3):539–544. doi: 10.1016/0092-8674(78)90240-4. [DOI] [PubMed] [Google Scholar]
- Muskavitch M. A., Hogness D. S. Molecular analysis of a gene in a developmentally regulated puff of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7362–7366. doi: 10.1073/pnas.77.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLING C. RIBONUKLEINSAEURE-SYNTHESE DER RIESENCHROMOSOMEN. AUTORADIOGRAPHISCHE UNTERSUCHUNGEN AN CHIRONOMUS TENTANS. Chromosoma. 1964 Apr 1;15:71–122. doi: 10.1007/BF00326915. [DOI] [PubMed] [Google Scholar]
- Plagens U., Greenleaf A. L., Bautz E. K. Distribution of RNA polymerase on Drosophila polytene chromosomes as studied by indirect immunofluorescence. Chromosoma. 1976 Dec 16;59(2):157–165. doi: 10.1007/BF00328484. [DOI] [PubMed] [Google Scholar]
- Sass H., Bautz E. K. Immunoelectron microscopic localization of RNA polymerase B on isolated polytene chromosomes of Chironomus tentans. Chromosoma. 1982;85(5):633–642. doi: 10.1007/BF00330777. [DOI] [PubMed] [Google Scholar]
- Sass H., Bautz E. K. Interbands of polytene chromosomes: binding sites and start points for RNA polymerase B (II). Chromosoma. 1982;86(1):77–93. doi: 10.1007/BF00330731. [DOI] [PubMed] [Google Scholar]
- Sass H. Features of in vitro puffing and RNA synthesis in polytene chromosomes of Chironomus. Chromosoma. 1980;78(1):33–78. doi: 10.1007/BF00291908. [DOI] [PubMed] [Google Scholar]
- Sass H. RNA polymerase B in polytene chromosomes: immunofluorescent and autoradiographic analysis during stimulated and repressed RNA synthesis. Cell. 1982 Feb;28(2):269–278. doi: 10.1016/0092-8674(82)90345-2. [DOI] [PubMed] [Google Scholar]
- Saumweber H., Symmons P., Kabisch R., Will H., Bonhoeffer F. Monoclonal antibodies against chromosomal proteins of Drosophila melanogaster: establishment of antibody producing cell lines and partial characterization of corresponding antigens. Chromosoma. 1980;80(3):253–275. doi: 10.1007/BF00292684. [DOI] [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Silver L. M., Elgin S. C. Distribution patterns of three subfractions of drosophila nonhistone chromosomal proteins: possible correlations with gene activity. Cell. 1977 Aug;11(4):971–983. doi: 10.1016/0092-8674(77)90308-7. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Gallinaro-Matringe H., Gattoni R., Jacob M. Complexity of the structure of particles containing heterogeneous nuclear RNA as demonstrated by ribonuclease treatment. Eur J Biochem. 1977 Apr 15;74(3):589–602. doi: 10.1111/j.1432-1033.1977.tb11428.x. [DOI] [PubMed] [Google Scholar]
- Velissariou V., Ashburner M. The secretory proteins of the larval salivary gland of Drosophila melanogaster: Cytogenetic correlation of a protein and a puff. Chromosoma. 1980;77(1):13–27. doi: 10.1007/BF00292038. [DOI] [PubMed] [Google Scholar]
- Walker V. K., Ashburner M. The control of ecdysterone-regulated puffs in Drosophila salivary glands. Cell. 1981 Oct;26(2 Pt 2):269–277. doi: 10.1016/0092-8674(81)90309-3. [DOI] [PubMed] [Google Scholar]
- Yang V. W., Lerner M. R., Steitz J. A., Flint S. J. A small nuclear ribonucleoprotein is required for splicing of adenoviral early RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1371–1375. doi: 10.1073/pnas.78.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij W. J., Janssen D. B. HnRNP particles. Mol Biol Rep. 1978 Feb 28;4(1):3–8. doi: 10.1007/BF00775172. [DOI] [PubMed] [Google Scholar]