Abstract
Purpose
Nationwide data has been lacking on drug abuse (DA) associated mortality. We do not know the degree to which this excess mortality results from characteristics of drug abusing individuals or from the effects of DA itself.
Method
DA was assessed from medical, criminal and prescribed drug registries. Relative pairs discordant for DA were obtained from the Multi-Generation and Twin Registers. Mortality was obtained from the Swedish Mortality registry.
Results
We examined all individuals born in Sweden 1955–1980 (n=2,696,253), 75,061 of whom developed DA. The mortality hazard ratio (mHR) (95% CIs) for DA was 11.36 (95% CIs, 11.07–11.66), substantially higher in nonmedical (18.15, 17.51–18.82) than medical causes (8.05, 7.77–8.35) and stronger in women (12.13, 11.52–12.77) than in men (11.14, 10.82–11.47). Comorbid smoking and alcohol use disorder explained only a small proportion of the excess DA-associated mortality. Co-relative analyses demonstrated substantial familial confounding in the DA-mortality association with the strongest direct effects seen in middle and late-middle age. The mHR was highest for opiate abusers (24.57, 23.46–25.73), followed by sedatives (14.19, 13.11–15.36), cocaine/stimulants (12.01, 11.36–12.69), and cannabis (10.93, 9.94–12.03).
Conclusion
The association between registry-ascertained DA and premature mortality is very strong and results from both non-medical and medical causes. This excess mortality arises both indirectly — from characteristics of drug abusing persons — and directly from the effects of DA. Excess mortality of opiate abuse was substantially hi3gher than that observed for all other drug classes. These results have implications for interventions seeking to reduce the large burden of DA-associated premature mortality.
Keywords: Drug abuse, mortality, alcohol use disorders, co-relative design, medical causes of death, age
The abuse of illicit psychoactive substances (hereafter drug abuse (DA)) contributes substantially to the global burden of disease [1–3] and is strongly associated with premature mortality [1,4–10]. DA represents one of the chief modifiable behavioral risk factors for mortality in the US [11] and is a major contributor to the recent increase in all-cause mortality in US working class white men [12].
While the aggregate evidence associating DA and premature mortality is strong, important limitations exist in many of the individual studies that have examined this question [6,7,13,14]. With important exceptions (e.g. [4,15,16]), follow-up periods are typically short giving a limited view of time-course of DA-associated mortality especially into middle and late adult life. Sample sizes tend to be modest, particularly given the relative rarity of death in early adulthood, the focus of most such studies. Ascertainment is rarely population-based and often involves a single treatment facility. Few studies are sufficiently powered to compare mortality associated with different classes of illicit substances.
Most critically, we are unaware of prior studies that have attempted to disentangle the causative relationship between DA and excess mortality. Individuals with DA are predisposed to a range of personality traits (e.g. novelty seeking and impulsivity [17–19]) and health habits (e.g. smoking and excess drinking [20]) that impact on mortality. To what extent is the excess mortality associated with DA an indirect effect of these predispositions versus a direct effect of the DA itself? This is a critical question because it indicates what proportion of the excess mortality might be prevented if the progression to DA could be prevented.
We here report a study which addresses several of these prior limitations. We examined prospectively a large population-based cohort in Sweden where illicit DA (excluding alcohol and tobacco abuse) was identified from medical and legal registries which we followed into late adulthood matching to national mortality records. In this cohort, we address six questions:
What is the overall magnitude of the DA-mortality association?
To what degree does this association differ for non-medical versus medical causes of death and between men and women?
What is the pattern of the DA-mortality association across the lifespan?
To what degree is the association between DA and mortality confounded by smoking or alcohol use disorder (AUD)?
Using an extended co-relative design, to what degree does the DA-mortality association arise indirectly from shared predispositions versus directly from the effects of DA?
Does mortality differ as a function of the class of abused substance?
METHODS
This prospective cohort study was based on Swedish population-based registers with national coverage. Several different registers were linked using each person’s unique identification number. To preserve confidentiality, this ID number was replaced by a serial number. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409).
Starting with the Multi-Generation register we selected all individuals born in Sweden 1955–1980 who had neither died nor migrated prior to 1973 or age 15. The Multi-Generation register is a register made up of persons who have been registered in Sweden at some time since 1961 and those who were born in 1932 or later. These are called index persons. This sample was merged with the Swedish Hospital Discharge Register, containing all hospitalizations for all Swedish inhabitants from 1964–2010; the Swedish Prescribed Drug Register, containing all prescriptions in Sweden picked up by patients from July 2005 to 2010; the Outpatient Care Register, containing information from outpatient clinics from 2001 to 2010; the Swedish Crime Register included complete national data on all convictions from 1973–2010; the Swedish suspicion register included complete national data on all individuals strongly suspected of crime from 1998–2010 and the Swedish Mortality Register, containing causes of death.
Based on information in the medical, criminal and prescribed drug Register we defined Drug Abuse (DA). In the Swedish medical registries DA was identified from primary and secondary ICD codes (ICD8: Drug dependence (304); ICD9: Drug psychoses (292) and Drug dependence (304), Nondependent abuse of drugs (305.1–305.9); ICD10: Mental and behavioral disorders due to psychoactive substance use (F10–F19), except those due to alcohol (F10) or tobacco (F17)); in the Suspicion Register by codes 3070, 5010, 5011, and 5012, that reflect crimes related to DA; and in the Crime Register by references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). DA was identified in individuals (excluding those suffering from cancer) in the Prescribed Drug Register who had retrieved (in average) more than four defined daily doses a day for 12 months from either Hypnotics and Sedatives (Anatomical Therapeutic Chemical (ATC) Classification System N05C and N05BA) or Opioids (ATC: N02A). An individual was considered as registered for DA if he/she fulfilled any of the criteria described above.
To investigate the association between DA and mortality, we utilized a Cox proportional hazards model in which the risk of death, from age 15 until end of follow-up (death, emigration, or 2010), was modeled as a function of their first DA registration. As first registration for DA could occur at varying ages, we treated DA as a time dependent covariate: i.e., from age 15 and until the year the individual was registered for DA this individual was considered as free of DA; from the year of DA and until end of follow-up this individual was considered registered for DA. In this model, the proportionality assumption was not fulfilled suggesting that the association between DA and mortality varied across ages. We therefore included several coefficients, based on age intervals, for DA. We selected the number of age intervals based on model fit value (lowest Akaike’s information criterion (AIC) [21]) and non-overlapping 95 % CIs for the resulting coefficients. In the final model we included a separate coefficient for ages 15–22, 23–39, 40–44, 45–49, and 50–56. Robust standard errors were used to adjust the 95% confidence intervals to reflect that the sample contained individuals from the same family. We also included mid-parent educational status [(1) <=9 years, (2) 10–11 years, (3) 12 years or more], sex and year of birth in the model.
The Multi-Generation register contains connections between index persons and their biological parents; hence pairs of twins, siblings, half-siblings, and cousins can be derived. The Swedish Twin Register was used to separate MZ-twin pairs from DZ-twin pairs. To clarify the degree to which the excess mortality associated with DA arises indirectly, from the predispositions of the kind of person who develops DA, versus directly from the DA itself, we used a co-relative design. From the Swedish Multi-Generation Register and the Swedish Twin Register, we identified all MZ twin pairs and all full-sibling, half-sibling pairs, and first-cousin pairs. Using stratified Cox proportional hazards models, we refit all analyses within strata of the specific relative set (MZ-twins (among the total number of MZ-twin pairs born 1955–1980, 164 pairs were discordant for DA and included in the co-relative models), full-siblings (40,645 discordant sibling sets), half-siblings (43,827 discordant pairs), and cousins (222,249 discordant pairs)). Within each strata, the Hazard ratio (HR) was adjusted for familial clustering thereby accounting for an array of unmeasured shared genetic and environmental factors.
Next, we combined all five samples (i.e., population, twin, full- and half- sibling, cousin) into one dataset on which we performed two analyses. The first allowed all coefficients for each sample to be independent. In the second we modeled the resemblance in longevity in relatives as arising from genetic factors, consistent with prior empirical findings [22]. For each DA coefficient (i.e., within each age interval) we assumed that the coefficient followed the genetic resemblance for each sample: i.e., 0 for the population, 0.125 for cousins, 0.25, for half-siblings, 0.5 for full-siblings, and 1 for the MZ twin sample. We compared this model, using the AIC, with the previous model. If the second model fitted the data well, we obtained a HR for the familial confounding within each age interval as well as an improved estimation of the DA-mortality association among all types of relatives, but especially MZ twins, where the data was sparser. We create from these estimates a confounding index which ranges from zero — reflecting no contribution of familial confounding to the observed association (consistent with the association arising entirely from direct effects of DA) – to unity indicating the entire association arises from familial confounding influences (consistent with DA having no causal impact on mortality). Our confounding index was calculated from the estimated mHR in the general population (mHRgp) and in discordant monozygotic twins (mHRmz) was defined as follows:
Total familial confounding would predict that the value of the mHRmz should equal unity. That is, the mHR would be the same in both members of a pair of MZ twins where one had DA and the other did not. Therefore, as confounding becomes total, the ratio of [mHRmz−1]/[mHRgp−1] goes to zero and the confounding index goes to unity. By contrast, no familial confounding predicts that the mHRmz and mHRgp will be the same. That is, the mHR would be the same in a person picked at random from the general population (controlling for age, sex and parental education) as in an unaffected member of an MZ twin pair where the cotwin had DA. Under those circumstances, the ratio of [mHRmz−1]/[mHRgp−1] goes to one and the value of the confounding index goes to zero. If familial confounding contributed approximately half of the overall association, we would predict a value of mHRmz−1 that would be around one half of the value of mHRgp−1 and produce a value of the confounding index of 0.5.
For those registered for DA, we could identify in 45% of cases, one or more of 4 classes of abused substances: Opiates, Stimulants, Sedatives, and Cannabis. Opiate abuse was defined by ICD10 code F11 and ICD9 codes 305.5, 304.0 and ICD8 code 304.0. In the Swedish Crime Registry, we used references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). Within those laws cannabis was defined by codes 9–13. Stimulant abuse was defined by ICD10 codes F14, F15 and ICD9 codes 305.7, 304.2, 304.4 and ICD8 code 304.4. In the Swedish Crime Registry, we used references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). Within those laws cannabis was defined by codes 1–7. Sedative abuse was defined by ICD10 code F13 and ICD9 codes 305.4, 304.1 and ICD8 code 304.1. In the Swedish Crime Registry, we used references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). Within those laws cannabis was defined by codes 17, 19. Cannabis abuse was defined by ICD10 code F12 and ICD8 and 9 codes 304.5 in the medical registers. In the Swedish Crime Registry, we used references to laws covering narcotics (law 1968:64, paragraph 1, point 6) and drug-related driving offences (law 1951:649, paragraph 4, subsection 2 and paragraph 4A, subsection 2). Within those laws cannabis was defined by code 8. We applied a hierarchy of the substances in the order noted above. This means, for example, that a cannabis registration would not be considered as such in our analysis unless it was the only registered drug noted. We then applied the same analytical framework, as described above, in which we included 5 dummy variables indicating substance (including one dummy variable for unknown substance).
In additional analyses, we divided causes of death into two groups: non-medical and medical. Non-medical causes of death were defined in Swedish Mortality register by the following ICD codes: ICD10: X, V, Y, S, T and in ICD 8 and 9 by E-codes. All other causes were defined as medical causes of death. We then replicated the analysis described above using a competing risk model as the two types of mortality were mutually exclusive.
Furthermore, in an additional analysis, we excluded cases of DA who also had a lifetime diagnosis of Alcohol Use Disorder (AUD). For a definition of AUD see [23].
Finally, in an additional sample of 49,463 males who underwent examination for army conscription in 1969–70, we had available to us their smoking status at age 18. (These individuals were born in 1951–1952 and were therefore slightly older than our main study cohort). The smoking variable was categorized into 3 groups; No smoking, Light smoking (1–10 cigarettes/day) and Heavy smoking (more than 10 cigarettes per day). On this sample we replicated the Cox regression models using DA as a time dependent covariate while controlling for smoking status. All statistical analyses were performed using SAS 9.3 [24].
RESULTS
Descriptive Findings
Key descriptive features of our sample are depicted in table 1. Our main study cohort, born 1955–1980, contained 2,696,253 individuals followed for a mean of 27.7 years. Of those, 75,061 developed DA for a prevalence of 2.78%.
Table 2.
The Degree of Familial Confounding (as assessed by the Confounding Index) in the Association between Mortality and Drug Abuse in Cohorts Born 1955–1990 and 1940–1980
| Cohort Born 1955–1980 | Cohort Born 1940–1980 | ||
|---|---|---|---|
| Confounding Index* | Confounding Index* | ||
| Age | Drug Abuse | Age | Drug Abuse |
| 15–22 | 0.85 | 15–22 | 0.95 |
| 23–39 | 0.77 | 23–39 | 0.70 |
| 40–44 | 0.72 | 40–44 | 0.60 |
| 45–49 | 0.69 | 45–49 | 0.53 |
| 50–56 | 0.64 | 50–54 | 0.66 |
| 55–59 | 0.59 | ||
| 60–64 | 0.66 | ||
| 65- | 0.85 | ||
DA was associated with a substantially elevated mortality with an unadjusted mortality HR (mHR) of 11.96 (95% CIs, 11.66–12.27). Controlling for sex, parental educational status, and year of birth, the mHR was reduced slightly to 11.36 (11.07–11.66). DA associated mortality was much higher for non-medical than for medical causes (18.15 [17.51–18.82] vs. 8.05 [7.77–8.35], respectively) and modestly higher in women (12.13, 11.52–12.77) than in men (11.14, 10.82–11.47). The mHR was substantially higher for DA ascertained in the medical registry (13.80, 13.40–14.21) than in the criminal registry (8.70, 8.29–9.12).
The mortality rate was not constant over the lifespan and demonstrated an inverted U-shape function, maximizing in early to mid-adulthood (figure 1.) Sex differences were similar across the lifespan with the ratio of the mHRs in males vs. females ranging across age-groups from 0.72 to 0.86 with no clear temporal trend. Higher mortality HRs from non-medical than medical causes were seen in all age groups and the ratio tended to increase with advancing age, reaching a maximum at ages 45–49.
Figure 1.
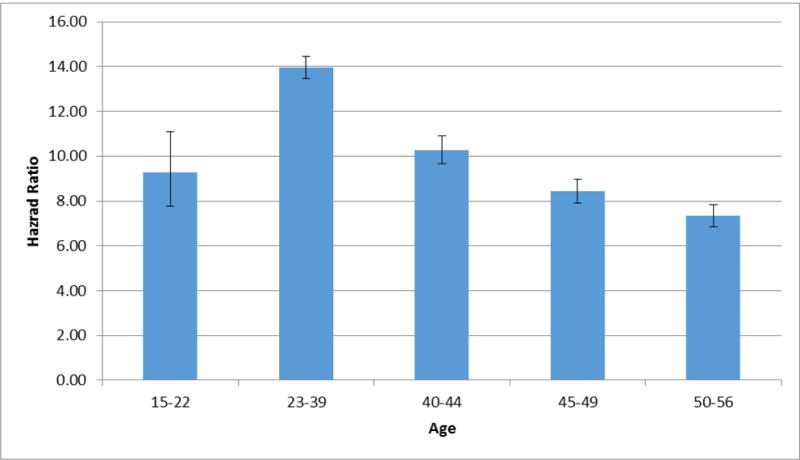
Observed Hazard Ratio in the General Population for Death as the Outcome With Drug Abuse Registration as a Time-Dependent Coefficient as a Function of Age, Controlling for Parental Education, Year of Birth and Sex.
Impact of Smoking and Alcohol Use Disorder
Smoking status was known at age 18 for a cohort of 49,463 males who underwent examination for army conscription in 1969–70. Light and heavy smoking in that group was associated with substantial elevations of mortality with mHRs of 1.41 (1.30–1.53) and 1.88 (1.74–2.04), respectively. In this select group, the unadjusted mHR for DA was 8.46 (7.58–9.43). Controlling for smoking status at conscription, the mHR for DA declined modestly to 7.03 (6.27–7.88). In our entire sample, if we excluded from analysis all cases of DA who also had a lifetime diagnosis of AUD, the mHR for DA declined only slightly to 11.31 (10.86–11.77).
Co-Relative Analyses
We next examined the association between DA and mortality by age group in the general population and then in first-cousins, half-siblings, full-siblings, and MZ twin pairs. Our co-relative model fitted the data well (the AIC of the raw data vs model were 1680540.2 and 1680537.6, respectively). The results of this model are seen in figure 2 and appendix table 1. This figure compares the mHR for DA in the general population to that observed within pairs of discordant relatives. If familial factors contribute to the DA-mortality association, then the mHR should be attenuated in those pairs compared to the general population and more attenuated in more closely related pairs. This is exactly the pattern we observe across all ages, consistent with some degree of familial confounding for the DA-mortality association. However, the mHRs were consistently greater than unity in MZ twins, suggesting a direct causal effect of DA on mortality. The degree of familial confounding can be seen visually by the slope of the declining HR moving from genetically unrelated individuals in the general population, through cousins, half-siblings, full-siblings, and MZ twins. Numerically, the slope is captured by the Confounding Index, values for which are seen in table 2. The proportion of the DA-mortality association that is the result of familial confounding decreased with advancing age. However, even in the 50s, the Confounding Index suggests that the majority of the DA-mortality association arose from confounding familial influences.
Figure 2.
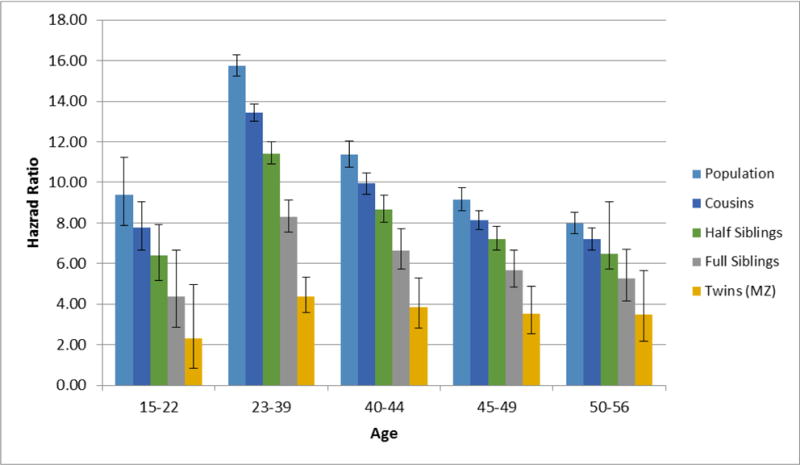
Estimated Hazard Ratio for Death as the Outcome With Drug Abuse Registration as a Time-Dependent Coefficient as a Function of Age in 5 Age Groups from Late Adolescence to Late Adulthood in our main study cohort born 1955 to 1980. For each age group, the estimated mortality hazard ratios are presented for the general population and for cousin, half-sibling, full-sibling, and monozygotic twin pairs discordant for drug abuse.
Specific Drugs of Abuse
One or more drugs of abuse were available for 44.8% of the individuals registered for DA. Using the hierarchy outlined above, the most to least commonly noted substances were: cocaine/stimulants−38.3%, opiates− 31.4%, sedatives−19.2%, and cannabis−11.0%. The mHR was highest for opiate abusers (24.57, 23.46–25.73), followed by sedatives (14.19, 13.11–15.36), cocaine/stimulants (12.01, 11.36–12.69), and cannabis (10.93, 9.94–12.03).
Cohort Born 1940–1980
We did not study subjects born before 1955 in our main cohort to prevent substantial left truncation for age at onset of DA, as DA case ascertainment was not fully in place from the medical and criminal registries until the mid-1970s. However, this meant that the oldest subjects we could study were aged 50–56. To investigate where patterns of DA-associated mortality changes in late adulthood, we moved the beginning birth date for our cohort from 1955 to 1940. Although there is more bias in this sample toward recurrent or late onset DA in these earlier birth years, it is the only way to examine DA-mortality association into late adulthood. Results including the co-relative analyses are seen in figure 3 and appendix table 2. The overall trends seen in figure 2 are continued into the late 50s and 60s. Mortality effect of DA declined further with advancing age. As can be seen by visual inspection of figure 3 and the numerical results presented in table 2, the degree of familial confounding did not decrease in late adult life but instead actually increased modestly.
Figure 3.
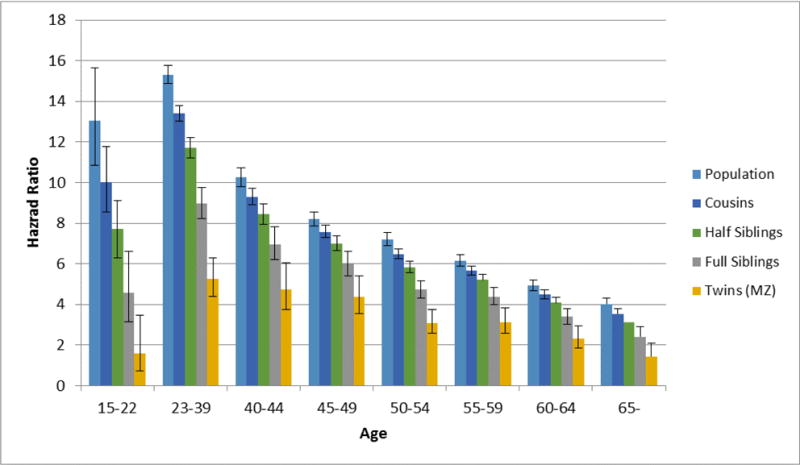
Estimated Hazard Ratio for Death as the Outcome With Drug Abuse Registration as a Time-Dependent Coefficient as a Function of Age in 8 Age Groups From Late Adolescence to Late Adulthood in our secondary study cohort born 1940 to 1980. For each age group, the estimated mortality hazard ratios are presented for the general population and for cousin, half-sibling, full-sibling, and monozygotic twin pairs discordant for drug abuse.
DISCUSSION
Our main goals of this report was to further clarify the nature and causes of the association between drug abuse and excess mortality. We addressed six questions which we review in order.
First, in the Swedish population with a mean follow-up of 28 years, DA was associated with a nearly twelve-fold increase in mortality. We are unaware of other methodologically similar studies to which our findings can be directly compared. Results from four reports can help contextualize our findings. In a cohort of 11,432 drug abusers (92% heroin users) attending treatment facilities in Rome, with a mean follow-up period of 7.1 years, the standardized mortality ratio (SMR±95%CIs) was estimated at 17.3 (16.5–18.2) [16]. A cohort of 4,817 drug abusers (30% opiate users) seeking treatment at one facility in Helsinki Finland with a mean follow-up of 9 years demonstrated an SMR of 8.9 (8.1–9.7) [25]. In 9,866 members of the National Drug Abuse Treatment Clinical Trials Network followed for around 1 year, the SMR was estimated to be 5.59 in females and 3.39 in males [10]. A 27-year follow-up of the Baltimore Epidemiologic Catchment Area Study — a general population sample assessed by interview — found much more modest increases in mortality associated with non-medical opioid pan reliever use (HR=1.60) and non-medical use of other drugs (HR=1.31) [26].
Second, elevated mortality from DA was more than twice as great for non-medical than medical causes of death and modestly higher in females than males. Similar findings have been noted previously. For example, an extensive review of opiate-associated mortality [7] noted the high proportion of deaths arising from over-dose, trauma and suicide. Other reports have observed higher DA-associated mortality rates in females than males [7,8,13,16,27].
Third, we found an inverted U-shaped function for DA-associated mortality across the life-span reaching its maximum in early adult life but with substantial excess mortality found well into late adulthood. Our curve for age-related mortality for DA closely mirrors that seen for illicit drug associated disability from the Global Burden of Disease study which also shows a sharp peak in early adult life. [2].
Fourth, we were able to examine the degree to which the increased mortality associated with DA arose due to comorbidity with smoking and alcohol misuse. While we could study AUD in our entire cohort, smoking data was available on a much smaller sub-sample. Nonetheless, both of these analyses suggested that little of the increased morality associated with the abuse of illicit substances could be explained by the use or misuse of the two harmful legal substances consumed for their psychoactive effects: nicotine and ethanol.
Fifth, for the first time to our knowledge, we demonstrated that a substantial proportion of the DA-mortality association arose from familial confounding. Across all ages, our analyses suggested (table 2) that a majority of this association arose indirectly from shared familial predispositions. However, at all ages, and especially in middle to middle-late adult life, our results also indicate a substantial, direct and probably causal effect of DA on mortality. While our design does not permit us to further specify the nature of the confounding factors, our guess is that both genetic and familial-environmental factors are involved. Important mediating variables might include personality traits such as impulsivity, low education, excess alcohol and tobacco use and other lifestyle choices that are associated both with DA and premature mortality.
Sixth, congruent with prior studies, we found mortality was substantially higher for opiate abuse than for other forms of DA. A broadly comparable national study of a Danish opiate addicts found, over a 5-year follow-up, an SMR of 12.3 (11.0–13.8) in males and 17.4 (13.9–21.4) in females [8]. Fugelstad et al found mortality rates from opiate abuse (largely heroin) was more than three times higher than that seen from amphetamines [28]. Particularly informative are three recent reviews estimating SMRs in largely treatment seeking cohorts and finding much higher estimates for opiates (14.66) [7] than for cocaine (4–8) [6] or amphetamines (6.22) [13]. In addition, intravenous use of drugs is strongly associated with excess mortality [29] and this form of administration is particularly common with opiates [30].
We conducted our main analyses on the cohort born 1955–1980 so we could detect early onset DA in the entire sample. However, this limited our ability to examine mortality effects of DA in late adult life. We therefore also examined a 1940–1980 cohort the older members of which, to be ascertained for DA, had to have either recurrent or late onset abuse. However, the pattern of results from this 1940–1980 cohort were reassuringly similar to those obtained using birth years 1955–1980, suggesting that we were correctly estimating the pattern of DA mortality into late adult life.
Limitations
These results should be interpreted in the context of five potential methodological limitations. First, our results only apply to the Swedish population which for DA associated mortality appears to be representative of Europe [8]. Second, DA was ascertained using official registries which are not dependent on subject cooperation or accurate recall. Compared to interview assessments, these methods likely generate both false positive and particularly false negative diagnoses. While large interview studies of DA prevalence do not exist in Sweden, lifetime prevalence of drug abuse/dependence in near-by Norway was estimated at 3.4% [31], close to our rates of DA (2.8%). Third, evidence that the mortality rate for DA ascertained in the medical registry was considerably higher than in the criminal registry raises the concerns that the former estimates are biased upward because drug abusing individuals with comorbid medical conditions would more likely be detected. This has sometimes been called the “unhealthy drug user effect.” We reduced this concern by restricting the ICD codes used to ascertain cases to only drug dependence, abuse and psychosis and counting only the primary and first secondary diagnosis, the former accounting for 75.3% of medically ascertained cases. However, mortality rates were modestly greater in cases where DA was the second vs. primary diagnosis (mHR=1.16, 1.10–1.23). But for most of our medically ascertained cases of DA, the DA was not an incidental finding in those who presented for care for other medical problems. Nonetheless, our estimates for DA-related mortality are much higher than those recently estimated from a US population-based sample [26]. Our cases of DA are probably, on average, more severe than those identified by interview surveys, and there might be a bias in our ascertainment which has enriched our sample of DA cases for those with higher levels of medical comorbidity.
Fourth, if our ascertainment for DA is substantially incomplete, given the known strong familial aggregation of DA [32–34], false negative cases will be common in relatives of DA probands, biasing upward our measure of familial confounding as mortality rates would be elevated in these undetected DA cases and biasing downward our estimates of the direct effects of DA on mortality. Fifth, we detected DA from four registries, two of which — medical and conviction — were constant over our period of study (from 1973 to 2010) while two — arrest and pharmacy registries — were added in, respectively, 1997 and 2005. To examine whether the addition of these two new registries biases our estimates, we calculated the mHR for DA using only cases ascertained in the medical and criminal conviction registries. The unadjusted mHR and that adjusted for sex, parental educational status, and year of birth were moderately higher than those obtained using all our registry data: 13.39 (13.03–13.76) and 12.37 (12.03–12.71), respectively.
Conclusion
In a large national sample followed for nearly three decades, DA, ascertained from nationwide criminal and medical registries, was associated with a nearly 12-fold increased mortality rate. This estimate may be biased upward for two reasons. First our use of registries rather than general population surveys may have identified more severe cases. Second, our use of medical registries may have included more DA cases with medical comorbidity. This increased mortality in DA was strongest in early adult life, arose from elevations of both medical and especially non-medical causes, and was greater in women than men. This excess mortality arose from both indirect causes (e.g., characteristics of DA-predisposed persons) and from the direct impact of DA. Indirect effects were overall more important, with direct effects strongest in middle to middle-late adult life. Opiate abuse was associated with substantially higher mortality rates than other drug classes. Little of the excess mortality associated with DA could be explained by comorbidities with smoking or AUD. Our findings have important implications for interventions seeking to reduce the large burden of DA-associated premature mortality. In particular, they suggest that preventing DA will reduce only a portion of the mortality burden as individuals who abuse drugs would be at increased risk for early death even if they do not actually misuse illicit psychoactive substances.
Table 1.
Descriptive Statistics of our Cohort of Individuals born in Sweden 1955–1980, alive at age 15 or in 1973 with DA Registration Measured from 1973 Onwards
| Sample Size | 2,696,253 |
| DA Registration | 2.78% (n = 75,061) |
| Age at first DA Registration (SD) | 32.0 (9.0) |
| 25–50–75 percentile | 25–31–39 |
| % Dead(DA/No DA) | 9.7 (n=7,313)/ 1.9 (n=49,306) |
| Mean age at Death (DA/No DA) | 37.9/33.9 |
| Mean follow-up time (SD) | 27.7 (8.1) |
Acknowledgments
This project was supported by grant R01DA030005 from the National Institutes of Health, the Swedish Research Council (K2012-70X-15428-08-3), the Swedish Research Council for Health, Working Life and Welfare (In Swedish: Forte; Reg.nr: 2013–1836), the Swedish Research Council (2012–2378; 2014–10134) and FORTE (2014–0804) as well as ALF funding from Region Skåne awarded.
APPENDIX
Table 1.
Exact Values of Mortality Hazard Ratios for Figure 2 – Results of Genetic Model Fitting for Co-Relative Design For Drug Abuse – Cohort Born 1955–1980
| Age Group | Population | Cousins | Half Siblings | Full Siblings | MZ Twins |
|---|---|---|---|---|---|
| 15–22 | 9.40 (7.88; 11.21) | 7.76 (6.66; 9.04) | 6.41 (5.18; 7.93) | 4.36 (2.86; 6.66) | 2.29 (0.83; 4.96) |
| 23–39 | 15.76 (15.25; 16.28) | 13.42 (13.01; 13.85) | 11.43 (10.9; 12) | 8.29 (7.54; 9.13) | 4.37 (3.58; 5.34) |
| 40–44 | 11.38 (10.74; 12.04) | 9.94 (9.42; 10.48) | 8.68 (8.04; 9.38) | 6.63 (5.71; 7.7) | 3.87 (2.83; 5.28) |
| 45–49 | 9.16 (8.62; 9.72) | 8.13 (7.67; 8.61) | 7.21 (6.64; 7.83) | 5.68 (4.85; 6.65) | 3.52 (2.54; 4.88) |
| 50–56 | 7.97 (7.46; 8.50) | 7.19 (6.66; 7.75) | 6.48 (5.74; 9.04) | 5.28 (4.16; 6.68) | 3.49 (2.16; 5.64) |
Table 2.
Exact Values of Mortality Hazard Ratios for Figure 3 – Results of Genetic Model Fitting for Co-Relative Design For Drug Abuse – Cohort Born 1940–1980
| Age Group | Population | Cousins | Half Siblings | Siblings | MZ twins |
|---|---|---|---|---|---|
| 15–22 | 13.04 (10.86; 15.66) | 10.03 (8.54; 11.78) | 7.71 (6.30; 9.44) | 4.56 (3.14; 6.62) | 1.59 (0.73; 3.47) |
| 23–39 | 15.31 (14.89; 15.75) | 13.40 (13.02; 13.78) | 11.72 (11.22; 12.24) | 8.97 (8.23; 9.77) | 5.25 (4.40; 6.27) |
| 40–44 | 10.25 (9.8; 10.71) | 9.30 (8.92; 9.70) | 8.45 (7.96; 8.97) | 6.97 (6.21; 7.82) | 4.74 (3.73; 6.03) |
| 45–49 | 8.19 (7.84; 8.56) | 7.58 (7.28; 7.89) | 7.01 (6.64; 7.39) | 5.99 (5.41; 6.63) | 4.38 (3.55; 5.40) |
| 50–54 | 7.20 (6.9; 7.52) | 6.48 (6.23; 6.74) | 5.83 (5.55; 6.13) | 4.72 (4.31; 5.17) | 3.09 (2.56; 3.73) |
| 55–59 | 6.16 (5.89; 6.45) | 5.66 (5.43; 5.09) | 5.20 (4.93; 5.48) | 4.39 (3.98; 4.83) | 3.12 (2.56; 3.82) |
| 60–64 | 4.93 (4.67; 5.19) | 4.49 (4.27; 4.71) | 4.09 (3.84; 4.35) | 3.39 (3.02; 3.80) | 2.33 (1.84; 2.95) |
| 65– | 4.02 (3.76; 4.29) | 3.54 (3.30; 3.79) | 3.11 (2.82; 3.43) | 2.41 (2.00; 2.90) | 1.45 (0.99; 2.11) |
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest to declare.
Ethical Standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. We secured ethical approval for this study from the Regional Ethical Review Board of Lund University (No. 2008/409).
Reference List
- 1.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, Flaxman A, Murray CJ, Vos T. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1564–74. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 3.Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora ME. Mental, Neurological, and Substance Use Disorders. 3rd. Washington, D.C: World Bank Group; 2015. [PubMed] [Google Scholar]
- 4.Larm P, Hodgins S, Larsson A, Samuelson YM, Tengstrom A. Long-term outcomes of adolescents treated for substance misuse. Drug Alcohol Depend. 2008;96(1–2):79–89. doi: 10.1016/j.drugalcdep.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Bargagli AM, Hickman M, Davoli M, Perucci CA, Schifano P, Buster M, Brugal T, Vicente J. Drug-related mortality and its impact on adult mortality in eight European countries. Eur J Public Health. 2006;16(2):198–202. doi: 10.1093/eurpub/cki168. [DOI] [PubMed] [Google Scholar]
- 6.Degenhardt L, Singleton J, Calabria B, McLaren J, Kerr T, Mehta S, Kirk G, Hall WD. Mortality among cocaine users: a systematic review of cohort studies. Drug Alcohol Depend. 2011;113(2–3):88–95. doi: 10.1016/j.drugalcdep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 7.Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 8.Perucci CA, Bargagli AM, Davoli M, Sperati A, Hartnoll R, Vicente J. Mortality of drug users in the EU: coordination of implementation of new cohort studies, follow-up and analysis of existing cohorts and development of new methods and outputs. Lisboa: European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); 2000. 2000. Report No.: EMCDDA Scientific report: EMCDDA CT.99.EP.07/CT.00.EP.13. [Google Scholar]
- 9.Ridolfo B, Stevenson C. The Quantification of drug-caused mortality and morbidity in Australia, 1998. Canberra: AIHW; 2001. (Drug Statistics Series no. 7. Cat. no. PHE 29.6-1-2016). [Google Scholar]
- 10.Lindblad R, Hu L, Oden N, Wakim P, Rosa C, VanVeldhuisen P. Mortality Rates Among Substance Use Disorder Participants in Clinical Trials: Pooled Analysis of Twenty-Two Clinical Trials Within the National Drug Abuse Treatment Clinical Trials Network. J Subst Abuse Treat. 2016;70:73–80. doi: 10.1016/j.jsat.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–45. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 12.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112(49):15078–83. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton J, Degenhardt L, Hall W, Zabransky T. Mortality among amphetamine users: a systematic review of cohort studies. Drug Alcohol Depend. 2009;105(1–2):1–8. doi: 10.1016/j.drugalcdep.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Calabria B, Degenhardt L, Hall W, Lynskey M. Does cannabis use increase the risk of death? Systematic review of epidemiological evidence on adverse effects of cannabis use. Drug Alcohol Rev. 2010;29(3):318–30. doi: 10.1111/j.1465-3362.2009.00149.x. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Quintero C, Roth KB, Eaton WW, Wu LT, Cottler LB, Bruce M, Anthony JC. Mortality among heroin users and users of other internationally regulated drugs: A 27-year follow-up of users in the Epidemiologic Catchment Area Program household samples. Drug Alcohol Depend. 2015;156:104–11. doi: 10.1016/j.drugalcdep.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bargagli AM, Sperati A, Davoli M, Forastiere F, Perucci CA. Mortality among problem drug users in Rome: an 18-year follow-up study, 1980–97. Addiction. 2001;96(10):1455–63. doi: 10.1046/j.1360-0443.2001.961014559.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez dF, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276(5321):2050–4. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 18.Fergusson DM, Horwood LJ. Cannabis use and dependence in a New Zealand birth cohort. N Z Med J. 2000;113(1109):156–8. [PubMed] [Google Scholar]
- 19.Agrawal A, Jacobson KC, Prescott CA, Kendler KS. A twin study of personality and illicit drug use and abuse/dependence. Twin Res. 2004;7(1):72–81. doi: 10.1375/13690520460741462. [DOI] [PubMed] [Google Scholar]
- 20.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM–5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–32. [Google Scholar]
- 22.Ljungquist B, Berg S, Lanke J, McClearn GE, Pedersen NL. The effect of genetic factors for longevity: a comparison of identical and fraternal twins in the Swedish Twin Registry. J Gerontol Med Sci. 1998;53A(6):M441–M446. doi: 10.1093/gerona/53a.6.m441. [DOI] [PubMed] [Google Scholar]
- 23.Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Alcohol Use Disorder and Mortality Across the Lifespan: A Longitudinal Cohort and Co-relative Analysis. JAMA Psychiatry. 2016;73(6):575–81. doi: 10.1001/jamapsychiatry.2016.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute SAS I. SAS/STAT User’s Guide, Version.3. Cary, NC: SAS Institute Inc, SAS Institute Inc.; 2011. [Google Scholar]
- 25.Onyeka IN, Beynon CM, Hannila ML, Tiihonen J, Fohr J, Tuomola P, Kuikanmaki O, Tasa N, Paasolainen M, Kauhanen J. Patterns and 14-year trends in mortality among illicit drug users in Finland: the HUUTI study. Int J Drug Policy. 2014;25(6):1047–53. doi: 10.1016/j.drugpo.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Cottler LB, Hu H, Smallwood BA, Anthony JC, Wu LT, Eaton WW. Nonmedical Opioid Pain Relievers and All-Cause Mortality: A 27-Year Follow-Up From the Epidemiologic Catchment Area Study. Am J Public Health. 2016;106(3):509–16. doi: 10.2105/AJPH.2015.302961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyefeso A, Ghodse H, Clancy C, Corkery J, Goldfinch R. Drug abuse-related mortality: a study of teenage addicts over a 20-year period. Soc Psychiatry Psychiatr Epidemiol. 1999;34(8):437–41. doi: 10.1007/s001270050166. [DOI] [PubMed] [Google Scholar]
- 28.Fugelstad A, Annell A, Rajs J, Agren G. Mortality and causes and manner of death among drug addicts in Stockholm during the period 1981–1992. Acta Psychiatr Scand. 1997;96(3):169–75. doi: 10.1111/j.1600-0447.1997.tb10147.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(2):102–23. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–45. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 31.Kringlen E, Torgersen S, Cramer V. A Norwegian psychiatric epidemiological study. Am J Psychiatry. 2001;158(7):1091–8. doi: 10.1176/appi.ajp.158.7.1091. [DOI] [PubMed] [Google Scholar]
- 32.Rounsaville BJ, Kosten TR, Weissman MM, Prusoff B, Pauls D, Anton SF, Merikangas K. Psychiatric disorders in relatives of probands with opiate addiction. Arch Gen Psychiatry. 1991;48(1):33–42. doi: 10.1001/archpsyc.1991.01810250035004. [DOI] [PubMed] [Google Scholar]
- 33.Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55(11):973–9. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- 34.Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking: a report from the Collaborative Study on the Genetics of Alcoholism. Arch Gen Psychiatry. 1998;55(11):982–8. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]


