Abstract
Background/Aims
This in vitro study compared the remineralization effect on white spot lesions of casein phosphopeptide-amorphous calcium phosphate crème, or CPP-ACP (MI Paste™), 1.1% NaF dentifrice containing 5000 ppm of fluoride (ControlRX™), or CPP-ACP crème with 900 ppm of fluoride (MI Paste Plus™) with that of a control.
Methods
Artificial white spot lesions were created on smooth enamel surfaces of sound molars using a previously reported demineralization model. Specimens were randomly assigned to four treatments (n=35) with a pH-cycling model over 30 days: Control (no treatment); MI Paste (10% CPP-ACP crème); F5000 (1.1% NaF dentifrice); or MI Paste Plus (10% CPP-ACP plus 900 ppm fluoride crème). Products were applied following manufacturers’ directions. Changes in mean lesion depth expressed by percent fluorescence loss (∆F%), and lesion area (mm2) from baseline to after treatment were measured with light-induced fluorescence (QLF). Mean values of each parameter were compared between groups (p<0.05).
Results
The remineralization pattern for the F5000 group was unique with marked initial remineralization during the first 10 days and little subsequent change. Based on mean lesion area, the F5000 demonstrated greater remineralization than Control, MI Paste and MI Paste Plus groups. Based on mean fluorescence loss, the F5000 group showed improved remineralization relative to MI Paste Plus, but did not differ statistically from the Control at the end of 30 days.
Conclusions
The 1.1% NaF dentifrice demonstrated overall greater remineralization ability than 10% CPP-ACP crème. However, the 1.1% NaF dentifrice was only as effective as the Control to reduce fluorescence loss.
Keywords: CPP-ACP, fluoride, white spot lesions, remineralization, QLF
INTRODUCTION
Dental caries is a multifactorial disease that results from an imbalance between pathological and protective factors. Cariogenic bacteria, fermentable carbohydrates, and salivary dysfunction are scientifically accepted as important pathological factors. The imbalance produced will result in a rupture of the physiological processes of remineralization and demineralization of the dental structure, favoring the latter.1 Consequently, dental caries management should also be concentrated on an understanding of the role of remineralization in preventing caries progression and reestablishing a healthy balance when demineralization occurs.2–4 Whole human saliva has calcium and phosphate ions in supersaturated state and therefore the potential to remineralize enamel.5 However, if acid challenges overcome this physiological remineralization process, alternative therapeutic approaches are necessary to enhance remineralization.
Fluoride is a widely recognized remineralizing agent, interacting with oral fluids on the enamel surface and subsurface, and combining with calcium and phosphate ions to form carbonate substituted hydroxyapatite and fluorapatite.6 When used in excess during tooth development, this element can cause dental fluorosis, and can be toxic if administered in high enough doses.7, 8
The possible cariostatic potential of dairy products has been reported by several authors.9–11 In 1991, the casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) complex, derived from a milk protein called casein, was patented in the United States.12 CPP-ACP is presented as an alternative remineralizing agent, capable of stabilizing calcium phosphate, maintaining a state of supersaturation of these ions in the oral environment. As a consequence, the tooth structure would benefit from the high levels of calcium phosphate in the biofilm, and remineralization would occur.13, 14 Although products containing CPP-ACP are commercially available, there is a lack of consensus regarding its remineralizing potential.15–19
The technology to detect and quantitative and qualitatively evaluate carious lesions in vitro has greatly evolved. Traditional methods of analysis such as histology, transverse microradiography, and polarized light microscopy are effective but destructive and inherently time consuming. The use of a quantitative light fluorescence (QLF) to evaluate changes before and after remineralization of artificial enamel lesions has been validated.20–22 This technique is non-destructive and performs analysis based on principles of light transmission, absorption, scattering.
The aim of this study was to measure the remineralizing effect of a topical tooth crème containing CPP-ACP, a fluoride containing dentifrice with 5000 ppm of fluoride, and a hybrid topical tooth crème containing CPP-ACP and 900 ppm of fluoride, using a QLF technique, on white spot lesions (WSL) in vitro.
MATERIALS AND METHODS
Specimen preparation
Thirty five extracted human third molars were collected from the Department of Oral & Maxillofacial Surgery, with approval from the Office of Human Research Ethics of the University of North Carolina (#09-1662), over a period of a month and refrigerated and hydrated in thymol 0.01% until specimen preparation. Teeth with fluorosis, enamel or dentin defects, or presenting any type of dental restoration or sealant were excluded.
The specimens were de-coronated and the crowns sectioned twice, splitting each tooth in 4 sections of approximately equal size, and providing a total of 140 enamel slabs. Each section of the same tooth was identified with the number of the corresponding tooth (1 to 35), as well as the location within the tooth structure (mesiofacial, mesiolingual, distofacial, and distolingual). This enabled each tooth to serve as its own control, reducing the effects of confounders related to inherent differences in the structure and composition of individual teeth.23 The enamel surfaces were protected with a polyvinyl siloxane material (Affinity, Clinician’s Choice, New Milford, CT), and all specimens were mounted in phenolic rings (Buehler, Lake Bluff, IL) with epoxy resin (Buehler). The aprismatic layer of enamel was removed from all specimens using an Ecomet Grinder-Polisher machine (Buehler). The thickness of each specimen was measured with a digital caliper to provide an initial reference. All specimens were flat-polished with water-cooled abrasive papers up to 1200-grit. During this process, the specimens were constantly measured to verify the amount of tooth structure removed, which was always around 100 µm.
To ensure that the demineralizing solution would contact only the polished enamel surface, all specimens were covered with a color-free acid-resistant nail varnish (Revlon Nail Enamel, Oxford, NC) using a brush under microscope. All surfaces were coated except for the flat-ground enamel area after which the specimens were subjected to demineralization.
Lesion creation (demineralization)
The demineralizing medium consisted of 15% G8 Gel (Fisher Scientific, Pittsburgh, PA), 0.1 m lactic acid-sodium lactate (Sigma-Aldrich, St. Louis, MO), and 1 ppm NaF (Fisher Scientific, Pittsburgh, PA), adjusted to pH 4.15.24 Gel systems produce lesions that are histologically comparable to natural lesions.25 The specimens were immersed in the demineralizing solution at 37°C for 8 weeks. Specimens were visually examined throughout the demineralization process to verify lesion formation and surface integrity of the new-formed caries-like lesions. After the incubation period, each specimen was cleaned from the demineralizing medium, superficially dried with absorbent paper, and observed under a dissecting microscope (Nikon, Melville, NY). Finally, specimens were placed in fresh deionized water (high-purity water) and kept at room temperature.
Experimental groups and Study Design
The four specimens extracted from each of the 35 teeth were randomly assigned to four groups (n=35 per group): (1) Control (no treatment); (2) MI Paste (10% CPP-ACP crème, MI Paste, GC America, Alsip, IL); (3) F5000 (1.1% NaF dentifrice, 5000 ppm, ControlRx, 3M ESPE, St. Paul, MN), or (4) MI Paste Plus (10% CPP-ACP plus 900 ppm fluoride crème, MI Paste Plus, GC America, Alsip, IL). The formulations of each product are described in Table 1. Computer-aided randomization was used in a manner that each specimen from the same tooth was distributed to one of the four different groups, akin to a split-plot design with each tooth constituting a plot. For the treatment phase of the experiment, each product was strictly applied using their respective manufacturers’ directions (Table 2), daily, for a period ranging from 10 to 30 days. Since amount of product for each treatment was limited, one third of the specimens for each treatment group were removed after 10 days of follow-up, with an additional one third removed after 20 days (exact numbers for each treatment at each time point are given in Table 3). This design allowed for the maximum amount of information at the early time points while still retaining sufficient sample size for later time points. The applications were performed once in the morning and again in the evening. The products were applied in the form of slurry, formed by one part of the product and three parts of artificial saliva, directly over the lesions as previously published.15 The artificial saliva consisted of 2.2 g/L gastric mucin, 0.381 g/L NaCl, 0.231 g/L CaCl2, 0.738 g/L KH2PO4, 1.114 g/L KCl, 0.02% sodium azide, and trace of NaOH to pH 7.0.26 Five liters of artificial saliva were freshly prepared on a daily basis. One liter was always removed to produce the slurries. The remaining 4 liters were equally divided among 4 containers, one for each group. Specimens from the same group were collectively stored in the same container. Once the treatment was completed, the specimens were rinsed in deionized water for 5 seconds to remove the treatment solution. Immediately after the morning treatment, all specimens were submitted to pH cycling, as described below. Before the evening treatment, all specimens were placed back in their own artificial saliva container for 10 minutes.
Table 1.
Products tested and respective compositions.
| Product | Composition |
|---|---|
| ControlRX | Sodium Fluoride 1.1% w/w, water, Sorbitol, Hydrated Silica, Glycerin, MICRODENT® 2.0% w/v – a patented ULTRAMULSION® of Dimethicone and Poloxamer 407, PEG 12, Flavor, Cellulose Gum, Sodium Lauryl Sulfate, Titanium Dioxide, Sodium Saccharin |
| MI Paste | Pure water, glycerol, 10% by weight of CPP-ACP, D-sorbitol, CMC-Na, propylene glycol, silicon dioxide, titanium dioxide, xylitol, phosphoric acid, flavoring, zinc oxide, sodium saccharin, ethyl p-hydroxybenzoate, magnesium oxide, guar gum, propyl p-hydroxybenzoate, butyl p-hydroxybenzoate |
| MI Paste Plus | Pure water, glycerol, 10% by weight of CPP-ACP, d-sorbitol, CMC-Na, propylene glycol, silicon dioxide, titanium dioxide, xylitol, phosphoric acid, sodium fluoride, flavoring, sodium saccharin, ethyl p-hydroxybenzoate, propyl p-hydroxybenzoate, butyl p-hydroxybenzoate |
Table 2.
Products tested and correspondent application procedures
| Product | Directions for use |
|---|---|
| ControlRx | Applied once every 12 hours, in a form of slurry, made with 1 part of toothpaste to 3 parts of artificial saliva. Product was applied for 2 minutes and rinsed with deionized water for 5 seconds. |
| MI Paste | Applied once every 12 hours, in a form of slurry, made with 1 part of toothpaste to 3 parts of artificial saliva. Product was applied for 3 minutes and rinsed with deionized water for 5 seconds. No application of fluoride was done prior to this treatment. |
| MI Paste Plus | Applied once every 12 hours, in a form of slurry, made with 1 part of toothpaste to 3 parts of artificial saliva. Product was applied for 3 minutes and rinsed with deionized water for 5 seconds. |
Table 3.
Summary statistics for fluorescence loss and lesion area for each treatment group at baseline and each follow-up time point. Entries in each cell are mean, standard deviation (σ), and sample size (n).
| Fluorescence Loss | Baseline | Day 10 | Day 20 | Day 30 |
|---|---|---|---|---|
| Control | −12.77 (5.42) (n=35) | −11.55 (2.58) (n=35) | −10.02 (2.24) (n=25) | −8.38 (1.40) (n=11) |
| F5000 | −13.36 (6.55) (n=35) | −8.10 (3.00) (n=35) | −7.98 (3.27) (n=23) | −7.81 (2.15) (n=11) |
| MI Paste Plus | −11.01 (3.52) (n=35) | −10.11 (2.61) (n=35) | −9.27 (2.57) (n=22) | −8.92 (1.23) (n=11) |
| MI Paste | −12.09 (4.83) (n=34) | −10.8 (3.01) (n=34) | −10.86 (2.86) (n=22) | −10.23 (2.31) (n=11) |
| Lesion Area | ||||
| Control | 7.03 (1.90) (n=35) | 3.71 (2.04) (n=35) | 2.89 (1.71) (n=25) | 2.07 (1.83) (n=11) |
| F5000 | 7.45 (2.04) (n=35) | 0.85 (1.01) (n=35) | 0.80 (1.08) (n=23) | 0.70 (0.98) (n=11) |
| MI Paste Plus | 7.43 (2.11) (n=35) | 2.90 (1.67) (n=35) | 2.06 (1.42) (n=22) | 2.27 (1.12) (n=11) |
| MI Paste | 6.70 (2.10) (n=34) | 3.04 (2.18) (n=34) | 3.21 (2.19) (n=21) | 3.23 (2.27) (n=10) |
pH-cycling
Standard pH-cycling conditions were used in a daily schedule of 3 cycles, each of 30 minutes of demineralization and 2.5 hours of remineralization27, followed by an “overnight” period of 6 hours in artificial saliva. Four liters of each pH-cycling solution were placed in different containers, which were also labeled accordingly. The deionized water was placed in a third container. Specimens underwent pH-cycling together. Before and between each remineralization-demineralization period, the specimens were thoroughly rinsed with deionized water. The pH of the artificial saliva was checked daily and the solution replaced weekly. Each group had its own container of artificial saliva to avoid any type of contamination by other treatment solutions. The remineralization solutions contained 1.5 mM CaCl2, 0.9 mM KH2PO4, 130 mM KCl, and 20 mM Hepes pH 7.0, and the demineralization solution 1.5 mM CaCl2, 0.9 mM KH2PO4, and 50 mM acetic acid adjusted to pH 5.0. The pH-cycle solutions were refreshed daily.28
QLF analysis and outcome measures
Specimen images were acquired using a quantitative light fluorescence (QLF) system, the Inspektor QLF System (Inspektor Research System BV, Amsterdam, The Netherlands), which comprises a light from a xenon arc lamp with blue filter (λ = 488 nm, 10–20 mW cm−2) and camera connected to a computer. Images were captured and analyzed with the software provided by the manufacturer (Inspektor™ Pro Software, version 2.0.0.32, Amsterdam, The Netherlands). To ensure that images were captured from the same angle and in the same camera position, the video-repositioning tool of the software was used (correlation set to 0.90). This software holds the baseline image as a reference when trying to correlate the subsequent images, based on their similar geometry of the fluorescence intensities.29 The importance of this step is to permit a very precise comparison between the images captured at different moments in time.30, 31 Additionally, a handmade polyvinyl siloxane (Express STD, 3M ESPE) matrix was fabricated to help standardize the position of the camera over the specimen. Specimens were kept hydrated in deionized water until immediately before analysis. For the image capturing process, each lesion was dried for 5 seconds with an air syringe and the image was acquired under completely darkened laboratory conditions.
The effect of the remineralization protocols on the WSL were quantified by mean fluorescence loss (ΔF in %), and the corresponding lesion area (mm2). Images were analyzed using the Inspektor™ Pro Software. Identification and delimitation of each lesion on the software were done manually. The visual identification of the lesion is made easy because the demineralized tissue appears as a dark area surrounded by bright green fluorescing sound tissue.32 To delimitate the lesion, a patch is drawn around each dark area with its borders in sound enamel.31 The software then virtually reconstructs the fluorescence levels of the lesion using the fluorescence radiance of the surrounding sound enamel as reference.31 Pixels inside the patch were considered part of the lesion when the relative fluorescence loss exceeded 5% threshold.33 Fluorescence loss is the difference in percentage between the intensity of the green fluorescence of the sound enamel (high) and the lesion (low). The corresponding lesion area is calculated based on the patch borders determined by the software. The analyses patch and the surface contours used in the baseline image are copied by the software for all the consequential images.
The images were captured after the lesions were created (baseline) and every 10 days until the last day of treatment (Figure 1) for a total of 2 to 4 images for each specimen, depending on the treatment group. Specimens removed from the groups for analysis did not have further images captured.
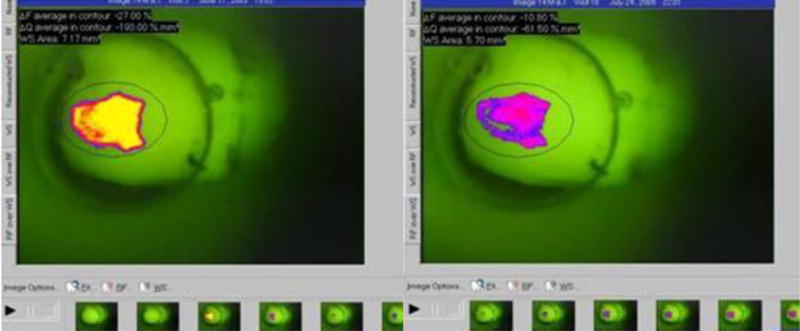
Figure 1.
QLF screen image representing the analysis process of the circumscripted white spot lesion at baseline and after treatment was applied overtime*
* Note the yellow color on the body of the lesion denoting area of higher scattering of light and, consequently, more demineralized tissue. On the second image, note the purple tone in the body of the lesion indicating less scattering of light and, consequently, a more remineralized tissue. In addition, the lesion area is slightly reduced, especially on the borders of the lesion.
Statistical analysis
The QLF assessments (fluorescence loss and lesion area) were analyzed using a hierarchical mixed effects model. Since interest was in assessing improvement from baseline, the outcome in each case was the difference between the follow-up (days 10, 20, and 30) and baseline values. To account for the repeated measurements on each specimen a random effect for tooth and a random effect for specimen nested within tooth was incorporated. The fixed effects in the model included treatment, time, and the treatment*time interaction. Contrasts for differences between treatment groups at each follow-up time point were estimated based on the model, along with 95% confidence intervals for the differences. P-values were adjusted for multiple comparisons to control the false-discovery rate using the method of Benjamini and Yekutieli, which controls for multiple dependent hypothesis tests.34 Level of significance for both analyses was set at 0.05. The random effects models were fit using the lme package with R version 3.0.3.35, 36
RESULTS
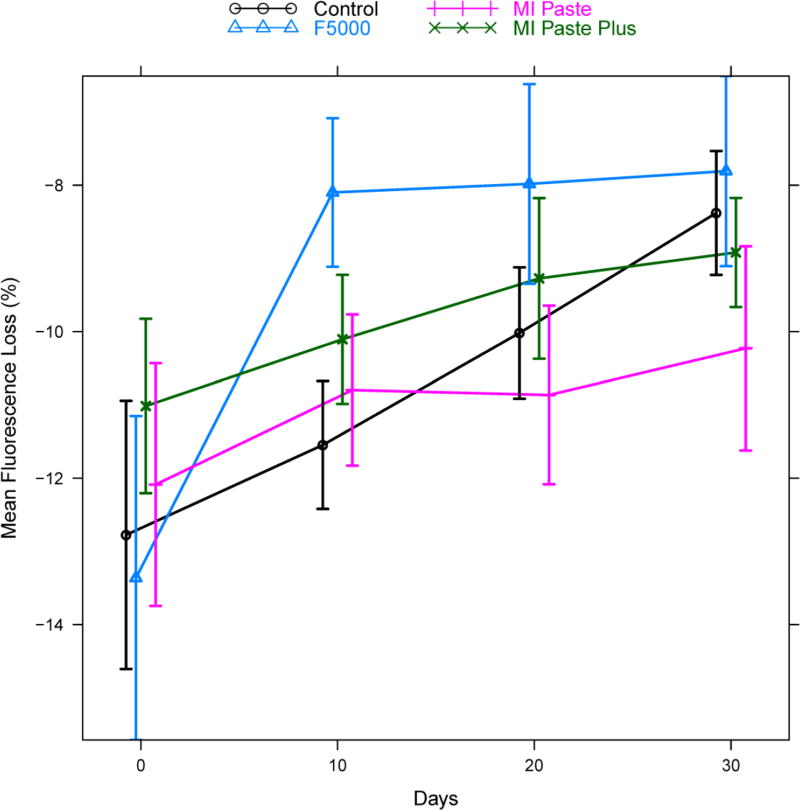
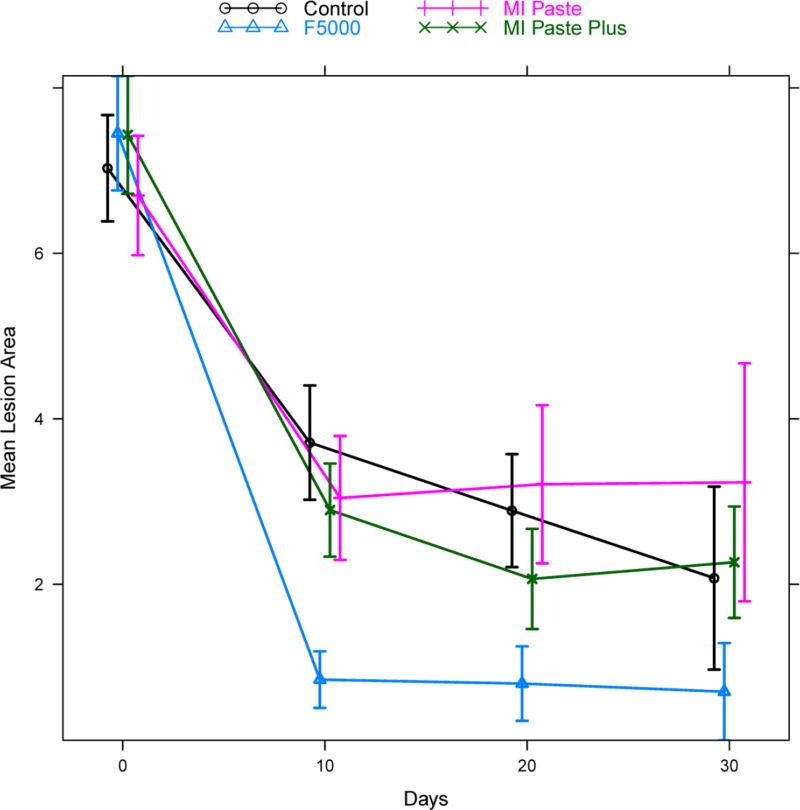
Summary statistics (mean, standard deviation (σ), and sample size (n)) for both fluorescence loss and lesion area are given in Table 3 for each treatment group at each follow-up time point. Figures 2 and 3 give corresponding plots of the mean fluorescence loss and mean lesion area, respectively, with vertical bars indicating +/− two standard errors to give a rough approximation of a 95% confidence interval for the mean.
Figure 2.
Mean fluorescence loss by treatment group and time. Vertical bars indicate +/− two standard errors and give a rough approximation of a 95% confidence interval for the mean. Points are slightly staggered at each time point for better visualization of the vertical bars.
Figure 3.
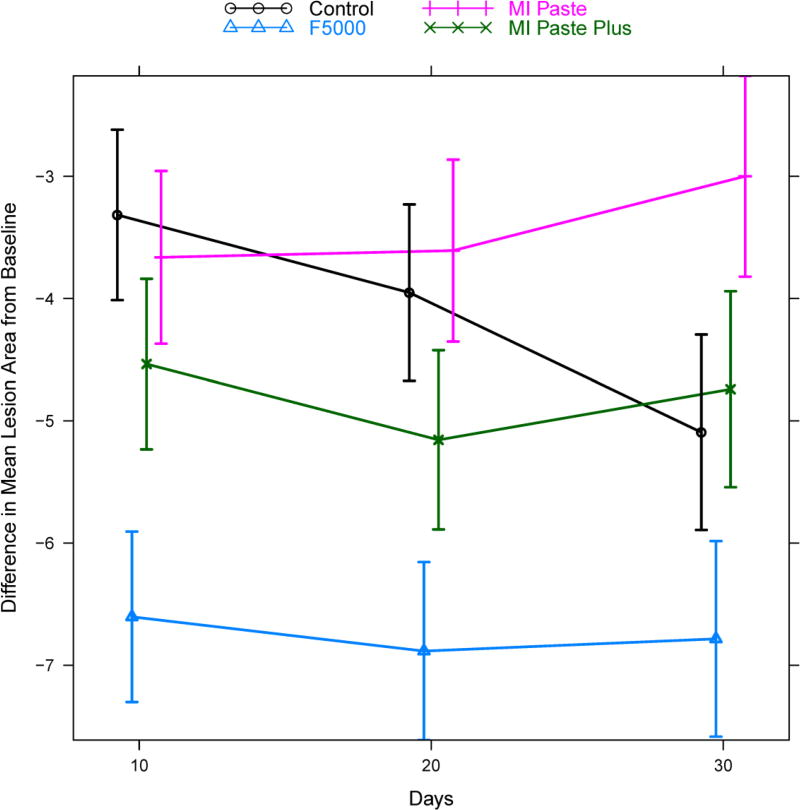
Mean lesion area by treatment group and time. Vertical bars indicate +/− two standard errors and give a rough approximation of a 95% confidence interval for the mean. Points are slightly staggered at each time point for better visualization of the vertical bars.
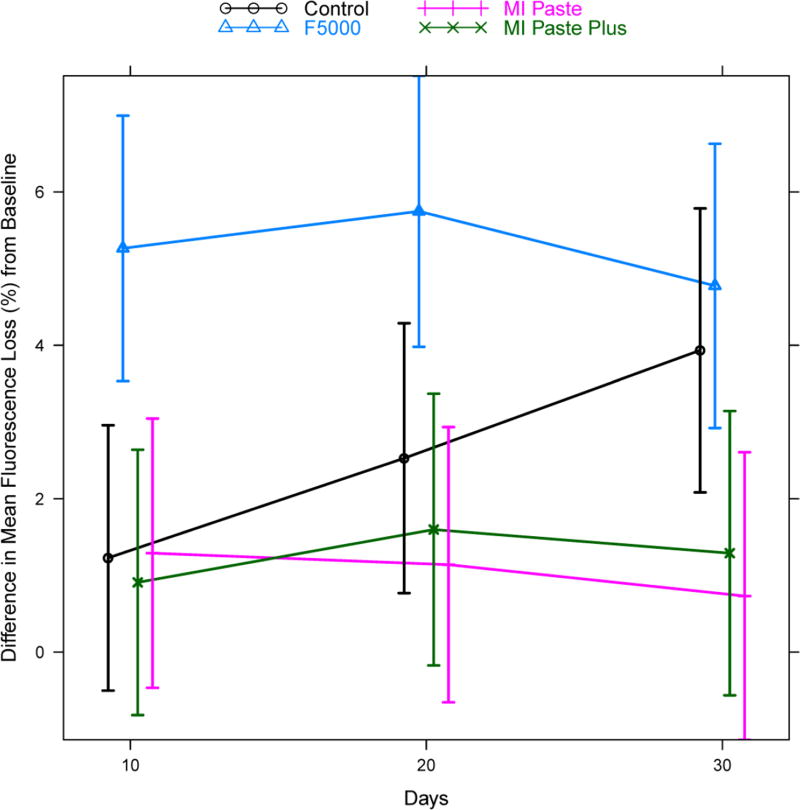
Analysis of difference from baseline for fluorescence loss data using a hierarchical mixed effects model indicated that the pattern of change in fluorescence loss was not the same for the four groups (significant treatment * time interaction: p<0.0001, see Figure 4). Hence, treatment comparisons were conducted separately at each follow-up time point. Table 4 displays the estimated difference between treatment groups at each time point, along with 95% confidence intervals for the difference and adjusted p-values. The F5000 treatment group showed rapid change by day 10 compared to all other groups (F5000 versus Control: adjusted p=0.02, MI Paste: adjusted p=0.02, MI Paste Plus: adjusted p=0.01). Differences between F5000 and MI Paste remained significant at days 20 and 30, while differences between F5000 and MI Paste Plus were significant at day 20 and only marginally significant at day 30 (adjusted p=0.08). Differences between F5000 and Control were only marginally significant at day 20 (adjusted p=0.08) and non-significant at day 30. The Control group showed some improvement relative to MI Paste and MI Paste Plus at day 30, though this difference was not statistically significant after adjustment for multiple comparisons (MI Paste: adjusted p=0.11, MI Paste Plus: adjusted p=0.29). The Control group was not different statistically from either MI Paste or MI Paste Plus on days 10 and 20. No statistically significant differences were found between MI Paste and MI Paste Plus at any of the follow-up time points.
Figure 4.
Model predicted estimates of the mean difference in fluorescence loss from baseline, for each treatment group at each follow-up time point. Estimates were based on the hierarchical random effects model with random effects for tooth and specimen nested within tooth and fixed effects for treatment, time, and treatment*time interaction. Vertical bars indicate model based estimates of 95% confidence intervals for the mean difference from baseline. Points are slightly staggered at each time point for better visualization of the vertical bars.
Table 4.
Estimated mean differences between treatments for differences from baseline for fluorescence loss. Estimates of treatment contrasts were based on a hierarchical random effects model with random effects for tooth and specimen nested within tooth and fixed effects for treatment, time, and treatment*time interaction. Point estimates for treatment comparisons are given for each time point, along with 95% confidence intervals for the differences, unadjusted p-values, and p-values adjusting for multiple comparisons.
| Treatment Comparison | Day | Estimate | 95% CI | P-value | Adj P-value |
|---|---|---|---|---|---|
| F5000 vs Control | 10 | 4.04 | (1.59, 6.48) | 0.001 | 0.02 |
| F5000 vs Control | 20 | 3.22 | (0.73, 5.71) | 0.01 | 0.08 |
| F5000 vs Control | 30 | 0.84 | (−1.78, 3.46) | 0.53 | 1.00 |
|
| |||||
| F5000 vs MI Paste Plus | 10 | 4.36 | (1.91, 6.8) | <0.001 | 0.01 |
| F5000 vs MI Paste Plus | 20 | 4.15 | (1.65, 6.65) | 0.001 | 0.02 |
| F5000 vs MI Paste Plus | 30 | 3.49 | (0.86, 6.11) | 0.008 | 0.08 |
|
| |||||
| F5000 vs MI Paste | 10 | 3.97 | (1.51, 6.44) | 0.001 | 0.02 |
| F5000 vs MI Paste | 20 | 4.61 | (2.09, 7.13) | <0.001 | 0.01 |
| F5000 vs MI Paste | 30 | 4.04 | (1.41, 6.68) | 0.002 | 0.03 |
|
| |||||
| MI Paste Plus vs Control | 10 | −0.32 | (−2.77, 2.13) | 0.80 | 1.00 |
| MI Paste Plus vs Control | 20 | −0.93 | (−3.43, 1.57) | 0.46 | 1.00 |
| MI Paste Plus vs Control | 30 | −2.64 | (−5.26, −0.02) | 0.05 | 0.29 |
|
| |||||
| MI Paste vs Control | 10 | 0.06 | (−2.4, 2.53) | 0.96 | 1.00 |
| MI Paste vs Control | 20 | −1.39 | (−3.9, 1.12) | 0.27 | 1.00 |
| MI Paste vs Control | 30 | −3.2 | (−5.84, −0.57) | 0.02 | 0.11 |
|
| |||||
| MI Paste Plus vs MI Paste | 10 | −0.38 | (−2.85, 2.08) | 0.76 | 1.00 |
| MI Paste Plus vs MI Paste | 20 | 0.46 | (−2.06, 2.98) | 0.72 | 1.00 |
| MI Paste Plus vs MI Paste | 30 | 0.56 | (−2.08, 3.2) | 0.68 | 1.00 |
The results for lesion area are presented in Table 5 and Figure 5. The pattern of change was again significantly different for the four groups (treatment * time interaction p<0.0001). The F5000 treatment group again presented rapid change at day 10 compared to all other groups (Control: adjusted p<0.001, MI Paste: adjusted p<0.001, MI Paste Plus: adjusted p<0.001). This difference remained statistically significant across days 20 and 30 as well (adjusted p<0.001 in nearly all cases). MI Paste Plus did not differ significantly from MI Paste at day 10, but did exhibit significant improvement relative to MI Paste at days 20 (adjusted p=0.008) and 30 (adjusted p=0.008). In contrast, while MI Paste Plus was significantly better relative to Control on days 10 (adjusted p=0.04) and 20 (adjusted p=0.05), this improvement was not evident on day 30. The MI Paste group did not differ significantly from the Control group on days 10 and 20, however the Control group was significantly better than MI Paste on day 30 (adjusted p=0.001).
Table 5.
Estimated mean differences between treatments for differences from baseline for lesion area. Estimates of treatment contrasts were based on a hierarchical random effects model with random effects for tooth and specimen nested within tooth and fixed effects for treatment, time, and treatment*time interaction. Point estimates for treatment comparisons are given for each time point, along with 95% confidence intervals for the differences, unadjusted p-values, and p-values adjusting for multiple comparisons.
| Treatment Comparison | Day | Estimate | 95% CI | P-value | Adj P-value |
|---|---|---|---|---|---|
| F5000 vs Control | 10 | −3.29 | (−4.19, −2.39) | <0.001 | <0.001 |
| F5000 vs Control | 20 | −2.93 | (−3.87, −1.99) | <0.001 | <0.001 |
| F5000 vs Control | 30 | −1.69 | (−2.75, −0.64) | 0.002 | 0.008 |
|
| |||||
| F5000 vs MI Paste Plus | 10 | −2.07 | (−2.97, −1.17) | <0.001 | <0.001 |
| F5000 vs MI Paste Plus | 20 | −1.73 | (−2.68, −0.78) | <0.001 | 0.002 |
| F5000 vs MI Paste Plus | 30 | −2.04 | (−3.10, −0.99) | <0.001 | 0.001 |
|
| |||||
| F5000 vs MI Paste | 10 | −2.94 | (−3.85, −2.04) | <0.001 | <0.001 |
| F5000 vs MI Paste | 20 | −3.28 | (−4.24, −2.32) | <0.001 | <0.001 |
| F5000 vs MI Paste | 30 | −3.79 | (−4.86, −2.71) | <0.001 | <0.001 |
|
| |||||
| MI Paste Plus vs Control | 10 | −1.22 | (−2.12, −0.32) | 0.007 | 0.04 |
| MI Paste Plus vs Control | 20 | −1.2 | (−2.15, −0.26) | 0.01 | 0.05 |
| MI Paste Plus vs Control | 30 | 0.35 | (−0.70, 1.41) | 0.51 | 1.0 |
|
| |||||
| MI Paste vs Control | 10 | −0.35 | (−1.25, 0.56) | 0.45 | 1.0 |
| MI Paste vs Control | 20 | 0.34 | (−0.61, 1.30) | 0.48 | 1.0 |
| MI Paste vs Control | 30 | 2.09 | (1.02, 3.17) | <0.001 | 0.001 |
|
| |||||
| MI Paste Plus vs MI Paste | 10 | −0.87 | (−1.78, 0.03) | 0.06 | 0.24 |
| MI Paste Plus vs MI Paste | 20 | −1.55 | (−2.51, −0.59) | 0.001 | 0.008 |
| MI Paste Plus vs MI Paste | 30 | −1.74 | (−2.82, −0.67) | 0.001 | 0.008 |
Figure 5.
Model predicted estimates of the mean difference in lesion area from baseline, for each treatment group at each follow-up time point. Estimates were based on the hierarchical random effects model with random effects for tooth and specimen nested within tooth and fixed effects for treatment, time, and treatment*time interaction. Vertical bars indicate model based estimates of 95% confidence intervals for the mean difference from baseline. Points are slightly staggered at each time point for better visualization of the vertical bars.
DISCUSSION
In this laboratory study, the methodologies used to quantify remineralization indicated that the fluoride group provides greater remineralizing effect over time as compared with the Control and the other treatment groups. Although we could not find other studies in the literature with strictly comparable methodology, it is reasonable to state that our findings are in agreement with those of Pulido and colleagues.15
The efficacy of fluoride as a remineralizing agent is well described in the literature although discussion about the relationship between dosage and effect persists.37 Presumably, the high concentration fluoride product used in this study (F5000) promotes some degree of remineralization over the period of 30 days. Indeed, this was first observed on the 10th day. The F5000 had its most pronounced effect during the first 10 days, with a rapid rate of remineralization that tended to plateau after that. This was observed for both outcomes. Such behavior was expected since it had been described before. Topical application of a high-concentration fluoride solution presented higher initial rates of mineral deposition, but lower subsequently. It was then hypothesized that a fluoride-enhanced deposition occurred primarily in the surface layer of the lesion, leading to blocking of the surface layer pores.38 Another study done using a computer simulation model concluded that lesions cannot be repaired by more than 80% of the mineral originally removed due to the surface layer being fully remineralized.39 More recently, these previous findings have been challenged. Elevated fluoride treatments (5000 ppm) were shown to give increased remineralization when compared with traditional fluoride products (1500 ppm) in subsurface lesions, using pH-cycling model.37 The authors explained the extended fluoride dose-response correlating fluoride deposition with lesion depth, pointing out that a fluoride diffusion would be responsible for driving fluoride deeper into the lesion, whenever the external level of fluoride is elevated. Although it is important to mention that the QLF presents greater sensitivity to detect surface changes20, that seemed to be the case in our study. Regarding the QLF sensitivity to mineral changes, a statistically significant correlation between QLF and Tranverse MicroRadiography (TMR), which is the gold-standard technique for quantifying mineral changes of early carious lesions in vitro, has been previously shown.20, 40
Similar remineralizing patterns were also observed for MI Paste and MI Paste Plus groups. At some point over the 30 days study period, all the treatment groups appeared to reach a turning point where the pace of the remineralization slowed down or stopped. This was especially true for the lesion area outcome, but less marked for fluorescence loss. The exception to this was the Control group, which exhibited a continuous and also effective remineralizing action for fluorescence loss, being exposed only to low concentrations of calcium and phosphate. The effect of calcium ion concentration on the remineralization process has been studied before. A solution with high calcium content is believed to rapidly precipitate ions on the superficial zones of the lesion, preventing the remineralization process from occurring in the body of the lesion.41, 42. In the MI Paste and MI Paste Plus groups, the presence of CPP in those products would inherently prevent rapid precipitation of calcium and phosphate. Indeed, this is the primary action of CPP. It stabilizes clusters of calcium, phosphate, and hydroxide ions at high concentrations as amorphous nanocomplexes (amorphous calcium phosphate, CPP-ACP), preventing spontaneous phase transformations.43 The same phenomenon can be observed with calcium, phosphate, and fluoride (amorphous calcium fluoride phosphate, CPP-ACFP).44 Neutral ions HF0 and CaHPO40 are then localized at the tooth surface, allowing the diffusion of these ions deep into the lesion and enabling remineralization to occur throughout the body of the lesion.45 That may help to explain the slower and attenuated remineralization patterns, when compared to F5000, observed in these groups for fluorescence loss. Additionally, the low concentration of calcium and phosphate in the pH-balanced artificial saliva were probably not enough to provoke rapid precipitation either.
Interestingly, both CPP-ACP products, MI Paste and MI Paste Plus, presented similar or even lower performances for mean fluorescence loss and lesion area when compared with the Control group. For this observation, several important factors may have played a role. Since the pH-balanced artificial saliva formulation purposely contained CaCl2 and KH2PO4, to better reproduce the oral environment, a certain degree of remineralization caused by exposure of the groups to this solution occurred. This potential remineralizing effect has been observed both in vitro and in vivo, causing regression of carious lesions.15, 31, 46 In fact, the effect of CPP-ACP on enamel surface has been previously shown to be similar to the remineralizing effect of saliva.47 Yet, the formation of a biofilm as seen in vivo, and its potential to directly influence the ionic changes in the oral environment are very difficult to recapitulate with an artificial saliva substitute48, leading to a second consideration. The lack of biofilm and, consequently, the optimal environment for the CPP-ACP to create a state of supersaturation of calcium and phosphate ions could have prevented this system from properly exerting a remineralizing action of these products.
In light of the issue stated above, it is logical to postulate that due to the lack of a biofilm, all products tested were completely washed away by the deionized water immediately after the treatment times were completed. As a result, the treatment times may have been too short to provide substantial remineralizing benefits. The treatment exposure time was applied following the manufacturer’s directions and should therefore reflect the exposure that would occur clinically. Despite the acid challenge that specimens were subjected to after the treatments were completed, the acidic activation of CPP-ACP molecules separating ACP from casein, as previously suggested49, was very unlikely because of the absence of material on the tooth surface, i.e. biofilm. If this is true, the ACP would not precipitate and, consequently, promote crystal growth. Then, the remineralization would come primarily from the artificial saliva.
Does the addition of fluoride to CPP-ACP enhance its remineralizing capacity? The outcome measures for MI Paste Plus compared to MI Paste group indicates that the addition of fluoride does enhance remineralization. Based on mean fluorescence loss, the F5000 group showed improved remineralization relative to MI Paste Plus group, but did not differ statistically from the Control group at the end of 30 days.
CONCLUSIONS
Based on the findings of this study and within its limitations, we conclude that 1.1% NaF dentifrice demonstrated greater remineralization ability than 10% CPP-ACP topical tooth crème. When compared to the fluoride-containing 10% CPP-ACP Plus, the QLF analysis also demonstrated a greater remineralization ability of the 1.1% NaF dentifrice for the outcome mean lesion area. However, the 1.1% NaF dentifrice was only as effective as the Control to reduce fluorescence loss at the end of the 30 days period. Saliva also has the ability to exert an important remineralization effect over time. Moreover, the period of 10 days may be enough to remineralize white spot lesions in vitro when a dentifrice containing 1.1% NaF (5000 ppm) is used as therapeutic agent. The addition of fluoride to 10% CPP-ACP seems to enhance its remineralization ability based on our fluorescence loss studies.
Supplementary Material
Clinical Significance.
This study showed that a 1.1% NaF dentifrice (5000 ppm) demonstrated greater remineralization ability than the CPP-ACP topical tooth crème and that the addition of fluoride to its formulation seems to enhance remineralization. Saliva also has the ability to exert an important remineralization effect over time.
Acknowledgments
This project was supported by Award Number UL1RR025747 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Gustavo M. S. Oliveira, University of Louisville.
André V. Ritter, University of North Carolina at Chapel Hill.
Harald O. Heymann, University of North Carolina at Chapel Hill.
Edward Swift, Jr., University of North Carolina at Chapel Hill.
Terry Donovan, University of North Carolina at Chapel Hill.
Guy Brock, University of Louisville.
Tim Wright, University of North Carolina at Chapel Hill.
References
- 1.Featherstone JD. Caries prevention and reversal based on the caries balance. Pediatr Dent. 2006;28(2):128–32. discussion 92-8. [PubMed] [Google Scholar]
- 2.Backer Dirks O. Posteruptive changes in dental enamel. Journal of Dental Research. 1966;45:503–11. [Google Scholar]
- 3.Silverstone LM. Remineralization phenomena. Caries Res. 1977;11(Suppl 1):59–84. doi: 10.1159/000260296. [DOI] [PubMed] [Google Scholar]
- 4.Hicks J, Flaitz C. Role of remineralizing fluid in in vitro enamel caries formation and progression. Quintessence Int. 2007;38(4):313–9. [PubMed] [Google Scholar]
- 5.Featherstone JD, Behrman JM, Bell JE. Effect of whole saliva components on enamel demineralization in vitro. Crit Rev Oral Biol Med. 1993;4(3–4):357–62. doi: 10.1177/10454411930040031401. [DOI] [PubMed] [Google Scholar]
- 6.ten Cate JM, Featherstone JD. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2(3):283–96. doi: 10.1177/10454411910020030101. [DOI] [PubMed] [Google Scholar]
- 7.Dean HT, McKay FS, Elvove E. Mottled enamel survey of Bauxite, Ark., ten years after a change in the public water supply. Publ Health Rep. 1938;53(1736) [Google Scholar]
- 8.Rose RK. Binding characteristics of Streptococcus mutans for calcium and casein phosphopeptide. Caries Res. 2000;34(5):427–31. doi: 10.1159/000016618. [DOI] [PubMed] [Google Scholar]
- 9.Shaw JH, Ensfield BJ, Wollman DH. Studies on the relation of dairy products to dental caries in caries-susceptible rats. J Nutr. 1959;67(2):253–73. doi: 10.1093/jn/67.2.253. [DOI] [PubMed] [Google Scholar]
- 10.Rosen S, Min DB, Harper DS, Harper WJ, Beck EX, Beck FM. Effect of cheese, with and without sucrose, on dental caries and recovery of Streptococcus mutans in rats. J Dent Res. 1984;63(6):894–6. doi: 10.1177/00220345840630061601. [DOI] [PubMed] [Google Scholar]
- 11.Jensen ME, Wefel JS. Effects of processed cheese on human plaque pH and demineralization and remineralization. Am J Dent. 1990;3(5):217–23. [PubMed] [Google Scholar]
- 12.Anticariogenic phosphopeptides. United States: 1991. Reynolds ECNB, AU), inventor The University of Melbourne (Victoria, AU),Victorian, Dairy Industry Authority (Victoria, AU), assignee. [Google Scholar]
- 13.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76(9):1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds EC, Cain CJ, Webber FL, Black CL, Riley PF, Johnson IH, et al. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J Dent Res. 1995;74(6):1272–9. doi: 10.1177/00220345950740060601. [DOI] [PubMed] [Google Scholar]
- 15.Pulido MT, Wefel JS, Hernandez MM, Denehy GE, Guzman-Armstrong S, Chalmers JM, et al. The inhibitory effect of MI paste, fluoride and a combination of both on the progression of artificial caries-like lesions in enamel. Oper Dent. 2008;33(5):550–5. doi: 10.2341/07-136. [DOI] [PubMed] [Google Scholar]
- 16.Azarpazhooh A, Limeback H. Clinical efficacy of casein derivatives: a systematic review of the literature. J Am Dent Assoc. 2008;139(7):915–24. doi: 10.14219/jada.archive.2008.0278. quiz 94-5. [DOI] [PubMed] [Google Scholar]
- 17.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bader JD. Casein phosphopeptide-amorphous calcium phosphate shows promise for preventing caries. Evid Based Dent. 2010;11(1):11–2. doi: 10.1038/sj.ebd.6400701. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Liu X, Dai J, Jiang Z, Guo T, Ding Y. Effect of remineralizing agents on white spot lesions after orthodontic treatment: a systematic review. Am J Orthod Dentofacial Orthop. 2013;143(3):376–82. e3. doi: 10.1016/j.ajodo.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Cochrane NJ, Walker GD, Manton DJ, Reynolds EC. Comparison of quantitative light-induced fluorescence, digital photography and transverse microradiography for quantification of enamel remineralization. Aust Dent J. 2012;57(3):271–6. doi: 10.1111/j.1834-7819.2012.01706.x. [DOI] [PubMed] [Google Scholar]
- 21.Hafstrom-Bjorkman U, Sundstrom F, de Josselin de Jong E, Oliveby A, Angmar-Mansson B. Comparison of laser fluorescence and longitudinal microradiography for quantitative assessment of in vitro enamel caries. Caries Res. 1992;26(4):241–7. doi: 10.1159/000261446. [DOI] [PubMed] [Google Scholar]
- 22.Emami Z, al-Khateeb S, de Josselin de Jong E, Sundstrom F, Trollsas K, Angmar-Mansson B. Mineral loss in incipient caries lesions quantified with laser fluorescence and longitudinal microradiography. A methodologic study. Acta Odontol Scand. 1996;54(1):8–13. doi: 10.3109/00016359609003502. [DOI] [PubMed] [Google Scholar]
- 23.Gladwell J, Simmons D, Wright JT. Remineralization potential of a fluoridated carbamide peroxide whitening gel. J Esthet Restor Dent. 2006;18(4):206–12. doi: 10.1111/j.1708-8240.2006.00021_1.x. discussion 12-3. [DOI] [PubMed] [Google Scholar]
- 24.Feagin FF, Clarkson BH, Wefel JS. Chemical and physical evaluation of dialyzed-reconstituted acidified gelatin surface lesions of human enamel. Caries Res. 1985;19(3):219–27. doi: 10.1159/000260847. [DOI] [PubMed] [Google Scholar]
- 25.Wefel JS, Heilman JR, Jordan TH. Comparisons of in vitro root caries models. Caries Res. 1995;29(3):204–9. doi: 10.1159/000262070. [DOI] [PubMed] [Google Scholar]
- 26.Wongkhantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent. 2006;34(3):214–20. doi: 10.1016/j.jdent.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.ten Cate JM, Duijsters PP. Alternating demineralization and remineralization of artificial enamel lesions. Caries Res. 1982;16(3):201–10. doi: 10.1159/000260599. [DOI] [PubMed] [Google Scholar]
- 28.ten Cate JM, Buijs MJ, Damen JJ. pH-cycling of enamel and dentin lesions in the presence of low concentrations of fluoride. Eur J Oral Sci. 1995;103(6):362–7. doi: 10.1111/j.1600-0722.1995.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 29.Romane D, Bendika Z, Senakola E, Davies RM, Ellwood RP, Pretty IA. The effect of video repositioning on the reliability of light-induced fluorescence imaging: an in vivo study. Caries Res. 2005;39(5):397–402. doi: 10.1159/000086847. [DOI] [PubMed] [Google Scholar]
- 30.Mattousch TJ, van der Veen MH, Zentner A. Caries lesions after orthodontic treatment followed by quantitative light-induced fluorescence: a 2-year follow-up. Eur J Orthod. 2007;29(3):294–8. doi: 10.1093/ejo/cjm008. [DOI] [PubMed] [Google Scholar]
- 31.van der Veen MH, Mattousch T, Boersma JG. Longitudinal development of caries lesions after orthodontic treatment evaluated by quantitative light-induced fluorescence. Am J Orthod Dentofacial Orthop. 2007;131(2):223–8. doi: 10.1016/j.ajodo.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 32.de Josselin de Jong E, Sundstrom F, Westerling H, Tranaeus S, ten Bosch JJ, Angmar-Mansson B. A new method for in vivo quantification of changes in initial enamel caries with laser fluorescence. Caries Res. 1995;29(1):2–7. doi: 10.1159/000262032. [DOI] [PubMed] [Google Scholar]
- 33.Al-Khateeb S, Forsberg CM, de Josselin de Jong E, Angmar-Mansson B. A longitudinal laser fluorescence study of white spot lesions in orthodontic patients. Am J Orthod Dentofacial Orthop. 1998;113(6):595–602. doi: 10.1016/s0889-5406(98)70218-5. [DOI] [PubMed] [Google Scholar]
- 34.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1–2):279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- 35.Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. 2. New York: Springer; 2000. [Google Scholar]
- 36.R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing; 2011. p. R is a free software environment for statistical computing and graphics. It can be used to generate species distribution models using as a base data such as those made available through GBIF. [Google Scholar]
- 37.ten Cate JM, Buijs MJ, Miller CC, Exterkate RA. Elevated fluoride products enhance remineralization of advanced enamel lesions. J Dent Res. 2008;87(10):943–7. doi: 10.1177/154405910808701019. [DOI] [PubMed] [Google Scholar]
- 38.ten Cate JM, Jongebloed WL, Arends J. Remineralization of artificial enamel lesions in vitro. IV. Influence of fluorides and diphosphonates on short- and long-term reimineralization. Caries Res. 1981;15(1):60–9. doi: 10.1159/000260501. [DOI] [PubMed] [Google Scholar]
- 39.Greene WM, Newbrun E. A theoretical study of in vivo lesion repair using a controlled-release device. J Dent Res. 1986;65(9):1169–72. doi: 10.1177/00220345860650091101. [DOI] [PubMed] [Google Scholar]
- 40.Damen JJ, Exterkate RA, ten Cate JM. Reproducibility of TMR for the determination of longitudinal mineral changes in dental hard tissues. Adv Dent Res. 1997;11(4):415–9. doi: 10.1177/08959374970110040601. [DOI] [PubMed] [Google Scholar]
- 41.Silverstone LM, Hicks MJ, Featherstone MJ. Dynamic factors affecting lesion initiation and progression in human dental enamel. II. Surface morphology of sound enamel and carieslike lesions of enamel. Quintessence Int. 1988;19(11):773–85. [PubMed] [Google Scholar]
- 42.Silverstone LM, Hicks MJ, Featherstone MJ. Dynamic factors affecting lesion initiation and progression in human dental enamel. Part I. The dynamic nature of enamel caries. Quintessence Int. 1988;19(10):683–711. [PubMed] [Google Scholar]
- 43.Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds EC. Physicochemical characterization of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J Biol Chem. 2005;280(15):15362–9. doi: 10.1074/jbc.M413504200. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds EC, Cai F, Shen P, Walker GD. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res. 2003;82(3):206–11. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 45.Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008;42(2):88–97. doi: 10.1159/000113161. [DOI] [PubMed] [Google Scholar]
- 46.Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of remineralization and fluoride in the dynamic process of demineralization and remineralization (part 3) J Clin Pediatr Dent. 2004;28(3):203–14. doi: 10.17796/jcpd.28.3.w0610427l746j34n. [DOI] [PubMed] [Google Scholar]
- 47.Kargul B, Altinok B, Welbury R. The effect of casein phosphopeptide-amorphous calcium phosphate on enamel surface rehardening. An in vitro study. Eur J Paediatr Dent. 2012;13(2):123–7. [PubMed] [Google Scholar]
- 48.Kautsky MB, Featherstone JD. Effect of salivary components on dissolution rates of carbonated apatites. Caries Res. 1993;27(5):373–7. doi: 10.1159/000261567. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds EC, Walsh LJ. Additional aids to the remineralisation of tooth structure. Preservation and Restoration of Tooth Structure.: Knowledge Books and Software. 2005 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.