Abstract
Introduction
Medial knee instability is a key clinical parameter for assessing ligament injury and arthroplasty success, but current methods for measuring stability are typically either qualitative or involve ionizing radiation. The purpose of this study was to perform a preliminary analysis of whether ultrasound (US) could be used as an alternate approach for quantifying medial instability by comparing an US method with an approach mimicking the current gold standard fluoroscopy method.
Materials and Methods
US data from the medial knee were collected while cadaveric lower limbs (n=8) were loaded in valgus (10 Nm). During post-processing, the US gap width was measured by identifying the medial edges of the femur and tibia and computing the gap width between these points. For comparison, mimicked fluoroscopy (mFluoro) images were created from specimen-specific bone models, developed from segmented CT scans, and from kinematic data collected during testing. Then, gap width was measured in the mFluoro images based on two different published approaches, with gap width measured either at the most medial or most distal aspect of the femur.
Results
Gap width increased significantly with loading (p<0.001), and there were no significant differences between the US method (unloaded: 8.7±2.4 mm, loaded: 10.7±2.2 mm) and the mFluoro method that measured gap width at the medial femur. In terms of the change in gap width with load, no correlation with the change in abduction angle was observed, with no correlation between the various methods. Inter-rater reliability for the US method was high (0.899–0.952).
Conclusions
Ultrasound shows promise as a suitable alternative for quantifying medial instability without radiation exposure. However, the outstanding limitations of existing approaches and lack of true ground-truth data require that further validation work is necessary to better understand the clinical viability of an US approach for measuring medial knee gap width.
Keywords: MCL, Quantitative ultrasound, Instability
Introduction
Medial knee stability is an important characteristic of overall knee health, and can be an indicator for injury and long-term success following knee arthroplasty. A key contributor to medial knee stability is the medial collateral ligament (MCL), which, in the intact knee, is the primary restraint to tibial abduction under valgus loading, prevents external rotation when the knee is flexed [1], and serves as a secondary restraint to anterior tibial translation [2–4]. For knee arthroplasty patients, the MCL may play an even more crucial role, as other stabilizing ligaments are often sacrificed during surgery (e.g. the anterior cruciate ligament) [5], and maintaining a stable knee can be critical to implant success [6–10] and patient satisfaction [11]. The MCL is also frequently injured as it is involved in 42% of all ligamentous injuries in the knee [12].
Likewise, the accurate measurement of knee laxity can be critical to diagnosing ligament injuries, properly balancing the knee during knee arthroplasty, and evaluating knee instability post-operatively. Recent evidence also suggests that better outcomes during high tibial osteotomies could be achieved with pre-operative laxity measurements [13]. Clinically, valgus laxity is typically assessed via the valgus stress test in which the knee is slightly flexed, abduction is applied to the limb, and the size of the joint opening is estimated in order to grade injury severity. However, such approaches can be highly subjective; scoring systems depend on the perception of millimeters of differences in joint opening [14, 15], which may actually exceed the discriminating capacity of human judgment [16, 17]. In one study, the overall agreement between five clinicians in their assessment of mediolateral instability was reported to be only moderate [18, 19].
There are some quantitative approaches for measuring knee laxity; one of the most common involves the fluoroscopic imaging of the knee under valgus stress with the post-hoc analysis of tibiofemoral joint angle [20–23], medial gapping [24, 25] or the ratio between gapping in the knee of interest and the contralateral knee [26]. With these methods, researchers have shown differences in valgus laxity for patients receiving unicompartmental knee arthroplasty via different techniques [21], differences in laxity when stress tests are performed under anesthesia [23], the increase in medial gapping with simulated MCL injury [24, 27], and improvement with MCL repair [25]. However, despite these successes, some limitations of the approach remain, most notably that fluoroscopy exposes patients to ionizing radiation, which, even in low doses, can increase the risk for cancer [28]. Routine radiation exposure for both patients and healthcare providers [29] have made radiation exposure a growing problem in orthopaedics [30], such that a radiation-free alternative would be of interest. Other limitations of fluoroscopic approaches include that it can be expensive, have a lengthy setup time, and inconsistent limb positioning can influence gap measurements [31, 32]. Finally, the exact measurement of gapping depends on the identification of anatomical landmarks within the image, and due to the nature of the imaging modality, 3D information is compressed onto a 2D radiograph, such that 3D distance information is lost, and measurements may inaccurately represent the anatomical situation.
Therefore, the goal of this in situ study was to investigate whether ultrasound (US), an imaging modality with no ionizing radiation, and well-regarded for its safety, may be a suitable alternative for evaluating gapping in the knee. To our knowledge, there is only one study that has used an US method for measuring medial gapping in the knee [33], and there is no comprehensive comparison between this and the gold standard. We hypothesized that an US method would be capable of measuring the increase in the medial gap of the knee with valgus loading. Secondly, the US gap width was compared with measures of gap width that mimicked the gold standard fluoroscopic approach and estimated gap width based on two published methods. In the first mimicked fluoroscopy (mFluoro) approach, the gap was measured at the most medial aspect of the femur (mFluoro-Medial), based on [25], and in the second, the gap was measured at the most distal aspect of the femur (mFluoro-Distal), based on [24]. We hypothesized that the US method would show similar absolute measures of gap width as the mFluoro-Medial method, as both measured gap width at approximately the same anatomical location, and we hypothesized that the change in gap width would be correlated in all three methods. We also hypothesized that the change in gap width would correlate with the change in abduction angle for all methods. Finally, inter-rater reliability was estimated using intra-class correlation coefficients for the US method.
Materials and Methods
Ethical approval was obtained for this study from the Committee on Medical Ethics of the university. Eight fresh-frozen lower limbs disarticulated at the level of the hip were obtained from six elderly specimens (3 left, 5 female, aged 68–101). Specimens were only included if they were found to be free from prior lower limb arthroplasty, had intact ligaments, and no obvious deformity. The specimens were thawed and bi-cortical bone pins were inserted into the femur and tibia. Rigid marker frames with four reflective spheres each were then attached to the bone pins, and the refrozen specimens were imaged in full extension in a volumetric CT scanner (slice thickness: 0.6 mm Somatom Force, Siemens Healthcare GmbH, Erlangen, Germany). Using segmentation software (Mimics 18, Materialise NV, Leuven, Belgium), 3D models of the femur and tibia were generated. The reflective markers were also identified within the images, such that bone positioning during the tests could be recreated in the 3D model, an approach which has been shown to be accurate within ±0.2 mm [34]. Finally, the coordinate systems of the femur and tibia were determined automatically based on the geometric and inertial properties of the bones [35].
On the day prior to experimental testing, specimens were removed from the freezer and allowed to thaw, such that each specimen underwent two freeze-thaw cycles in total. For testing, intact specimens were positioned on a work bench with the knee in approximately 20 deg of flexion to mimic a clinically relevant posture [24, 36]. To reduce any undesired motion, a 5 mm bicortical bone pin was drilled through the femur to secure the bone to the bench, and rigid blocks were used to reduce rotation and translation. A 38 mm, 10 MHz ultrasound transducer (Ultrasonix Corp. Richmond, BC) was then positioned over the tibiofemoral gap and aligned with the MCL. With a depth setting of 2 mm, the probe has an approximate spatial resolution of 0.0019 mm through the depth and 0.297 mm along the ligament axis. Next, a researcher applied a 10 Nm valgus load to the knee [24, 37] by pulling on a load cell (Series 4, Mark-10, Copiague, NY, USA) that was attached to the tibia at the approximate level of the malleoli and positioning the other hand at the lateral femoral condyle (Fig. 1). During collections, LabVIEW (National Instruments Corporation, Austin, TX) was used to synchronize the data collected from the ultrasound transducer, and load cell, as well as the motion data from a six-camera motion capture system (MX40+, Vicon Motion Systems, Oxford, UK). Between 8–13 trials were collected for each specimen.
Fig. 1.
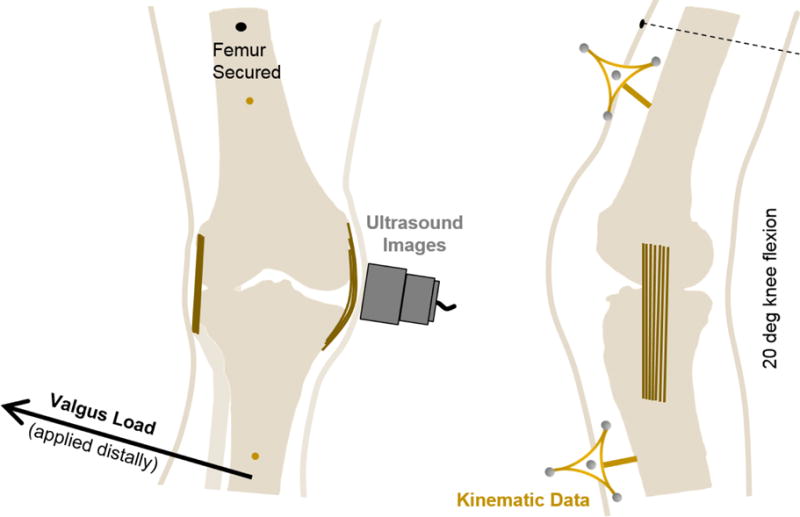
A schematic of the experimental setup. The femur was secured to a work bench at a flexion angle of approximately 20 deg. A 10 Nm valgus load was then applied to the tibia at the approximate level of the malleoli (please note that the arrow showing the application of load has been moved proximally for illustrative purposes). Simultaneously, ultrasound images were collected from the probe which was positioned over the MCL aligned along its proximal/distal axis, and motion data were collected from rigid marker frames attached to bone pins drilled through the femur and tibia.
During post-processing, the motion data were processed using a custom pipeline (Nexus 1.8.5, Vicon Motion Systems) and then transformed into anatomical translations and rotations using the Grood and Suntay convention [38]. Synchronized load cell data were used to identify the kinematic data point that corresponded with the unloaded and loaded state of the knee.
To create the mimicked fluoroscopy images, an automated method was developed that utilized the segmented CT scans and kinematic data (Fig. 2). First, the relative positioning of the femur and tibia at a single moment (e.g. unloaded) was determined from the motion data, and the bones segmented from the CT data were transformed into these positions using MATLAB (R2015B, Mathworks, Natick, MA). Next, the bones were reoriented to a neutral position, so that the projections were made from a consistent orientation for all specimens. Then, the femurs from both the unloaded and loaded timepoints were aligned, and a regular matrix of binary data points was constructed to create point clouds for the positions that fell within the boundaries of the bones. Finally, mimicked fluoroscopy images were made by summing all of the points within these clouds along the z-direction.
Fig. 2.
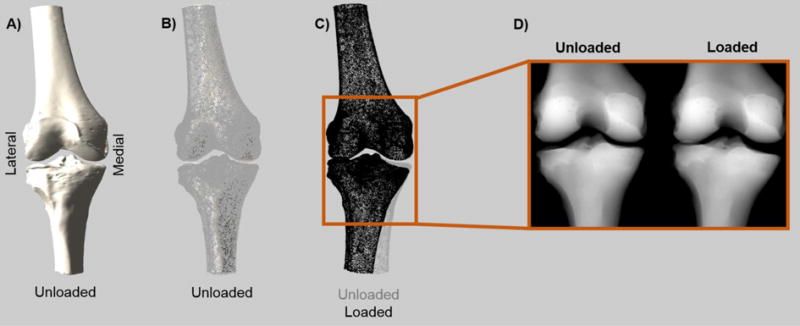
An overview of the method for creating the mimicked fluoroscopy images. A) Bones segmented from the CT scan are loaded into MATLAB and positioned based on kinematic data. In this case, the unloaded bone is shown. B) Based on the outline of the bone, a regular matrix of binary data points was created that identified which points fell within the bone. Here, the points on the edge of that point cloud are shown overlaid onto the bone. C) Comparison of point clouds (edges only) in the unloaded and loaded states. The perfectly aligned femurs can be seen. D) Mimicked fluoroscopy (mFluoro) images were created by projecting these point clouds onto a 2D image.
After creating the mFluoro images, two methods were used to compute gap width, both of which were entirely automated. First, the edges of the tibia and femur were automatically determined. Then, for the mFluoro-Distal method, which was based on the approach of LaPrade et al. [24], the most distal aspect of the medial femoral condyle was automatically identified and the distance between this and the corresponding point on the tibial plateau was taken as the gap width. Because it is unclear from the prior publication how the corresponding point on the tibial plateau was identified, in this study, the point was defined by projecting a line parallel to the tibial axis from the femoral point and finding where this line and the edge of the tibial plateau intersected. For the mFluoro-Medial approach, the femoral and tibial edges were identified as defined above, and the most medial aspect of the bone surfaces were selected automatically (Fig. 3).
Fig. 3.
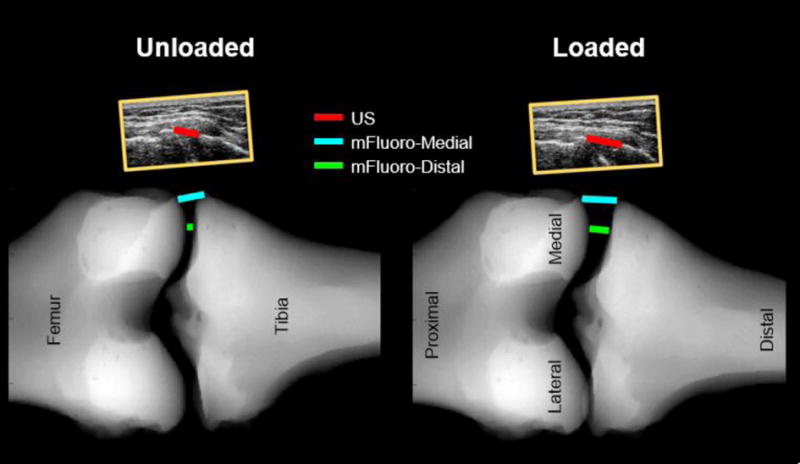
Comparison of the measurement locations of the US, mFluoro-Medial and mFluoro-Distal approaches. The ultrasound images can be seen (to scale) above the mFluoro images. The US gap width was estimated by manually selecting the most medial point on the femur and tibia. mFluoro gap widths were estimated automatically from the mFluoro images. For the mFluoro-Medial method, the most medial aspects of the femur and tibia were selected. For the mFluoro-Distal approach the most distal aspect of the femur was identified and then the corresponding point on the tibia plateau was found by projecting a line parallel to the axis of the tibia and finding the intersection between this line and the edge of the tibial plateau.
The US gap width was measured manually. First, the quality of the ultrasound data for each trial was evaluated. Because the US gap width was measured by identifying the hyperechoic bony outline of the femur and tibia, any trial in which the bony outline was not clear in either the unloaded or loaded image was not considered further. Following this quality assessment, the remaining trials were included in analysis. Next, the specific ultrasound images corresponding with the unloaded and loaded states were found from the synchronized data. To measure gap width, the most superficial (i.e. medial) aspects of the distal femur and proximal tibia relative to the skin were identified manually in the ultrasound images, and the distance between these points was taken as the US measure of gap width (Fig. 3). To assess reliability of this approach, a second rater was supplied with a sample image of the gap width for each specimen, and then independently measured gap width for all high-quality trials.
For each specimen, the average gap width values were computed and used in the statistical analysis. Because both limbs were not available for all specimens, collateral limbs were treated as independent samples. Average gap width was compared between the unloaded and loaded state for all three measures using paired t-tests. To evaluate differences between the approaches in terms of absolute gap width in the unloaded and loaded states, three repeated measures ANOVAs were performed. Significant effects were followed up with pairwise comparisons with a Sidak adjustment for multiple comparisons. Regression analyses were used to evaluate correlations between the measures in terms of the change in gap width, and also to test for a correlation between change in gap width and change in abduction angle for all three measures. Inter-rater reliability was assessed for the US method by computing the absolute agreement Intraclass Correlation Coefficient (ICC) using a two-way random effects model and average measures (SPSS 23, Armonk, NY, USA). Because the mFluoro methods were entirely automated, inter-rater reliability was not tested for them. P-values of less than 0.05 were taken as significant.
Results
The gap width increased significantly from the unloaded to the loaded condition for all three measures (p<0.001, Table 1). No significant differences between the US and the mFluoro-Medial measures of gap width were found in the unloaded (p=0.95) and loaded (p=0.11) conditions, though the mFluoro-Distal approach differed significantly from both (p<0.001). There were no correlations between any measures for the change in gap width, with p-values of 0.10, 0.23 and 0.07 for the comparison between US and mFluoro-Medial, US and mFluoro-Distal, and mFluoro-Medial and mFluoro-Distal respectively.
Table 1.
Average ± standard deviation for gap width measures for the US, mFluoro-Medial and mFluoro-Distal measures in the unloaded and maximally loaded conditions, as well as the average change with load.
| Location | Method | Unloaded | Max. Loaded | Change | ||
|---|---|---|---|---|---|---|
| Current Study | US | Medial | Ultrasound | 8.7 ± 2.4 mm | 10.7 ± 2.2 mm | 2.1 ± 0.7 mm |
| mFluoro-Medial | Medial | Mimicked Fluoroscopy | 9.0 ± 2.5 mm | 12.4 ± 2.5 mm | 3.4 ± 0.6 mm | |
| mFluoro-Distal | Distal | Mimicked Fluoroscopy | 2.1 ± 1.9 mm | 5.2 ± 2.4 mm | 3.0 ± 0.9 mm | |
|
| ||||||
| Literature | Kleinbaum and Blankstein [33] | Medial | Ultrasound | 6.7 ± 0.7 mm | 9.6 ± 0.7 mm | 3.0 ± 0.5 mm |
| Whelan et al. [25] | Medial | Fluoroscopy | – | 10.9 ± 2.1 mm | – | |
| LaPrade et al. [24] | Distal | Fluoroscopy | – | 5.1 ± 2.1 mm | – | |
Also included in the table are data from the literature using similar methods. The location column refers to whether data points were identified at the most medial or most distal aspect of the femur. Some differences between the results from the current study and those in the literature may be due to differences in loading. LaPrade et al. [24] reported that gap width was measured during the application of a 10 Nm load, as in our study, whereas Kleinbaum and Blankstein [33] and Whelan et al. [25] either did not control load or did not report it
The change in gap width was significantly correlated with the change in abduction angle for the mFluoro-Distal measure (R2=0.62, p=0.02) and the mFluoro-Medial measure (R2=0.74, p=0.03), but not the US measure (R2=0.4, p=0.1).
Inter-rater reliability for the US method was found to be high for the absolute measures of gap width in the unloaded (0.952) and loaded measures (0.928), as well as the change in gap width (0.899).
Discussion
Consistent with our first hypotheses, the US method was found to be capable of measuring an increase in gap width with applied valgus load, with similar values for measures of absolute gap width between the US and the mFluoro-Medial method that both measured gap width from the most medial aspect of the femur. In addition, the US measure was found to have a high reliability, particularly for absolute measures of gap width, with inter-rater reliability values that are well above the suggested minimum for basic research (0.8 [39]), and are higher than values for other tools currently used clinically to assess stability (e.g. the KT-2000 [40]). The results from this study were also found to closely match previously published measures of gap width (Table 1), showing that the mFluoro methods sufficiently mimicked the gold standard approach, and were a reasonable comparison from which to assess the US measure. These findings, combined with the high safety and accessibility of ultrasound, suggest that an US approach may be a suitable alternative for evaluating medial gapping, particularly in terms of absolute gap width. This has clinical implications. For example, there is evidence that MCL-deficient knees show significantly greater values of absolute gap width compared with intact knees [24, 25, 33], and medial gap width may be a suitable method for assessing the efficacy of MCL repair [25]. Varus/valgus instability post-arthroplasty, which has been linked to patient satisfaction [11], and is often assessed clinically with radiographic examination, may also be possible to evaluate with ultrasound, though reverberation artifacts off of the metallic prosthesis [41] may make gap width measures more challenging in these cases.
In addition to the clinical value of measuring absolute gap width, a reliable method for measuring the change in gap width with applied load could provide additional information regarding MCL tissue properties. Because the MCL is the primary restraint to tibial abduction [1, 42, 43], the change in gap width with load is related to the change in MCL length, from which ligament stiffness could be estimated, though assumptions regarding the contributions of other soft tissues (e.g. the posterior cruciate ligament [43]) would be necessary. Clinically, a noninvasive measurement of MCL stiffness could provide important insight into disease and healing. For example, there is evidence that MCL stiffness may be increased in cases of severe OA [44] and may change following knee arthroplasty [45], with animal models further suggesting that MCL stiffness increases with age [46], is reduced immediately following MCL injury and repair [47], and returns toward normal during healing [47]. Such measurements of MCL tissue quality are not possible with absolute measures of gap width, as absolute gap width depends on both the tissue mechanical properties and slack length. Thus, an US measure of the change in gap width could provide a noninvasive way for evaluating MCL tissue quality.
However, in contrast to our expectations, no correlation between the change in abduction angle and the change in gap width with the US method was found, and likewise there was no correlation between the three measures in terms of the change in gap width, suggesting that the US method may not be a suitable approach for measuring the change in gap width with applied load. The lack of correlation between the measures likely relates to the fact that the three methods are measuring fundamentally different anatomical distances that are linked with the specific anatomy of each knee joint. In other words, if a simple model was used to mathematically derive the relationship between the two mFluoro measures, this correlation would be dependent on the perpendicular distance along the femur from which the two measures are compared (e.g. the distance between most distal aspect of the femur and the most medial aspect of the femur). Due to normal anatomical variation between specimens, this distance varies between knees and likewise, there is no general linear relationship that describes the link between the two mFluoro gap width measures for all specimens. Further, the fact that all three methods are attempting to measure 3D bone gapping in a single 2D image, that is produced differently in the cases of the US and mFluoro methods, also likely contributes to the differences. Whereas the US method measures a 2D distance aligned with the MCL, the mFluoro methods use a 2D projection that is created by compressing 3D bone models, such that any perpendicular distance information is lost, potentially contributing to non-real distance measures. Likewise, differences in how the anatomical landmarks are defined, or how the corresponding point on the tibia was found in the mFluoro-Distal approach, could all contribute to substantial variations in measurements.
Given these limitations, it becomes unclear whether a lack of correlation between the US method and the mFluoro methods arises from inaccuracies in the US method, variations in the plane used for measuring gap width, or another factor. As a starting point, future studies could add motion capture markers to the US probe in order to exactly reproduce the US plane within bone models such that mFluoro images could be made from along the same plane, but more broadly, the challenge is a lack of ground-truth data. Although the mFluoro methods were found to correlate with the change in abduction angle, and the US method did not, this does not necessarily indicate that the US method is inferior. Further, it should be noted that the mFluoro images were created by repositioning the bones within the bone model based on the kinematic data, and thus there is an inherent link between the mFluoro measures and the kinematic data. As above, regular anatomical variation, and differences in MCL stiffness, mean that a one-to-one correlation between change in gap width and change in abduction angle would not be expected, and without ground-truth data it is impossible to determine with certainty which method is superior for this purpose.
There are some limitations to this study, including its small sample size, the fact that cadaveric specimens enabled an easier application of a controlled loading scenario, and the aforementioned lack of true ground-truth measures. In this study, mimicked fluoroscopy images were created for computing gap width to mimic the current gold standard, and indeed average gap width values were found to be similar to those in prior publications that have used the gold standard approach. However, there are some differences between the mFluoro methods and the gold standard. For example, because the mFluoro images were recreated during post-processing, it was possible to reorient the bone models so that all trials, and all specimens, were evaluated in the same orientation; in fluoroscopic approaches, patient shifting or misalignment could significantly influence gap width measures between multiple scans, such that the mFluoro measures may actually out-perform the gold standard. On the other hand, given the lack of clarity in prior studies, lost 3D distance information, differences in resolution between techniques, and fundamental differences in the anatomical distances measured between different approaches, it remains unclear whether the comparison with mFluoro methods is the best approach for validation. Further, because the ultrasound transducer can be manually aligned directly over the MCL, an US approach has the potential to be better for evaluating MCL tissue properties. Nevertheless, additional work will be necessary to assess whether there is a link between the US measures of change in gap width and true MCL strain. For example, future studies could pair the collection of in situ data with post-hoc mechanical testing of the MCL, or take advantage of techniques like digital image correlation to measure MCL strain during loading.
One of the most important findings of this work is that ultrasound may be suitable for measuring absolute gap width in the MCL, however, more work is necessary in order to fully assess the clinical viability of this approach. In this cadaveric study, the loading was controlled such that specimens only underwent small amounts of knee flexion (average: −0.3 ± 0.78 deg) and tibial rotation (average: −1.5 ± 3.2 deg). However, in vivo, without the use of bone pins, and considering other factors like inadvertent muscle contraction, achieving such isolated motion may be challenging, though it would be similar to what is required for the clinical valgus stress test [48]. In this first study, the focus was on the medial side of the knee because of its greater clinical relevance, but it would be relevant to consider whether such an approach could also evaluate lateral gapping, as the paired evaluation of medial and lateral laxity is of clinical interest, particularly in knee arthroplasty patients, and it can be measured with the gold standard approach [27]. Further, there are some challenges specific to ultrasound that may limit its clinical translation, most notably that image quality can depend on the experience of the sonographer, and that the imaging of collateral ligaments during motion does require some amount of training. To combat these challenges, in this study, a large number of repeat trials were collected, and an assessment of image quality was performed prior to analysis, however, in a clinical scenario such extra measures would ideally be unnecessary. High values for inter-rater reliability were observed, but it should be noted that the second rater was provided with sample measures of gap width for each specimen, and the repeat analysis was performed on the same data set. In order for this technique to transition to clinical use, the reliability of the entire process in an in vivo setting should be assessed, and the next step in validation should include the assessment of reliability when multiple sets of images are collected.
Conclusions
The results of this study suggest that ultrasound has potential as a method for measuring absolute medial gapping of the knee, though further work is necessary to fully assess the clinical viability of this technique. The US approach was found to have a high reliability and measured similar values of gap width as the mFluoro-Medial method which mimicked the gold standard and assessed medial gapping at the same anatomical location as the US method. Further, the measures in this study closely matched those from the literature. The measurement of change in gap width measure, which may be relevant to the assessment of MCL tissue properties, was inferior in the US method. Future work should include a comparison of US measures with other techniques for evaluating MCL strain (e.g. digital image correlation), and an assessment of the reliability of the entire approach when performed in vivo, from the image collection to post-hoc processing, which will provide important information regarding clinical viability. Overall, these results show that ultrasound has promise as a technique for enabling the radiation-free assessment of medial/lateral knee stability, but further work is necessary prior to clinical translation.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
The authors gratefully acknowledge funding from the National Institute of Arthritis and Musculoskeletal Skin Diseases (F32 AR069459), the contributions of Félix Dandois, Darryl Thelen, Michael Vignos, and the support of the Vesalius Institute including Goedele Liégeois, Kristof Reyniers, and Jo Verbinnen.
Footnotes
Limitation of Authorship
The authors declare that they have no conflict of interest.
References
- 1.Robinson JR, Bull AMJ, Thomas RRD, Amis AA. The role of the medial collateral ligament and posteromedial capsule in controlling knee laxity. Am J Sports Med. 2006;34:1815–1823. doi: 10.1177/0363546506289433. [DOI] [PubMed] [Google Scholar]
- 2.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62:259–270. [PubMed] [Google Scholar]
- 3.Sullivan D, Levy IM, Sheskier S, et al. Medical restraints to anterior-posterior motion of the knee. J Bone Jt Surg. 1984;66:930–936. doi: 10.2106/00004623-198466060-00015. [DOI] [PubMed] [Google Scholar]
- 4.Sakane M, Livesay GA, Fox RJ, et al. Relative contribution of the ACL, MCL, and bony contact to the anterior stability of the knee. Knee Surgery, Sport Traumatol Arthrosc. 1999;7:93–97. doi: 10.1007/s001670050128. [DOI] [PubMed] [Google Scholar]
- 5.Athwal KK, Hunt NC, Davies AJ, et al. Clinical biomechanics of instability related to total knee arthroplasty. Clin Biomech. 2014;29:119–128. doi: 10.1016/j.clinbiomech.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Fehring TK, Odum S, Griffin WL, et al. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001:315–318. doi: 10.1097/00003086-200111000-00041. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey PF, Hozack WJ, Rothman RH, et al. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Mulhall KJ, Ghomrawi HM, Scully S, et al. Current Etiologies and Modes of Failure in Total Knee Arthroplasty Revision. Clin Orthop Relat Res. 2006;446:45–50. doi: 10.1097/01.blo.0000214421.21712.62. [DOI] [PubMed] [Google Scholar]
- 9.Schroer WC, Berend KR, Lombardi AV, et al. Why Are Total Knees Failing Today? Etiology of Total Knee Revision in 2010 and 2011. J Arthroplasty. 2013;28:116–119. doi: 10.1016/j.arth.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 10.Sharkey PF, Lichstein PM, Shen C, et al. Why Are Total Knee Arthroplasties Failing Today—Has Anything Changed After 10 Years? J Arthroplasty. 2014;29:1774–1778. doi: 10.1016/j.arth.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Kuster MS, Bitschnau B, Votruba T. Influence of collateral ligament laxity on patient satisfaction after total knee arthroplasty: a comparative bilateral study. Arch Orthop Trauma Surg. 2004;124:415–417. doi: 10.1007/s00402-004-0700-7. [DOI] [PubMed] [Google Scholar]
- 12.Bollen S. Epidemiology of knee injuries: diagnosis and triage. Br J Sports Med. 2000;34:227–228. doi: 10.1136/bjsm.34.3.227-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa H, Matsumoto K, Ogawa T, et al. Preoperative varus laxity correlates with overcorrection in medial opening wedge high tibial osteotomy. Arch Orthop Trauma Surg. 2016;136:1337–1342. doi: 10.1007/s00402-016-2521-x. [DOI] [PubMed] [Google Scholar]
- 14.Wijdicks CA, Griffith CJ, Johansen S, et al. Injuries to the Medial Collateral Ligament and Associated Medial Structures of the Knee. J Bone Jt Surg. 2010;92 doi: 10.2106/JBJS.I.01229. [DOI] [PubMed] [Google Scholar]
- 15.Hefti E, Müller W, Jakob RP, Stäubli H-U. Evaluation of knee ligament injuries with the IKDC form. Knee Surgery, Sport Traumatol Arthrosc. 1993;1:226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 16.Clarke JV, Wilson WT, Wearing SC, et al. Standardising the clinical assessment of coronal knee laxity. Proc Inst Mech Eng H. 2012;226:699–708. doi: 10.1177/0954411912451814. [DOI] [PubMed] [Google Scholar]
- 17.Noyes FR, Grood ES, Butler DL, Raterman L. Knee Ligament Tests. Phys Ther. 1980;60 doi: 10.1093/ptj/60.12.1578. [DOI] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical Data. Biometrics. 1977;33:159. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 19.Cushnaghan J, Cooper C, Dieppe P, et al. Clinical assessment of osteoarthritis of the knee. Ann Rheum Dis. 1990;49:768–770. doi: 10.1136/ARD.49.10.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stähelin T, Kessler O, Pfirrmann C, et al. Fluoroscopically assisted stress radiography for varus-valgus stability assessment in flexion after total knee arthroplasty. J Arthroplasty. 2003;18:513–515. doi: 10.1016/S0883-5403(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 21.ten Ham AM, Heesterbeek PJC, van der Schaaf DB, et al. Flexion and extension laxity after medial, mobile-bearing unicompartmental knee arthroplasty: a comparison between a spacer- and a tension-guided technique. Knee Surgery, Sport Traumatol Arthrosc. 2013;21:2447–2452. doi: 10.1007/s00167-012-2021-7. [DOI] [PubMed] [Google Scholar]
- 22.Heesterbeek PJC, Verdonschot N, Wymenga AB. In vivo knee laxity in flexion and extension: A radiographic study in 30 older healthy subjects. Knee. 2008;15:45–49. doi: 10.1016/j.knee.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Tsukeoka T, Tsuneizumi Y. Varus and valgus stress tests after total knee arthroplasty with and without anesthesia. Arch Orthop Trauma Surg. 2016;136:407–411. doi: 10.1007/s00402-015-2405-5. [DOI] [PubMed] [Google Scholar]
- 24.Laprade RF, Bernhardson AS, Griffith CJ, et al. Correlation of valgus stress radiographs with medial knee ligament injuries: an in vitro biomechanical study. Am J Sports Med. 2010;38:330–338. doi: 10.1177/0363546509349347. [DOI] [PubMed] [Google Scholar]
- 25.Whelan D, Leiter J, Sasyniuk T, et al. Double-row repair of the distal attachment of the superficial medial collateral ligament: a basic science pilot study. Knee Surgery, Sport Traumatol Arthrosc. 2015;23:2820–2824. doi: 10.1007/s00167-015-3773-7. [DOI] [PubMed] [Google Scholar]
- 26.Sawant M, Narasimha Murty A, Ireland J. Valgus knee injuries: evaluation and documentation using a simple technique of stress radiography. Knee. 2004;11:25–28. doi: 10.1016/S0968-0160(03)00009-7. [DOI] [PubMed] [Google Scholar]
- 27.LaPrade RF, Heikes C, Bakker AJ, Jakobsen RB. The Reproducibility and Repeatability of Varus Stress Radiographs in the Assessment of Isolated Fibular Collateral Ligament and Grade-III Posterolateral Knee Injuries. J Bone Jt Surg. 2008;90:2069–2076. doi: 10.2106/JBJS.G.00979. [DOI] [PubMed] [Google Scholar]
- 28.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giordano BD, Grauer JN, Miller CP, et al. Radiation Exposure Issues in Orthopaedics. J Bone Jt Surg. 2011;93 doi: 10.2106/JBJS.J.01328. [DOI] [PubMed] [Google Scholar]
- 30.Bhargavan M. Trends in the utilization of medical procedures that use ionizing radiation. Health Phys. 2008;95:612–627. doi: 10.1097/01.HP.0000327659.42618.c1. [DOI] [PubMed] [Google Scholar]
- 31.Siu D, Cooke TD, Broekhoven LD, et al. A standardized technique for lower limb radiography. Practice, applications, and error analysis. Invest Radiol. 1991;26:71–7. doi: 10.1097/00004424-199101000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Krackow KA, Pepe CL, Galloway EJ. A mathematical analysis of the effect flexion and rotation on apparent varus/valgus alignment at the knee. Orthopedics. 1990;13:861–868. doi: 10.3928/0147-7447-19900801-09. [DOI] [PubMed] [Google Scholar]
- 33.Kleinbaum Y, Blankstein A. Mild to Moderate Medial Collateral Ligament (MCL) Injuries of the Knee: Sonographic Findings and Sonographic Valgus Stress Test. J Musculoskelet Res. 2008;11:9–14. doi: 10.1142/S0218957708001912. [DOI] [Google Scholar]
- 34.Victor J, Van Glabbeek F, Vander Sloten J, et al. An Experimental Model for Kinematic Analysis of the Knee. J Bone Jt Surg. 2009;91:150–163. doi: 10.2106/JBJS.I.00498. [DOI] [PubMed] [Google Scholar]
- 35.Miranda DL, Rainbow MJ, Leventhal EL, et al. Automatic determination of anatomical coordinate systems for three-dimensional bone models of the isolated human knee. J Biomech. 2010;43:1623–1626. doi: 10.1016/j.jbiomech.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aronson PA, Gieck JH, Hertel J, et al. Tibiofemoral joint positioning for the valgus stress test. J Athl Train. 2010;45:357–363. doi: 10.4085/1062-6050-45.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shultz SJ, Shimokochi Y, Nguyen A-D, et al. Measurement of varus–valgus and internal–external rotational knee laxities in vivo—part I: assessment of measurement reliability and bilateral asymmetry. J Orthop Res. 2007;25:981–988. doi: 10.1002/jor.20397. [DOI] [PubMed] [Google Scholar]
- 38.Grood ES, Suntay WJ. A Joint Coordinate System for the Clinical Description of Three-Dimensional Motions: Application to the Knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 39.Nunnally JC. Psychometric theory. 2nd. McGraw-Hill; New York: 1978. [Google Scholar]
- 40.Myrer JW, Schulthies SS, Fellingham GW. Relative and absolute reliability of the KT-2000 arthrometer for uninjured knees. Testing at 67, 89, 134, and 178 N and manual maximum forces. Am J Sports Med. 1996;24:104–108. doi: 10.1177/036354659602400119. [DOI] [PubMed] [Google Scholar]
- 41.Sofka CM, Adler RS, Laskin R. Sonography of Polyethylene Liners Used in Total Knee Arthroplasty. Am J Roentgenol. 2003;180:1437–1441. doi: 10.2214/ajr.180.5.1801437. [DOI] [PubMed] [Google Scholar]
- 42.Grood ES, Noyes FR, Butler DL, Suntay WJ. Ligamentous and capsular restraints preventing straight medial and lateral laxity in intact human cadaver knees. J Bone Joint Surg Am. 1981;63:1257–1269. [PubMed] [Google Scholar]
- 43.Seering WP, Piziali RL, Nagel DA, Schurman DJ. The function of the primary ligaments of the knee in varus-valgus and axial rotation. J Biomech. 1980;13:785–794. doi: 10.1016/0021-9290(80)90240-7. [DOI] [PubMed] [Google Scholar]
- 44.Fishkin Z, Miller D, Ritter C, Ziv I. Changes in human knee ligament stiffness secondary to osteoarthritis. J Orthop Res. 2002;20:204–207. doi: 10.1016/S0736-0266(01)00087-0. [DOI] [PubMed] [Google Scholar]
- 45.Delport H, Labey L, De Corte R, et al. Collateral ligament strains during knee joint laxity evaluation before and after TKA. Clin Biomech. 2013;28:777–782. doi: 10.1016/j.clinbiomech.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Woo SL-Y, Orlando CA, Gomez MA, et al. Tensile properties of the medial collateral ligament as a function of age. J Orthop Res. 1986;4:133–141. doi: 10.1002/jor.1100040201. [DOI] [PubMed] [Google Scholar]
- 47.Gijssen Y, Sierevelt IN, Kooloos JGM, Blankevoort L. Stiffness of the Healing Medial Collateral Ligament of the Mouse. Connect Tissue Res. 2004;45:190–195. doi: 10.1080/03008200490514158. [DOI] [PubMed] [Google Scholar]
- 48.Malanga GA, Andrus S, Nadler SF, McLean J. Physical examination of the knee: A review of the original test description and scientific validity of common orthopedic tests. Arch Phys Med Rehabil. 2003;84:592–603. doi: 10.1053/apmr.2003.50026. [DOI] [PubMed] [Google Scholar]


