Abstract
Purpose
To relate the concept of the retinal neurovascular unit and its alterations in diabetes to the pathophysiology of diabetic retinopathy.
Methods
Case illustrations and conceptual frameworks are presented that illustrate adaptive and maladaptive “dis-integration” of the retinal neurovascular unit with the progression of diabetes.
Results
Retinopathy treatment should address pathophysiologic processes rather than pathologic lesions as is current practice.
Conclusions
Future improvements in the treatment of diabetic retinopathy requires deeper understanding of the cellular and molecular changes induced by diabetes, coupled with the use of quantitative phenotyping methods that assess the pathophysiologic processes.
Keywords: diabetic retinopathy, neurovascular unit, sensory neuropathy, spectral domain optical coherence tomography (SD-OCT), frequency doubling perimetry
Introduction
This presentation is the product of many colleagues, notably Dr. George Blankenship, who provided the opportunity to develop a research program and to participate in the Club Jules Gonin. Drs. David Antonetti, Steven Abcouwer and Patrice Fort are long-standing colleagues who provide critical expertise and rigor for the research. Dr. Gregory Jackson's expertise in visual function has been essential to the clinical evaluation of diabetic retinopathy. This talk is dedicated to my brothers, Tim and Ted, and to all persons with type 1 diabetes who endure their disease every day.
Our work has focused on understanding the normal cellular interrelationships of the retinal neurovascular unit of and how they are altered to produce the clinical phenotype of diabetic retinopathy. Conventional clinical assessment reveals typical microvascular features of hemorrhages, lipid exudates, cotton wool spots and neovascularization. However, this perspective does not reveal the complex organization of the neurosensory retina. The term, “neurovascular unit,” was first applied to the blood-brain barrier (1), then to the retina by Dr. Eric Newman (2). The term refers to the intricate functional coupling between neurons, glial cells and blood vessels (Figure 1). These cells work in close coordination to integrate retinal vascular flow with metabolic activity, recognized clinically as autoregulation. Under normal circumstances flickering light stimulation causes retinal blood vessels to dilate, whereas breathing 100% oxygen causes them to constrict. Multiple studies have shown that these physiologic responses are impaired early in the course of diabetes prior to the onset of the clinical features of diabetic retinopathy (3, 4). These changes clearly show the interrelationships between the neurosensory retina and its blood vessels. Newman and colleagues (5) have determined that alterations in small molecules such as ATP, lactate, nitric oxide, arachidonic acid and other lipids mediate these interactions.
Figure 1.
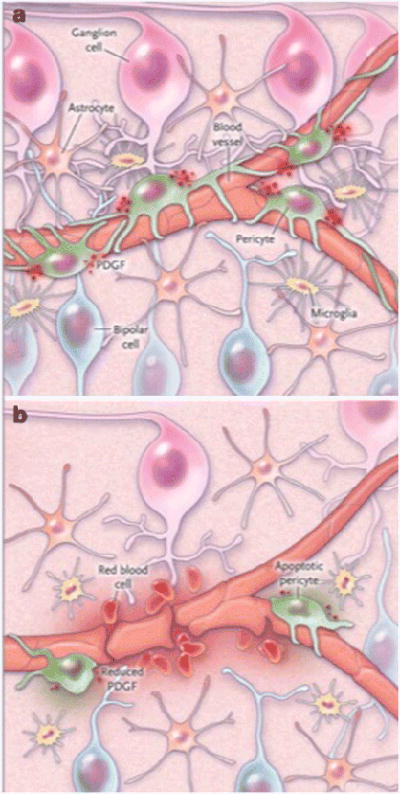
The neurovascular unit in the retina. Panel A shows pericytes and glial cells which support neuronal function and help form to the blood-retina barrier. Panel B shows an altered cellular environment with increased VEGF from glial cells, loss of platelet-derived growth factor (PDGF), and release of inflammatory cytokines leading to a breakdown of the blood-retina barrier and, in some cases, angiogenesis. From Antonetti (14), with permission.
Numerous studies have also shown that all components of the neurovascular unit are disrupted by diabetes. Neurons are altered as reflected by impaired glutamatergic and dopaminergic neurotransmitter signaling (6), their altered dendritic fields (7) and reduced synaptic protein expression (8). Ultimately neurons undergo apoptosis in response to persistent uncontrolled diabetes (9). Glial cells also change in diabetes as reflected by impaired interconversion of glutamate and glutamine (10), their regulation of potassium channels (11), and expression of the glutamate-aspartate transporter and intermediary filament proteins such as glial fibrillary acidic protein. Astrocytes which wrap around blood vessels of the nerve fiber retina and make contacts with synapses (5) are also altered early in the course of diabetes (12) although many of the details of their dysfunction are yet to be elucidated. Microglial cells, resident macrophages that survey the retinal environment and respond to injuries, probably serve an adaptive role initially but may contribute to retinal damage when chronically stimulated (13). Progressive damage to the neurovascular unit (Figure 1) results in features clinicians recognize as diabetic retinopathy; i.e., cotton wool spots, hemorrhages, and swelling of the macula due to vascular leakage. Therefore, diabetes causes the retinal neurovascular unit to “dis-integrate”, and diabetic retinopathy may be considered as a sensory neuropathy or neurovascular degeneration, not merely a microvascular disease (14).
Thus, while the progression of diabetic retinopathy through stages described by the accumulation of pathologic features, progression can also be considered as an evolution along a continuum of neurovascular unit disintegration. Figure 2 shows a model by which diabetes might induce insidious progression from a normal retina to one that is blind. Within the first few weeks to months after the onset of diabetes, the retina resets its normal high metabolic activity to a lower steady state set point with subtle adaptive changes (15), including reduced electrical activity (16), reduced biosynthetic activity involving less protein and lipid synthesis (17-19), and adaptive changes of autophagy (20) and apoptosis (9). This diminished anabolic activity may be a strategy to reduce retinal energy demand and allow retinal neurons to survive in the face of diabetes (17). By such a means the retina can remain compensated to the state of diabetes and there is no clinically detectable retinopathy and vision remains intact. However, after approximately 5 to 10 years, adaptive changes begin to fail and early decompensation follows, with the appearance of venous dilation, retinal hemorrhages, microaneurysms, cotton wool spots, macular edema and vascular nonperfusion, clinically recognized as nonproliferative diabetic retinopathy. By this point impairment of retinal function is detectable as reduced contrast sensitivity, impaired color vision and visual field sensitivity. Over time adaptive changes become maladaptive and there is aberrant repair in stages of proliferative retinopathy with neovascularization, gliosis, fibrosis, and loss of visual acuity. Remarkably, repair and recovery can occur during these decompensated and repair stages if the diabetes is controlled and with treatment of retinopathy (21).
Figure 2.
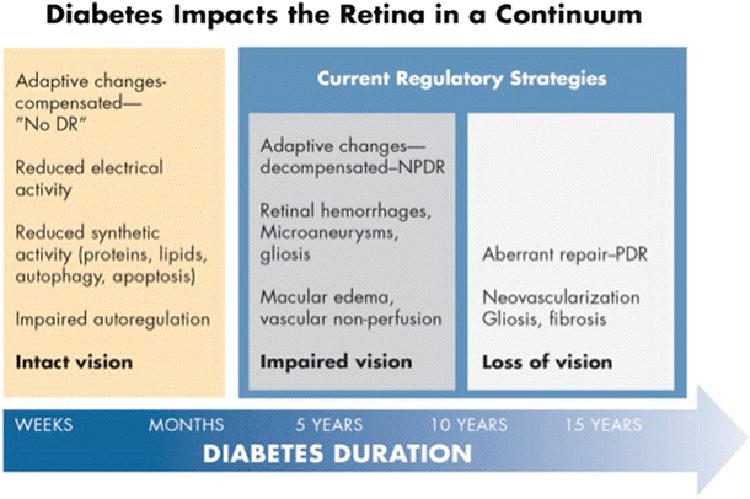
A model of adaptive and maladaptive stages of diabetic retinopathy development. Within the first weeks to months of diabetes the retina adapts to a lower metabolic steady state with reduced electrical and biosynthetic activity, and adaptive autophagy and apoptosis, and impaired autoregulation. Vision remains intact and there is no clinical evidence of diabetic retinopathy. After 5 – 10 years of diabetes adaptive mechanisms begin to decompensate with the appearance of mild nonproliferative retinopathy, and early impairment of vision. Aberrant repair can ensue with the onset of proliferative retinopathy and loss of vision. Regulatory strategies are currently limited to the latter two stages. Artwork by David Murrel, MFA.
Current regulatory strategies focus on the latter two stages and have provided the structure for treating advanced retinopathy, but are not yet established for patients with mild disease to enable prevention of advanced retinopathy. Figure 3 illustrates mild retinal decompensation (mild nonproliferative retinopathy) and the stages of retinopathy we treat, namely diabetic macular edema and proliferative retinopathy. These stages, by definition, represent loss of adaptive mechanisms such that there is “retinal failure” (22). Likewise, diabetes causes renal failure and cardiac failure, and our internal medicine colleagues measure cardiac ejection fraction and creatinine clearance, respectively. They treat those pathophysiologic parameters, whereas at present, ophthalmologists lack analogous validated, quantitative and reversible endpoints for diabetic retinopathy.
Figure 3.
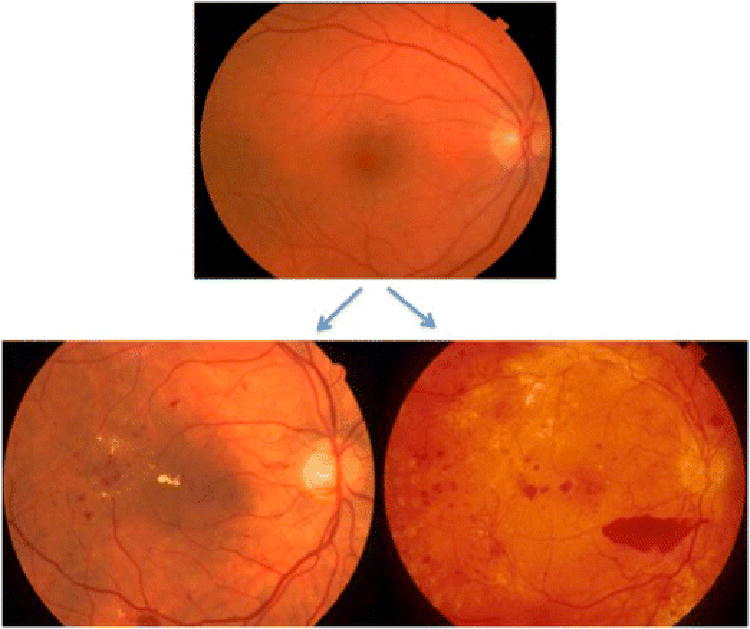
Progression of retinal failure in diabetes. Retinal failure occurs after long-term decompensated adaptive responses to metabolic dysfunction. The top image shows compensated diabetic retinopathy in a patient with mild non-proliferative diabetic retinopathy. The bottom images are examples of decompensated non-proliferative retinopathy with macular edema (lower left) and proliferative diabetic retinopathy with preretinal hemorrhage (lower right). From Gray (22) with permission.
For 5 decades numerous investigators have recognized that diabetes impairs not only blood vessels but also impairs visual function (23, 24). Figure 4 shows how diabetes affects multiple aspects of retinal function. This patient exhibited reduced dark adaptation, meaning her photoreceptors and pigment epithelial cells are impaired. She also had marked loss of visual field sensitivity both with frequency doubling perimetry, which assesses primarily the inner retina, and with standard photopic perimetry. If these field changes occurred in a patient suspected of having glaucoma ophthalmologists would consider treatment. At present we lack strategies that address this aspect of retinal function.
Figure 4.
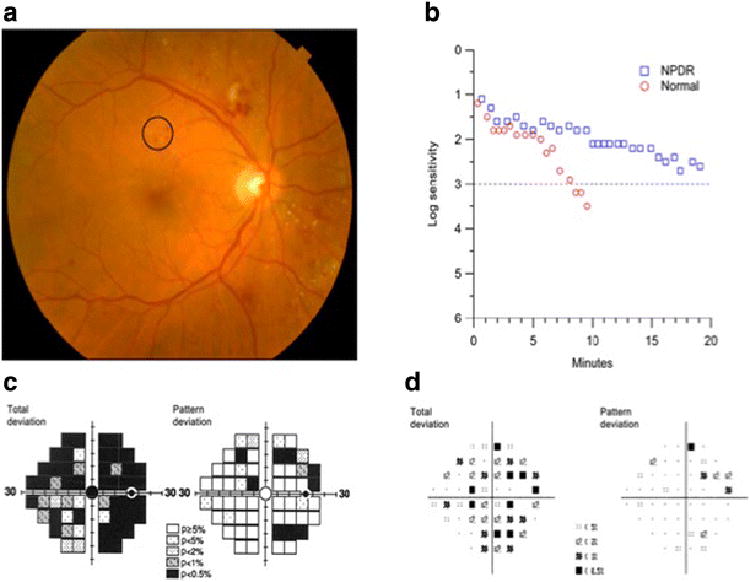
A patient with 21 years of type 2 diabetes mellitus, 20/25 visual acuity and moderate nonproliferative diabetic retinopathy (A) exhibits severe visual function abnormalities. Dark adaptation (B) was severely delayed compared to normal adult responses. Frequency doubling perimetry fields (C) exhibited deep deficits in sensitivity that exceeded those revealed by standard photopic visual fields (D). From Jackson et al (34) with permission.
Figure 5 illustrates the profound impact of diabetes on the neurosensory retina. The left image is from a healthy person with normal retinal anatomy, 20/20 visual acuity, normal foveal thickness of almost 300 microns and > 400 microns in the parafoveal region, and normal frequency doubling perimetry sensitivity. The patient in the middle column had 13 years of type 1 diabetes but no visual symptoms and 20/20 acuity. Nevertheless she had profound thinning of the fovea and the parafoveal regions between 50 and 75 microns, 1/6th to 1/4th of the retina, and 9 dB of sensitivity loss in the fovea. The patient in the right column had a 17-year history of type 1 diabetes without visual symptoms, but 20/20 acuity and early proliferative changes. She also had more profound reduction of retinal thickness and 15 dB loss of visual field loss sensitivity. These two cases illustrate important features of diabetic retinopathy that are not embodied in photographic grading schemes. Although ophthalmologists recognize retinal thickening as part of diabetic macular edema, retinal thinning has received much less attention. Clearly, standard spectral domain optical coherence tomography and frequency doubling visual field testing can reveal these profound structure and function changes.
Figure 5.
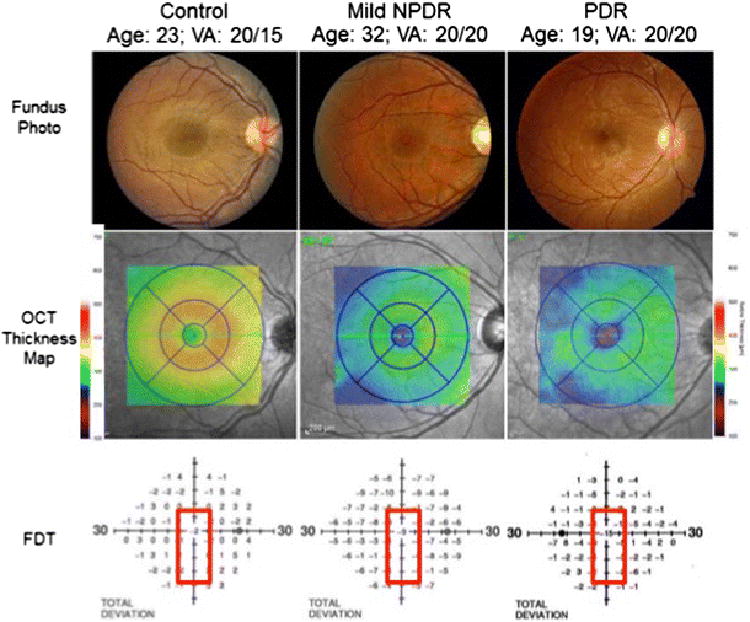
Color fundus photographs (top panel) of a healthy person (left), a person with mild non-proliferative diabetic retinopathy (NPDR) (middle), and a person with proliferative diabetic retinopathy (PDR) (right) demonstrate preserved macular anatomy without gross vascular compromise and visual acuities 20/20 or better. Spectral-domain optical coherence tomography (OCT) macular thickness mapping (central panel) demonstrates foveal and parafoveal thicknesses reduced by between 50 and 75 microns comparing healthy eyes to those with mild NPDR or PDR. The lower panel demonstrates a concomitant decline in foveal sensitivities in the diabetic patients as measured by frequency doubling perimetry, with a 9 decibel (dB) visual field sensitivity loss in the patient with mild NPDR and a 15 dB sensitivity loss in the patient with PDR.
Diabetes mellitus is a complex multi-faceted metabolic disease with widely varied patterns of complications. For example, diabetic neuropathies and renal failure are heterogenous with subsets of patients vulnerable to tissue injury (25, 26). Approximately 25% of persons with long-standing diabetes develop macular edema and 5% develop proliferative retinopathy (14). While risk factors such as diabetes duration, HbA1c levels and hypertension predict the likelihood of complications in populations of patients, Practicing clinicians recognize that certain patients with advanced diabetic retinopathy may not exhibit such marked retinal thinning or functional decline on visual field testing. Indeed, there is significant heterogeneity in the phenotypic expression of patients' diabetic retinopathy, and their individual risk for progression to diabetic macular edema, neovascularization, or frank visual loss varies. In an era focused increasingly on precision medicine, awareness and utilization of additional tools, such as SD-OCT and frequency doubling perimetry, may help better characterize and risk stratify patients in terms of their disease status, progression and predicted response to therapy.
Dr. Toke Bek (27) first recognized the relationship between loss of vascular and neural structures 22 years ago when he used perfusion casting in post-mortem eyes to reveal profound loss of the ganglion cells and the inner nuclear layer in regions of vascular non-perfusion. All regions of nonperfusion exhibited loss of the nerve fiber layer and ganglion cell layer. More recently, Dodo and colleagues (28) used OCT to show that regions of non-perfusion are accompanied by profound thinning of the inner retina. Sun and colleagues (29, 30) have shown that disorganization of inner layers of the fovea is a poor prognostic sign in patients with diabetic macular edema. Scarinci (31) found both inner and outer retinal atrophy in patients with macular nonperfusion. Davila et al (unpublished data) have found that retinal thinning correlates with reduced visual field sensitivity, so retinal thinning is clinically important. Indeed, patients with ETDRS level 43 (moderate nonproliferative) retinopathy have reduced NEI-VFQ quality of life measures (32), so it is likely that studies of these persons will reveal important insights into the pathophysiology of diabetic retinopathy.
Conclusion
Collectively, diabetes imposes profound impact on the neurovascular unit as it impairs vision (33). Still, many questions remain about the temporal and topographical distribution of retinal neurodegeneration, and the mechanisms by which it develops and impairs vision. Nevertheless, the retinal sensory neuropathy of diabetes can be detected with standard clinical instruments, SD-OCT and frequency doubling perimetry. These tests can help us understand patients' symptoms, and may help us counsel patients about the need to optimize metabolic control. These approaches will hopefully lead to quantitative phenotyping to provide comprehensive analysis of the disease state and means to develop new therapies to prevent retinal failure and vision loss.
Acknowledgments
Presented as the Jules Gonin Lecture sponsored by the Retina Research Foundation, Bordeaux, France, July, 2016
Funding: This work was supported by R01EY20582, DP3DK094292, P30EY007003, The Taubman Institute, and Research to Prevent Blindness, and previously by the Jack and Nancy Turner Professorship at Penn State University.
References
- 1.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol. 2007;92:635–640. doi: 10.1113/expphysiol.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lott ME, Slocomb JE, Shivkumar V, Smith B, Gabbay RA, Quillen D, Gardner TW, Bettermann K. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta ophthalmologica. 2012;90:e434–441. doi: 10.1111/j.1755-3768.2012.02445.x. [DOI] [PubMed] [Google Scholar]
- 4.Pemp B, Garhofer G, Weigert G, Karl K, Resch H, Wolzt M, Schmetterer L. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Investigative ophthalmology & visual science. 2009;50:4029–4032. doi: 10.1167/iovs.08-3260. [DOI] [PubMed] [Google Scholar]
- 5.Newman EA. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aung MH, Park HN, Han MK, Obertone TS, Abey J, Aseem F, Thule PM, Iuvone PM, Pardue MT. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34:726–736. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gastinger MJ, Kunselman AR, Conboy EE, Bronson SK, Barber AJ. Dendrite remodeling and other abnormalities in the retinal ganglion cells of Ins2 Akita diabetic mice. Invest Ophthalmol Vis Sci. 2008;49:2635–2642. doi: 10.1167/iovs.07-0683. [DOI] [PubMed] [Google Scholar]
- 8.D'Cruz TS, Weibley BN, Kimball SR, Barber AJ. Post-translational processing of synaptophysin in the rat retina is disrupted by diabetes. PLoS One. 2012;7:e44711. doi: 10.1371/journal.pone.0044711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investigative ophthalmology & visual science. 2011;52:1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieth E, LaNoue KF, Antonetti DA, Ratz M Group TPSRR. Diabetes reduces glutamate oxidation and glutamine synthesis in the retina. Experimental Eye Research. 2000;70:723–730. doi: 10.1006/exer.2000.0840. [DOI] [PubMed] [Google Scholar]
- 11.Pannicke T, Iandiev I, Wurm A, Uckermann O, vom Hagen F, Reichenbach A, Wiedemann P, Hammes HP, Bringmann A. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006;55:633–639. doi: 10.2337/diabetes.55.03.06.db05-1349. [DOI] [PubMed] [Google Scholar]
- 12.Barber AJ, Antonetti DA, Gardner TW. Altered expression of retinal occludin and glial fibrillary acidic protein in experimental diabetes. The Penn State Retina Research Group. Investigative Ophthalmology & Visual Science. 2000;41:3561–3568. [PubMed] [Google Scholar]
- 13.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. The New England journal of medicine. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 15.Reiter CEN, Wu X, Sandirasegarane L, Nakamura M, Gilbert KA, Singh RSJ, Fort PE, Antonetti DA, Gardner TW. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [DOI] [PubMed] [Google Scholar]
- 16.Harrison WW, Bearse MA, Jr, Ng JS, Jewell NP, Barez S, Burger D, Schneck ME, Adams AJ. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Investigative ophthalmology & visual science. 2011;52:772–777. doi: 10.1167/iovs.10-5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fort PE, Losiewicz MK, Pennathur S, Jefferson LS, Kimball SR, Abcouwer SF, Gardner TW. mTORC1-independent reduction of retinal protein synthesis in type 1 diabetes. Diabetes. 2014;63:3077–3090. doi: 10.2337/db14-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox TE, Han X, Kelly S, Merrill AH, Jr, Martin RE, Anderson RE, Gardner TW, Kester M. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–3580. doi: 10.2337/db06-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tikhonenko M, Lydic TA, Wang Y, Chen W, Opreanu M, Sochacki A, McSorley KM, Renis RL, Kern T, Jump DB, et al. Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes. 2010;59:219–227. doi: 10.2337/db09-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piano I, Novelli E, Della Santina L, Strettoi E, Cervetto L, Gargini C. Involvement of Autophagic Pathway in the Progression of Retinal Degeneration in a Mouse Model of Diabetes. Front Cell Neurosci. 2016;10:42. doi: 10.3389/fncel.2016.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. The New England journal of medicine. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 22.Gray EJ, Gardner TW. Retinal failure in diabetes: a feature of retinal sensory neuropathy. Curr Diab Rep. 2015;15 doi: 10.1007/s11892-015-0683-5. [DOI] [PubMed] [Google Scholar]
- 23.Simonsen SE. ERG in Juvenile Diabetics: a prognostic study. In: Goldberg MF, Fine SL, editors. Symposium on the Treatment of Diabetic Retinopathy. Arlington: US Department of Health, Education and Welfare; 1969. pp. 681–689. [Google Scholar]
- 24.Bresnick GH, Korth K, Groo A, Palta M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Arch Ophthalmol. 1984;102:1307–1311. doi: 10.1001/archopht.1984.01040031057023. [DOI] [PubMed] [Google Scholar]
- 25.Orlov S, Cherney DZ, Pop-Busui R, Lovblom LE, Ficociello LH, Smiles AM, Warram JH, Krolewski AS, Perkins BA. Cardiac autonomic neuropathy and early progressive renal decline in patients with nonmacroalbuminuric type 1 diabetes. Clin J Am Soc Nephrol. 2015;10:1136–1144. doi: 10.2215/CJN.11441114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC., 3rd From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57:1439–1445. doi: 10.2337/db08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bek T. Transretinal histopathological changes in capillary-free areas of diabetic retinopathy. Acta Ophthalmol (Copenh) 1994;72:409–415. doi: 10.1111/j.1755-3768.1994.tb02787.x. [DOI] [PubMed] [Google Scholar]
- 28.Dodo Y, Murakami T, Uji A, Yoshitake S, Yoshimura N. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56:2012–2020. doi: 10.1167/iovs.14-15924. [DOI] [PubMed] [Google Scholar]
- 29.Sun JK, Lin MM, Lammer J, Prager S, Sarangi R, Silva PS, Aiello LP. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–1316. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 30.Sun JK, Radwan SH, Soliman AZ, Lammer J, Lin MM, Prager SG, Silva PS, Aiello LB, Aiello LP. Neural Retinal Disorganization as a Robust Marker of Visual Acuity in Current and Resolved Diabetic Macular Edema. Diabetes. 2015;64:2560–2570. doi: 10.2337/db14-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarinci F, Jampol LM, Linsenmeier RA, Fawzi AA. Association of Diabetic Macular Nonperfusion With Outer Retinal Disruption on Optical Coherence Tomography. JAMA Ophthalmol. 2015;133:1036–1044. doi: 10.1001/jamaophthalmol.2015.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP Los Angeles Latino Eye Study, G. Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118:649–655. doi: 10.1016/j.ophtha.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sohn EH, van Dijk HW, Jiao C, Kok PH, Jeong W, Demirkaya N, Garmager A, Wit F, Kucukevcilioglu M, van Velthoven ME, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113:E2655–2664. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson GR, Scott IU, Quillen DA, Walter LE, Gardner TW. Inner retinal visual dysfunction is a sensitive marker of non-proliferative diabetic retinopathy. The British journal of ophthalmology. 2012;96:699–703. doi: 10.1136/bjophthalmol-2011-300467. [DOI] [PubMed] [Google Scholar]


