Abstract
Biofunctionalization of nanoparticles (NPs) is an essential component in targeted drug delivery. However, current nanotechnology remains inadequate to imitate complex intercellular interactions existing in physiological conditions in human bodies. Emerging concepts have been explored to utilize human cells to generate cell membrane-formed NPs because cells retain inherent abilities to interact with human tissues compared with synthetic nanomaterials. Neutrophils, red blood cells (RBCs), platelets and monocytes have been employed to form therapeutic NPs to treat vascular disease and cancer, and these novel drug delivery platforms show the translation potential to improve patient quality of life. In this review, we will discuss the concept of cell membrane-formed NPs, the molecular mechanisms of their disease targeting and the potential of personalized nanomedicine.
Keywords: : cell-derived nanovesicles, inflammation, nanotechnology
Synthetic nanoparticles (NPs) have been used in diagnosis and drug delivery to treat a wide range of diseases [1–8]. In particular, NPs are made from lipids [9,10], polymers [11], proteins [12–15] and metals [16–18]. To deliver NPs to pathological sites in the body, NPs are often conjugated with targeting ligands or antibodies [19]. However, biofunctionalization of NPs still remains insufficient to reproduce complex and multicellular interactions present in a human body, thus possibly limiting the efficacy of drug delivery using those nanotechnologies for translation [20,21].
Intercellular communication is fundamental to survival and maintenance of homeostasis in all multicellular systems. The communication is involved with two major pathways via direct cell interactions and soluble signaling molecules secreted by cells. Recent studies indicate that an additional mechanism plays a central role in regulating the intercellular interactions over a short and long distance via extracellular vesicles (EVs) [22,23]. EVs are spherical and nanoscale vesicles derived from cell membrane, and they contain various membrane proteins and lipids [24]. A range of cellular adhesion molecules are highly expressed on EVs, therefore they can mediate intercellular communications via endocytosis, and they could be utilized to selectively deliver therapeutics to a target [25]. EVs could overcome the limitation of synthetic NPs because EVs are endogenous cellular compartments and immune tolerance. However, inefficient production of EVs and drug loading remain problematic for drug delivery and therapies [26,27]. The main component of EVs is made of cell plasma membrane, therefore EV-like nanovesicles can be constructed using physical or chemical approaches to directly disrupt cell membrane to form vesicles [25,28–30].
Combining individual advantages of synthetic NPs and cell membrane nanovesicles, several drug delivery systems have been explored based on a hybrid structure. This novel concept could impact personalized nanomedicine. For example, human cell membrane-coated NPs can be made after human cells are isolated from patients and are loaded with therapeutic NPs. The drug delivery formulation is able to avoid detection by a patient's immune system while displaying all of the beneficial disease-targeting functions of the parent cells. For instance, a red blood cell (RBC) membrane-coated NP possesses the similar long circulation time to a natural RBC [28].
Inflammation is the natural response from infection and tissue injury [31–33]. If inflammation consistently operates, it results in acute and chronic diseases such as acute lung injury [34–36], cancer [37,38] and atherosclerosis [39,40]. During acute inflammation, vascular endothelium activates to recruit neutrophils (a type of white blood cell) and platelets through several adhesion molecules [41]. The interaction between endothelial cells and neutrophils is regulated by the binding partners of intercellular adhesion molecule-1 (ICAM-1)/integrin β2, E-selectin/E-selectin ligand and PSGL-1/P-selectin. Platelets interact with endothelial cells via the binding of GPIbα to P-selectin [42]. Based on intercellular interactions, several cell types have been exploited to generate cell membrane-derived NPs for targeted drug delivery.
However, cell membrane-derived NPs are not limited to those that constitute the blood stream. For instance, a recent study utilized EV-mimetic nanovesicles derived from murine pancreatic β cells [43]. This study focuses on restoring the loss and dysfunction of pancreatic β cells that result in impaired glucose homeostasis in diabetes mellitus [44]. Since sources of β cells are limited there has been a great interest in developing an alternative source. In diabetic immunocompromised mice, they constructed a 3D in vivo microenvironment consisting of Matrigel mixed with bone marrow (BM) cells that were transplanted into the subcutaneous space. Subsequently, β-cell-derived nanovesicles were administered into this Matrigel platform and guided the differentiation of BM cells into insulin-producing cells, which could maintain physiological glucose levels for over 60 days. This study further demonstrates the diversity of cellular sources that can be utilized for generating NPs and additional applications in regenerative medicine.
Here, we focus on four types of cell membrane-derived NPs from neutrophils, RBCs, platelets or monocytes (Figure 1A). We will focus on how cell membrane-derived NPs are created, and the role in which these NPs play for treating vascular inflammation, cancer and detoxification. Finally, we will discuss the perspective of nanotechnology and possible future applications.
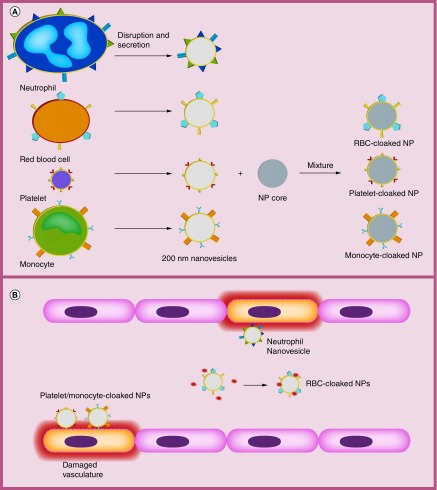
Figure 1. . Schematic of general preparation of membrane-coated nanoparticles and inflamed vasculature.
(A) Specific preparation of NPs varies depending on study but overall process remains the same to generate empty membrane nanovesicles. Vesicles are extracted from parent cells such as neutrophils, RBCs and platelets by disruption/secretion. These membrane-derived vesicles that retain the cell membrane antigens of parent cells can be combined with a nanoparticle core to form the final camouflaged nanoparticle. (B) Neutrophil, platelet and monocyte membrane-camouflaged nanovesicles can directly target and bind inflamed vasculature. RBC membrane-camouflaged nanovesicles can absorb cell membrane-damaging toxins (PFTs) in blood.
NP: Nanoparticle; PFT: Pore-forming toxin; RBC: Red blood cell.
Neutrophil membrane-formed NPs
Neutrophils are the most abundant circulating leukocytes in humans and the first immune cells that migrate to the tissues infected by bacteria or viruses [45]. This response is called acute inflammation involved with neutrophil infiltration and cytokine release [41]. Neutrophil infiltration is regulated by the intercellular interaction between vascular endothelium and neutrophils through several adhesion molecules. For example, ICAM-1 expressed on endothelium interacts with integrin β2 expressed on neutrophils to facilitate neutrophil vascular transmigration [14,32].
Excessive vascular inflammation could cause organ failure and damage such as acute lung inflammation/injury and sepsis [46]. Targeting inflammatory vasculature could reverse the disease progression [32]. NPs conjugated with anti-ICAM-1 or vascular targeting peptides were exploited to deliver inflammation inhibitors to vasculature [47,48], but the conjugation approach often could not achieve the targeting properties of theoretical design because of complex in vivo environments [21].
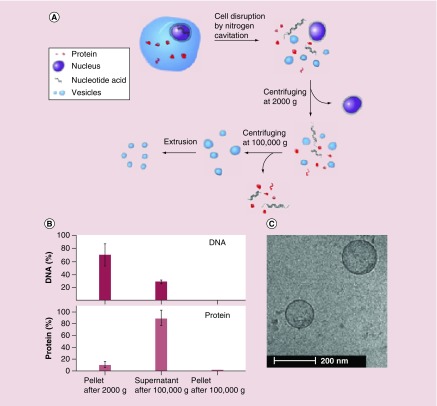
Inspired with the interaction between neutrophils and vascular endothelium during acute inflammation, a new strategy has been proposed in which activated neutrophils are disrupted by a physical force to form cell membrane nanovesicles [25,30]. Recent studies show that nitrogen cavitation is able to generate cell membrane-formed nanovesicles using HL60 cell line (neutrophil-like cells) [25]. Nitrogen cavitation is a process in which cells are placed in a vessel containing nitrogen gas kept at a high pressure. When the pressure is rapidly released, nitrogen bubbles are formed in a cell until it breaks the cell, and subsequently intracellular contents are quickly released leaving the cell membrane to spontaneously form nanosized vesicles (Figure 2A). After the centrifugation at 100,000 × g, there was no measurable amount of DNA left in the resulting pellet, which would be a safety concern, but 1.3% of whole cell proteins remained (Figure 2B). Upon further examination of proteins with a bichinchoninic acid assay, the pellet was found to be 50% protein, which is consistent with studies that have shown that cell membranes are 50% protein and 50% lipid [49]. This demonstrates that the pellet composition is mainly made of cell plasma membrane. When cryo-TEM along with dynamic light scattering were used to visualize this pellet, the spherical and hollow structure was observed, and the diameter was 200 nm with a shell thickness of 3–4 nm (Figure 2C). The double lipid layer of a cell is 3–4 nm in thickness. Collectively, the biochemistry studies and TEM image clearly indicate that nitrogen cavitation is a novel means to generate cell membrane-derived nanovesicles [25,30].
Figure 2. . Generation of nanovesicles utilizing nitrogen cavitation on HL60 neutrophil-like cells.
(A) Schematic shows the disruption of a cell into its cellular components, centrifugation that separates nanovesicles from other contents and extrusion. (B) Percentage of DNA and protein during varying steps of centrifugation in part A. (C) Cryo-TEM image of nanovesicles derived from HL60 cells.
Reproduced with permission from [25].
Because nitrogen cavitation provides the physical force to quickly break a cell, membrane proteins can maintain their natural properties compared with cell lysis approaches. Unlike other lysis, there is no heat or chemical damage to membrane proteins during nitrogen cavitation, and the nanovesicles are protected from oxidation by nitrogen gas. Nitrogen cavitation is fast and uniform because the same disruption force is applied within each cell and throughout the sample, ensuring reproducible cell-free homogenates [50]. The nitrogen cavitation approach is controllable, easy to scale up and leaves important cell membrane proteins intact for targeting specificity [25,50].
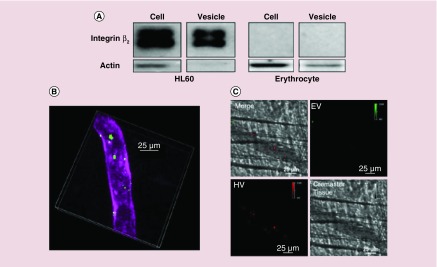
To investigate whether neutrophil membrane-derived nanovesicles expressed adherent molecules required to bind to activated endothelium, the western blot of nanovesicles and their resource cells were performed (Figure 3A). Neutrophil-derived nanovesicles highly expressed integrin β2 compared with their parent cells and RBCs as control did not express integrin β2. The intravital microscopy of cremaster venules showed that neutrophil-formed nanovesicles were adherent to inflamed endothelium treated with TNF-α (Figure 3B). When simultaneously injected with neutrophil nanovesicles and RBC nanovesicles, only neutrophil nanovesicles were adherent to endothelium but not RBC nanovesicles. The result indicates that integrin β2 is required for neutrophil nanovesicles to attach to endothelial vessels via ICAM-1. This specificity enables them to deliver therapeutics to activated endothelium during inflammation. To examine their usefulness, the nanovesicles were loaded with anti-inflammation drug (TPCA-1, [5-(p-Fluorophenyl)-2-ureido]thiophene-3-carboxaminde) to reverse acute lung inflammation/injury [51]. TPCA-1 targets the NF-κB pathway so that expression of ICAM-1 on endothelium was dramatically reduced and cytokine production was limited in a mouse model of acute lung inflammation induced by lipopolysaccharide [25]. This represents a novel and new strategy to treat vascular diseases.
Figure 3. . Neutrophil-derived nanovesicles target to inflamed vasculature induced by TNF-α.
(A) Western blot shows the presence of integrin β2 on neutrophils (HL60 cells) and erythrocyte cell membrane nanovesicles (EV) as control for comparison of targeting ability of integrin β2. (B) Intravital image of inflamed mouse cremaster venules shows the bound HL60 membrane-formed nanovesicles (HV) in green and the vasculature labeled with PECAM-1. (C) Image comparing binding of HV and EV to inflamed endothelium using intravital microscopy.
EV: Extracellular vesicle; HV: HL60 neutrophil-like vesicles; PECAM-1 (platelet endothelial cell adhesion molecule-1): endothelium marker
Reproduced with permission from [25].
Although there are limited studies on neutrophil NPs, a recent study was reported that polymeric NPs were coated with neutrophil membrane for targeting of endothelium and circulation tumor cells (CTCs) in the breast cancer mouse model [52]. In this study, the natural targeting ability of neutrophils for both CTCs and premetastatic niche inspired the creation of neutrophil membrane-coated NPs. The NPs demonstrated continuous capture of CTCs in circulation and when loaded with carfilzomib, a proteasome inhibitor, they showed therapeutic potential in prevention of early metastasis as well as inhibition in the progression of already-formed metastasis. Furthermore, this core-shell structure may allow the efficient loading of therapeutics into nanovesicles.
Erythrocyte membrane-cloaked NPs
Erythrocytes, or RBCs, are the body's natural delivery vehicles that possess an innate long circulation time. They possess a noteworthy design that allows them to flow through capillaries smaller than their own diameter [53]. In order to create an erythrocyte-coated nanovesicle, the membrane must be separated from the contents of the cell while preserving the original membrane antigens, therefore maintaining their natural targeting ability.
Using hypotonic medium or chemical agents, RBC ghost vesicles are formed without hemoglobin [28]. Once the membrane has formed vesicles, it can then be combined with a NP such as a poly(lactic-co-glycolic acid) polymer (PLGA) as a core loaded with a drug of choice. Transmission electron microscopy showed this core-shell structure, implying that the cellular membrane is translocated to a NP surface. The biodistribution study reveals that NPs are present in the blood even 72 h after injection of NPs [28]. Compared with the state-of-the-art PEG-coated PLGA NPs, RBC-coated NPs show the superior circulation half-lifetime.
Due to the longer circulation of RBC-coated NPs in the blood, they were designed to become a nanosponge that absorbs pore-forming toxins (PFTs) for detoxification (Figure 1) [54]. PFTs disrupt the cell membrane and alter its permeability as a major virulence mechanism to attack cells, leading to systemic toxicity, for example, various bacterial infections [55] and in the pathogenesis of venomous bites [56]. Cell membrane-coated NPs could competitively absorb PFTs in the blood, thus preventing the severe and systemic spreading of toxins. In a mouse model, the nanosponges significantly decreased the toxicity of staphylococcal alpha-hemolysin [57,58].
The same group reported that RBC-coated NPs containing toxins were applied to toxoid vaccination. Immunization against bacterial PFTs is a novel means to prevent infections, such as Escherichia coli, Helicobacter pylori and Staphylococcus aureus. The improvement of vaccine potency and safety is a key factor to develop vaccines. RBC-coated NPs can neutralize the membrane-damage activity of PFTs after RBC membrane detains the PFTs, and this NP system will activate the adaptive immune system to generate immunization against PFT-induced infections. For example, a vaccine against staphylococcal alpha-hemolysin, a specific type of PFT in mice, has been shown to be superior to standard vaccination with heat-denatured toxins [59]. Antitoxin vaccines are currently synthesized from either chemical or heat-denatured toxin, which inactivates the toxins. This denaturing process, however, can lead to disrupted antigenic presentation and lowered vaccine immunogenicity [60,61]. By using a NP-based toxoid vaccine, the natural structure of PFTs is preserved for enhanced protection of immunity. These findings open up the possibility of using membrane-coated NPs for the administration of vaccinations against other bacterial toxins.
Recently, there is increasing interest in research surrounding erythrocyte-camouflaged NPs for synergistic chemo-photodynamic therapy of cancers [62–65]. In one recent study of interest, erythrocyte-derived nanoprobes were functionalized with antibodies [66]. The nanostructures were loaded with indocyanine green (ICG) which is the near infrared dye that is US FDA approved. Free ICG is easily degradable in plasma resulting in a half-life of 2–4 min and does not have targeting specificity. Erythrocytes naturally have a long circulation time; therefore, when they are encapsulated with ICG, imagining will be allowed over a longer period. With the addition of successful functionalization of antibodies, these NPs demonstrate a new generation of molecular imaging in cancer.
Furthermore, erythrocyte polymeric NPs are being used to carry therapeutic agents for antigen delivery to dendritic cells (DCs) for an efficient induction of cytotoxic T lymphocyte-mediated response against tumors [67]. DCs are specifically targeted because of their role in collecting tumor antigens and secreting pro-inflammatory cytokines that promote T-cell activation. When antigens are encapsulated by nanocarriers, they are protected from degradation, improve uptake by DCs and generate a stronger T-cell response in situ. One of the findings were that the nanovaccine increased IFN-γ production and CD8+ T-cell response. Additionally, the nanovaccine retarded tumor occurring time, prevented tumor growth and inhibited tumor metastasis in various melanoma mouse models. This research shows promise for the future development of biomimetic vaccines that elicit tumor-specific immune responses.
Reported recently was the development of an erythrocyte-platelet hybrid NP [68]. This study shows that it is indeed possible to fuse two different types of cell membrane, and the fused membrane NPs carry properties from both cell types. Additionally, this study gives light to future combinations that will potentially display the advantages of both cell types. For instance, combination of platelet and erythrocyte membranes. Erythrocytes have the longest circulation life of all the cells discussed, but they do not target inflamed vasculature. On the other hand, platelets have a shorter half-life but do target inflammation sites. Fusing them together can provide a NP with targeting ability and a long half-life. Countless combinations are about to be explored and there are seemingly endless possibilities.
Platelet membrane-cloaked NPs
Platelets are essential components to maintain hemostasis. Platelets are small, non-nucleated membrane cell fragments that circulate in the blood. Platelets possess a wide array of cell membrane antigens and are responsible for immune defense [69]. When blood vessels are destroyed, the injury exposes proteins, such as collagen, that are rich in the subendothelial layer underneath the vessel wall. The platelets can bind to these proteins with high affinity and then release blood-clotting factors, promoting the formation of a platelet plug that helps to heal the wound.
Inspired with the unique intercellular interaction, the platelet membrane is coated to NPs so that the hybrid structure can target the injured vasculature. A platelet is an important component of the blood with a natural ability to target vascular injury sites (Figure 1) [70–72]. In a recent experiment with the mouse models of both coronary restenosis and systemic bacterial infections, platelet membrane-coated polymeric PLGA NPs showed increased therapeutic efficacy when compared with uncoated particles [29]. Interestingly, when injected in the blood, the platelet-coated NPs showed the reduced uptake by macrophage-like cells, thus possibly increasing the NP deposition in the target.
In addition, platelets can adhere to other disease-relevant substrates such as CD44 receptors upregulated on cancer cell surfaces. Platelet membrane is coated to polymeric NPs to exploit both the extracellular and intracellular components of platelet nanovesicles in the targeting of tumor cells. The inside is loaded with a small molecular drug (doxorubicin) that targets an intrinsic apoptosis pathway in tumor cells while the outside is decorated with TNF-related apoptosis inducing ligand that induces extrinsic apoptosis by binding to the cell surface [73]. This way acts to synergistically attack tumor cells from the inside-out and displays the broad spectrum of functions that cell membrane nanovesicles possess.
Furthermore, an exciting application of platelet NPs is in the development of thrombus-targeted delivery of plasminogen activators [74]. Plasminogen activators facilitate the conversion of plasminogen to plasmin which acts to break down fibrin in a clot. Clinically, plasminogen activators are used in the treatment of occlusive vascular conditions such as heart attack and stroke. Their action is therapeutic at a blood clot site, however systemically a major side effect is hemorrhagic risk. Another issue with free thrombolytic agents is that they are readily deactivated by plasma components in the blood resulting in the short circulation lifetime. This study shows that their thrombolytic-loaded platelet NPs can protect and selectively deliver their cargo to the clot site of a carotid artery thrombosis mouse model without altering systemic hemostasis.
Platelet NPs have also been studied as theragnostic tools used for cancer diagnosis and therapy [75]. In this study, platelet vesicles with intact membrane proteins were generated and subsequently coated onto Fe3O4 magnetic NPs. The platelet membrane provides long circulation lifetime, immune compatibility and targeting abilities while the magnetic NP contributes optical absorption properties for MRI and photothermal therapy (PTT). In addition, they found that the enhanced targeting to the PTT site is attributed to both properties of PTT and targeting of platelet membrane. This study shows that nanotherapeutics have the potential to revolutionize cancer theranostics.
Monocyte-derived NPs
Monocytes are a subset of circulating white blood cells that have also shown great promise as cell-derived nanovesicles for drug delivery. Their precursors originate from the bone marrow (BM), and they can further differentiate into macrophages [76]. They represent 2–8% of leukocytes in human blood [77] and in the blood monocytes have a half-life of 1–3 days [78]. Similar to neutrophils and platelets, they play an important role in inflammation and vascular injury. Due to their biological functions, they possess intrinsic targeting toward inflammatory sites. Monocytes have a variety of deleterious roles in tissue damages, but recent studies show that monocytes also support the tissue regeneration [79].
Currently monocyte-derived nanovesicles have shown a variety of capabilities such as preclinical use as a diagnostic imaging tool for brain targeting cancer and inflammation [80]. In this study, macrophage-derived exosome-mimetic nanovesicles (ENVs) were radiolabeled with 99mTc-hexamethylpropyleneamineoxime in order to trace these ENVs in vivo. By developing this approach to monitor the biodistribution of ENVs, it enables us to investigate the in vivo behavior of nanovesicles, thus furthering their biomedical application. For example, loading NPs with imaging molecules and chemotherapeutics together could result in combined diagnostics and therapy.
Additionally, monocytic nanovesicles were developed as delivery vehicles for therapeutic RNA molecules [81]. Naked RNA is easily degraded and cannot readily pass through biological membranes to reach the cytoplasm of diseased cells. When an effective intracellular delivery system is achieved, therapeutic RNA is delivered to recipient cells and cause RNA interference. RNA interference is a process where RNA can modify gene expression and inhibit the function of specific mRNA. This could be particularly useful in attenuating single mutated genes or overexpressed oncogenes in cancer. This study shows that the monocyte-derived nanovesicles were efficiently delivered RNAi to recipient cells, which subsequently suppressed target gene expression.
Naturally, tumors exhibit abnormal angiogenesis that acts to support tumor growth [82]. Chemotherapeutic-loaded monocyte nanovesicles are able to bind to the abnormally proliferating endothelial cells and directly release chemotherapeutics in tumor tissues [83]. In this study, monocyte and macrophage nanovesicles were generated using serial extrusion through a series of nanosized filters. The nanovesicles maintained their plasma membrane topology, and therefore retained the natural targeting ability of their parent cells. Importantly, the chemotherapeutic drug-loaded nanovesicles reduced tumor growth to the same extent as 20-fold higher doses of free drug without adverse side effects. Plasma membrane topology of nanovesicles is crucial for their targeting ability because the therapeutic effect is reduced when plasma membrane proteins were removed using tyrosination. Furthermore, circulating monocytes are naturally targeted by cancers as a source of cell-proliferating growth factors [84]. Knowing this, one study utilized monocyte-derived cell membrane-coated PLGA loaded with doxorubicin via nanoprecipitation for targeted cancer therapy [85].
Potential translation of cell membrane-derived NPs
Standard pharmacokinetics generally dictate that dose increases correspond to an increase in both therapeutic effects and drug toxicity. With nanomedicine, a drug is encapsulated within a nanocarrier and as a result this increases its effectiveness as compared with traditional dosing formulations. Nanoparticle-based drug therapy increases circulation half-life and targeting specificity while decreases drug metabolism and drug toxicity. This is especially important for drugs that have high toxicities such as anticancer drugs, which have been a major focus of NP drug delivery. EVs, which are involved in intercellular communication, have been investigated as possible delivery platforms for therapeutic intervention. While there are some advantages of EVs, such as targeting specificity, blood stability and immune compatibility, they can also produce unwanted adverse effects due to their complicated and heterogenic nature [24]. In addition, EVs do not have suitable method for generation and purification needed for clinical translation [26]. In contrast, man-made nanovesicles using physical forces have shown to be more easily controlled and suitable for drug delivery, as shown above. With the use of these individual cellular carriers we would be able to have a patient-derived drug delivery system that would lead to personalized nanomedicine.
Despite the promise of the nanovesicle drug delivery, there are still some challenges that will need to be addressed before clinical application. For all cell types isolated from blood, it will be important that they go through a stringent sterilization process to avoid infection risks and if using a nonautologous blood source donor blood screening followed by blood type matching will need to be done in order to ensure maximum compatibility [86]. Challenges that platelet membrane-coated NPs have encountered moving forward into clinical translation include developing large-scale purification and derivation techniques [29]. This is a potential future opportunity to expand nitrogen cavitation to generation and purification of platelet membrane vesicles [25,30]. Upon review, neutrophil-derived nanovesicles may possess the advantage of ease of generation for clinical application. In circulating blood, human neutrophils make up 50–70% of all leukocytes [87]. Matched with an efficient technique such as nitrogen cavitation where 50–75% of cell membranes form nanovesicles [25,30], it would be possible to generate a large quantity of patient-derived nanovesicles from a single blood sample.
Conclusion & future perspective
Cell membrane-derived nanovesicles have the advantages of diverse biological entities that can be used for drug delivery. We summarize some key characteristics and challenges when we exploit them in a wide range of applications in medicine (Table 1). These biocompatible vesicles are naturally present in the human body, therefore they are not immediately attacked and removed. This enhances therapeutic efficacy by increasing its circulation time and selective targeting ability, ultimately leading to sustained drug concentrations within therapeutic windows. This also limits potential side effects and toxicity, which has always been the treatment goal for healthcare. Using the nitrogen cavitation approach for generating nanovesicles maintains membrane antigens of interest while providing a process that is able to scale up. With these recent advances in nanovesicle drug delivery, it is now fathomable for a patient to confidently manage their disease states with personalized treatment.
Table 1. . Summary of membrane-derived nanovesicles from different cell types.
| Cell types | Disease models/applications | Characteristics | Challenges |
|---|---|---|---|
| Neutrophils |
Acute lung inflammation/injury Cancer Sepsis |
First leukocyte at site of inflammation Most prevalent leukocyte in blood (50–75%) High-yield production of nanovesicles Targeting of inflamed vasculature |
Short circulating half-life |
| Erythrocyte |
Nanosponge for detoxification Diagnostic imaging Nanovaccine |
Longest circulating half-life Most abundant blood cells |
Less tissue targeting |
| Platelet |
Cancer Thrombosis Diagnostic imaging |
Targeting of inflamed/injured vasculature |
Isolation and purification Scalability |
| Monocyte |
Cancer Diabetes Diagnostic imaging RNAi delivery vehicles |
Higher production yield than exosomes Targeting of inflamed vasculature |
Less prevalent leukocyte in blood (2–8% of leukocytes) |
| EVs | Cancer Small RNA delivery vehicles Anti-inflammatory delivery Parkinson's disease |
Secretion from any types of cells Naturally involved in intercellular communication |
Low production yield; Difficult purification; Less efficient drug loading; Low scalability; Heterogeneity in size and composition |
EV: Extracellular vesicle.
Due to the variety of existing biological cells and their unique membrane antigens, there are many novel, high-specificity nanovesicle drug delivery systems left to be developed. In the process of developing future NP delivery systems, it is beneficial to use a top–down approach that is first disease driven from which we can identify the appropriate biological cells involved in order to target that particular disease state [25]. Once the biological cells of interest are identified, nanovesicles can be generated and purified using nitrogen cavitation. Nitrogen cavitation is not only reliable but also scalable to allow nanovesicle-based drug delivery systems to be transitioned into clinical use. Another direction that NP technology is making great strides in are vaccinations. Currently, research is being done to develop an approach to treat cancer prophylactically using anticancer vaccinations [88]. The idea behind these vaccinations is to train the human body to recognize and eliminate cancer cells that are normally able to avoid detection from the immune system [89]. Because of this, we can expect that future treatments become more personalized to patients. Patient blood specimens can be collected and the appropriate biological cells extracted and further purified to generate patient-derived nanovesicles. These nanovesicles possess natural stability in blood due to evasion of the immune system providing drug delivery that is superior to previous forms. With this proposed technology, it is possible to use the patient's own tumor cells or stem cells to spearhead a personalized cancer treatment [90,91].
In summary, cell membrane-derived nanovesicles and their loading with therapeutic NPs show the potential translation to treat a wide range of diseases. While the concepts are novel and the drug delivery systems can be tailored for individuals, new technologies are needed to scale up their production for clinical use.
Executive summary.
Background
While some advances in design and bioengineering of synthetic nanoparticles (NPs), exposure of NPs to complex and multicellular systems in vivo cannot avoid immune surveillance, thus decreasing therapeutic effects.
Extracellular vesicles, membrane-formed NPs, could overcome the limitations of synthetic NPs.
Extracellular vesicles-like nanovesicles can be made from a wide range of cell types.
Neutrophil membrane-formed NPs
Neutrophils are the most abundant circulating leukocytes in human, and the first immune cells that migrate to the tissues infected by bacteria or viruses.
Excessive vascular inflammation could cause organ failure and damage such as acute lung inflammation/injury, sepsis and cancers.
Inspired with the interaction between neutrophils and vascular endothelium during acute inflammation, a new strategy is proposed to generate neutrophil membrane-formed vesicles to target inflamed lungs, thus reversing acute lung injury.
Nitrogen cavitation can generate homogeneous size of neutrophil-derived nanovesicles with targeting ligand, integrin β2.
Intravital microscopy enables real-time visualization of interactions between neutrophil-derived nanovesicles and inflamed vasculature in a live mouse.
Erythrocyte membrane-cloaked NPs
Erythrocytes (red blood cells [RBCs]) are the body's natural delivery vehicles that possess an innate long circulation time.
Due to the longer circulation of RBC-cloaked NPs in the blood, they are designed to become a nanosponge that absorbs pore-forming toxins for detoxification.
RBC-cloaked NPs containing toxins are used in vaccination.
RBC-cloaked NPs are used for synergistic chemo-photodynamic therapy and immunotherapy in cancers.
Platelet membrane-cloaked NPs
Platelets are essential components to maintain hemostasis.
Inspired with the unique intercellular interactions, the platelet membrane is coated to NPs for targeting to injured vasculature and tumor microenvironments.
Hybrid structure of erythrocyte and platelet membrane combines both advantages.
Monocyte-derived nanovesicles
Monocytes are subset of circulating white blood cells and respond to tissue injury and infection.
Monocyte-derived nanovesicles are utilized for diagnostics in brain, cancer and inflammation.
Potential translation of cell membrane-formed NPs
Personalized nanomedicine using cell membrane-formed NPs.
Despite the promise of nanovesicles drug delivery, there are still some challenges needed to address, such as stringent sterilization, blood source, production yield and scalability.
Potential opportunity for nitrogen cavitation to generate membrane-formed nanovesicles, compared with other cell lysis techniques.
Human neutrophils are accounted for 50–75% of all leukocytes, therefore there is the translational opportunity for neutrophils as a novel drug delivery platform.
Footnotes
Financial & competing interests disclosure
The work was supported in part by NIH grant K25HL111157 to Z Wang. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Tiruppathi C, Minshall RD, Malik AB. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3(12):4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat. Rev. Drug Discov. 2014;13(9):655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Malik AB. Nanoparticles squeezing across the blood-endothelial barrier via caveolae. Ther. Deliv. 2013;4(2):131–133. doi: 10.4155/tde.12.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Tiruppathi C, Cho J, Minshall RD, Malik AB. Delivery of nanoparticle: complexed drugs across the vascular endothelial barrier via caveolae. IUBMB Life. 2011;63(8):659–667. doi: 10.1002/iub.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong X, Chu D, Wang Z. Leukocyte-mediated delivery of nanotherapeutics in inflammatory and tumor sites. Theranostics. 2017;7(3):751–763. doi: 10.7150/thno.18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z. Imaging nanotherapeutics in inflamed vasculature by intravital microscopy. Theranostics. 2016;6(13):2431–2438. doi: 10.7150/thno.16307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barenholz Y, Peer D. Liposomes and other assemblies as drugs and nano-drugs: from basic and translational research to the clinics. J. Control. Release. 2012;160(2):115–116. doi: 10.1016/j.jconrel.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 11.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Chen Q, Wang X, Wang C, Feng L, Li Y, Liu Z. Drug-induced self-assembly of modified albumins as nano-theranostics for tumor-targeted combination therapy. ACS Nano. 2015;9(5):5223–5233. doi: 10.1021/acsnano.5b00640. [DOI] [PubMed] [Google Scholar]
- 13.Chu D, Zhao Q, Yu J, Zhang F, Zhang H, Wang Z. Nanoparticle targeting of neutrophils for improved cancer immunotherapy. Adv. Healthc. Mater. 2016;5(9):1088–1093. doi: 10.1002/adhm.201500998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu D, Gao J, Wang Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS Nano. 2015;9(12):11800–11811. doi: 10.1021/acsnano.5b05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat. Nanotechnol. 2014;9(3):204–210. doi: 10.1038/nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M, Zheng J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano. 2015;9(7):6655–6674. doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song J, Yang X, Jacobson O, et al. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano. 2015;9(9):9199–9209. doi: 10.1021/acsnano.5b03804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu D, Dong X, Zhao Q, Gu J, Wang Z. Photosensitization priming of tumor microenvironments improves delivery of nanotherapeutics via neutrophil infiltration. Adv. Mater. 2017 doi: 10.1002/adma.201701021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014;13(11):813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvati A, Pitek AS, Monopoli MP, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013;8(2):137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat. Rev. Drug Discov. 2015;14(4):239–247. doi: 10.1038/nrd4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol. Med. 2014;20(7):385–393. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zomer A, Maynard C, Verweij FJ, et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom. Rev. 2015;34(4):474–490. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Chu D, Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J. Control. Release. 2016;224:208–216. doi: 10.1016/j.jconrel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates a novel approach to generate neutrophil membrane nanovesicles using nitrogen cavitation, and drug-loaded nanovesicles show the increased therapy in a mouse model of acute lung inflammation/injury.
- 26.Ingato D, Lee JU, Sim SJ, Kwon YJ. Good things come in small packages: overcoming challenges to harness extracellular vesicles for therapeutic delivery. J. Control. Release. 2016;241:174–185. doi: 10.1016/j.jconrel.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 28.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu CM, Fang RH, Wang KC, et al. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that platelet-cloaked nanoparticles can target inflamed vasculature and infection sites.
- 30.Gao J, Wang S, Wang Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials. 2017;135:62–73. doi: 10.1016/j.biomaterials.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates high yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles using nitrogen cavitation for anti-inflammation therapy such as acute lung injury and sepsis.
- 31.Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140(6):771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Anti-inflammatory agents: present and future. Cell. 2010;140(6):935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu. Rev. Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N. Engl. J. Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 36.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 37.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 39.Hansson GK. Mechanisms of disease – inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 40.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 41.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat. Med. 2011;17(11):1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21(2):99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Oh K, Kim SR, Kim DK, et al. In vivo differentiation of therapeutic insulin-producing cells from bone marrow cells via extracellular vesicle-mimetic nanovesicles. ACS Nano. 2015;9(12):11718–11727. doi: 10.1021/acsnano.5b02997. [DOI] [PubMed] [Google Scholar]
- 44.Vetere A, Choudhary A, Burns SM, Wagner BK. Targeting the pancreatic beta-cell to treat diabetes. Nat. Rev. Drug Discov. 2014;13(4):278–289. doi: 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- 45.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J. Clin. Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muro S, Dziubla T, Qiu W, et al. Endothelial targeting of high-affinity multivalent polymer nanocarriers directed to intercellular adhesion molecule 1. J. Pharmacol. Exp. Ther. 2006;317(3):1161–1169. doi: 10.1124/jpet.105.098970. [DOI] [PubMed] [Google Scholar]
- 48.Khondee S, Olsen CM, Zeng Y, Middaugh CR, Berkland C. Noncovalent PEGylation by polyanion complexation as a means to stabilize keratinocyte growth factor-2 (KGF-2) Biomacromolecules. 2011;12(11):3880–3894. doi: 10.1021/bm2007967. [DOI] [PubMed] [Google Scholar]
- 49.Cooper GM. The Cell: A Molecular Approach. ASM Press; DC, USA: 2000. [Google Scholar]
- 50.Simpson RJ. Disruption of cultured cells by nitrogen cavitation. Cold Spring Harb. Protoc. 2010;2010(11):pdb prot5513. doi: 10.1101/pdb.prot5513. [DOI] [PubMed] [Google Scholar]
- 51.Podolin PL, Callahan JF, Bolognese BJ, et al. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell proliferation. J. Pharmacol. Exp. Ther. 2005;312(1):373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]
- 52.Kang T, Zhu Q, Wei D, et al. Nanoparticles coated with neutrophil membranes can effectively treat cancer metastasis. ACS Nano. 2017;11(2):1397–1411. doi: 10.1021/acsnano.6b06477. [DOI] [PubMed] [Google Scholar]
- 53.Dzierzak E, Philipsen S. Erythropoiesis: development and differentiation. Cold Spring Harb. Perspect. Med. 2013;3(4):a011601. doi: 10.1101/cshperspect.a011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013;8(5):336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosado CJ, Kondos S, Bull TE, et al. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 2008;10(9):1765–1774. doi: 10.1111/j.1462-5822.2008.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andreeva-Kovalevskaya ZI, Solonin AS, Sineva EV, Ternovsky VI. Pore-forming proteins and adaptation of living organisms to environmental conditions. Biochemistry (Mosc.) 2008;73(13):1473–1492. doi: 10.1134/s0006297908130087. [DOI] [PubMed] [Google Scholar]
- 57.Hu C-MJ, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv. Healthc. Mater. 2012;1(5):537–547. doi: 10.1002/adhm.201200138. [DOI] [PubMed] [Google Scholar]
- 58.Hu C-MJ, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat. Nanotechnol. 2013;8(5):336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes a new biomimetic nanosponge that absorbs pore-forming toxins for detoxification.
- 59.Hu C-MJ, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat. Nanotechnol. 2013;8(12):933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metz B, Kersten GFA, Hoogerhout P, et al. Identification of formaldehyde- induced modifications in proteins: reactions with model peptides. J. Biol. Chem. 2004;279(8):6235–6243. doi: 10.1074/jbc.M310752200. [DOI] [PubMed] [Google Scholar]
- 61.Cryz SJJ, Furer E, Germanier R. Effect of chemical and heat inactivation on the antigenicity and immunogenicity of Vibrio cholerae . Infect. Immun. 1982;38(1):21–26. doi: 10.1128/iai.38.1.21-26.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen WS, Zeng K, Liu H, et al. Cell membrane camouflaged hollow prussian blue nanoparticles for synergistic photothermal-/chemotherapy of cancer. Adv. Funct. Mater. 2017;27(11) Epub ahead of print. [Google Scholar]
- 63.Su JH, Sun HP, Meng QS, et al. Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Adv. Funct. Mater. 2016;26(41):7495–7506. [Google Scholar]
- 64.Su JH, Sun HP, Meng QS, Zhang PC, Yin Q, Li YP. Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics. 2017;7(3):523–537. doi: 10.7150/thno.17259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ren XQ, Zheng R, Fang XL, et al. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials. 2016;92:13–24. doi: 10.1016/j.biomaterials.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Mac JT, Nunez V, Burns JM, Guerrero YA, Vullev VI, Anvari B. Erythrocyte-derived nano-probes functionalized with antibodies for targeted near infrared fluorescence imaging of cancer cells. Biomed. Opt. Express. 2016;7(4):1311–1322. doi: 10.1364/BOE.7.001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo YY, Wang D, Song QL, et al. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9(7):6918–6933. doi: 10.1021/acsnano.5b01042. [DOI] [PubMed] [Google Scholar]
- 68.Dehaini D, Wei XL, Fang RH, et al. Erythrocyte-platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater. 2017;29(16) doi: 10.1002/adma.201606209. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jenne CN, Urrutia R, Kubes P. Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013;35(3):254–261. doi: 10.1111/ijlh.12084. [DOI] [PubMed] [Google Scholar]
- 70.Doshi N, Orje JN, Molins B, Smith JW, Mitragotri S, Ruggeri ZM. Platelet mimetic particles for targeting thrombi in flowing blood. Adv. Mater. 2012;24(28):3864–3869. doi: 10.1002/adma.201200607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anselmo AC, Modery-Pawlowski CL, Menegatti S, et al. Platelet-like nanoparticles: mimicking shape, flexibility, and surface biology of platelets to target vascular injuries. ACS Nano. 2014;8(11):11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 73.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv. Mater. 2015;27(44):7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pawlowski CL, Li W, Sun M, et al. Platelet microparticle-inspired clot-responsive nanomedicine for targeted fibrinolysis. Biomaterials. 2017;128:94–108. doi: 10.1016/j.biomaterials.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rao L, Bu LL, Meng QF, et al. Antitumor platelet-mimicking magnetic nanoparticles. Adv. Funct. Mater. 2017;27(9) Epub ahead of print. [Google Scholar]
- 76.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 78.Yang J, Zhang L, Yu C, Yang X-F, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker Res. 2014;2(1):1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Apostolakis S, Lip GYH, Shantsila E. Monocytes in heart failure: relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc. Res. 2010;85(4):649–660. doi: 10.1093/cvr/cvp327. [DOI] [PubMed] [Google Scholar]
- 80.Hwang DW, Choi H, Jang SC, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using Tc-99m-HMPAO. Sci. Rep. 2015;5:15636. doi: 10.1038/srep15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lunavat TR, Jang SC, Nilsson L, et al. RNAi delivery by exosome-mimetic nanovesicles – implications for targeting c-Myc in cancer. Biomaterials. 2016;102:231–238. doi: 10.1016/j.biomaterials.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 82.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 83.Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 84.Laoui D, Van Overmeire E, De Baetselier P, Van Ginderachter JA, Raes G. Functional relationship between tumor-associated macrophages and macrophage colony-stimulating factor as contributors to cancer progression. Front. Immunol. 2014;5:489. doi: 10.3389/fimmu.2014.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krishnamurthy S, Gnanasammandhan MK, Xie C, Huang K, Cui MY, Chan JM. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale. 2016;8(13):6981–6985. doi: 10.1039/c5nr07588b. [DOI] [PubMed] [Google Scholar]
- 86.Naeem S, Kiew LV, Yong CL, Yin YT, Bin Misran M. Drug delivery and innovative pharmaceutical development in mimicking the red blood cell membrane. Rev. Chem. Eng. 2015;31(5):491–508. [Google Scholar]
- 87.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang RH, Kroll AV, Zhang L. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small. 2015;11(41):5483–5496. doi: 10.1002/smll.201501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mellstedt H, Gaudernack G, Gerritsen WR, et al. Awareness and understanding of cancer immunotherapy in Europe. Hum. Vaccin. Immunother. 2014;10(7):1828–1835. doi: 10.4161/hv.28943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toledano Furman NE, Lupu-Haber Y, Bronshtein T, et al. Reconstructed stem cell nanoghosts: a natural tumor targeting platform. Nano Lett. 2013;13(7):3248–3255. doi: 10.1021/nl401376w. [DOI] [PubMed] [Google Scholar]
- 91.Fang RH, Hu CM, Luk BT, et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]