Abstract
The disruptive effects of severe stress on reproductive function are well documented, but surprisingly few studies exist that demonstrate milder psychosocial stressors interfere with the ovarian cycle in females. We hypothesized repeated application of psychosocial stress would disrupt estrous cycles in mice. Mice were transferred to a new cage, transported to a new room, and restrained (2 hours) for 21 consecutive days. Contrary to our hypothesis, this paradigm did not affect estrous cycles. We next tested the hypothesis that a single exposure to mild stress disrupts a specific aspect of the cycle: the proestrous luteinizing hormone (LH) surge. We developed a model of acute, layered psychosocial stress (sequential application of new cage, transport to new room, restraint and predator cues lasting 5 hours total) that consistently increased circulating corticosterone. Application of this stress paradigm on midmorning of proestrus disrupted the LH surge measured near lights out in 14 of 24 mice; there was no evidence for a 24-hour delay of the surge. Following stress, mice continued to have normal estrous cycles, even when the LH surge was disrupted. Stressed mice failing to exhibit an LH surge had uterine masses suggesting the proestrous estradiol rise occurred. To test specifically whether the layered stress paradigm blocks estradiol-dependent positive feedback mechanisms, we examined the estradiol-induced LH surge. Stress blocked the estradiol-induced LH surge in all mice. These results suggest exposure to mild, acute psychosocial stress on proestrus can severely disrupt the generation of the LH surge in mice without affecting the overall estrous cycle.
Exposure to psychosocial stress is often linked to infertility. We show repeated stress did not affect estrous cycles, whereas a single exposure during proestrus disrupted the LH surge.
Stress is often associated with disruptions in fertility in women (1, 2). A large body of literature demonstrates the deleterious effects of severe disruptions in homeostasis (e.g., endotoxin, caloric restriction, hypoglycemia) on the hypothalamo–pituitary–gonadal (HPG) axis, specifically reduced gonadotropin-releasing hormone (GnRH) and/or luteinizing hormone (LH) release (3–7). Direct evidence is limited, however, that milder but more prevalent psychosocial stressors can affect reproduction. Psychosocial stress has been attributed as an underling cause of functional hypothalamic amenorrhea (8–10), a common menstrual cycle disorder, but many reports are anecdotal and a clear cause-and-effect relationship has not been identified.
Studies using controlled stress paradigms in animal models have provided evidence that psychosocial stress alters female reproductive cycles, although effects vary with species and stress paradigm/duration. Most reports suggest that a prolonged or repeated exposure to mild stress is needed to affect ovarian cycles. In monkeys, an extended psychosocial stress (spanning at least one menstrual cycle) combined with other mild stressors interferes with progesterone and LH secretion and disrupts the cycle by lengthening the follicular phase and/or increasing incidence of insufficient luteal function (11, 12). In mice, a daily 3-hour restraint applied for 18 days lengthens estrous cycles (13). Longer stress durations are not always needed, however, to impair ovarian cycles. For example, in Syrian hamsters, a single exposure to a novel object, such a new running wheel or movement to a new cage, on the morning of proestrus can delay the onset of estrus by 24 hours (14, 15).
In addition to disruptions in the ovarian cycle being attributed to psychosocial stress, specific aspects of reproductive control such as pulsatile release of GnRH and LH (16, 17), pituitary responsiveness to GnRH (16), and the preovulatory LH surge have been examined. These studies have largely used single exposures to acute stress to investigate effects on gonadotropins. In sheep, a 4-hour transport stress delayed LH surge onset when applied during the interval between corpus luteum regression and the expected time of the LH surge (18). Restraint (5 to 7 hours) on the day of proestrus in rats blocked the LH surge in ∼50% of animals, with the remaining animals showing a blunted surge amplitude compared with nonstressed controls (19).
Given the persistent nature of psychosocial stress in modern society, it is important to determine the impact this type of stress may have on overall reproductive fitness as well as the underlying mechanisms. To accomplish this, we conducted a series of experiments to test effects of either daily repeated or single exposure to acute psychosocial stress on estrous cyclicity and the LH surge in female mice. In the course of these studies, we developed and validated a model of layered, acute psychosocial stressors in mice. The results suggest the estrous cycle is resistant to disruptions by psychosocial stress, but a specific component of the cycle, the LH surge, is more sensitive.
Materials and Methods
Animals
Adult female C57Bl6/J or CBA/B6F1 mice aged 45 to 162 days were used for all experiments. All mice were provided with water and Teklad 2916 chow (Envigo, Madison, WI) ad libitum and were housed on a 14:10 light/dark cycle with lights on at 4:00 am Eastern Standard Time. Animals were group housed before any experimental treatment; nonstress control mice were never singly housed at any time to avoid any stress associated with social isolation. Estrous cycle stage was determined via vaginal cytology and was monitored for at least 7 days before experiments involving the natural estrous cycle. For studies using an estradiol-induced LH surge model (OVX+E), mice were ovariectomized and received a subcutaneous silastic implant (Dow Corning, Midland, MI) that contained 0.625 µg of 17β-estradiol (Sigma-Aldrich, St. Louis, MO) in sesame oil (20). Surgery was done under isoflurane general anesthesia with bupivacaine as a local analgesic. Studies were performed 2 days after surgery. The Institutional Animal Care and Use Committee of the University of Michigan approved all procedures.
Repeated stress paradigm
To investigate effects of repeated exposure to psychosocial stress on the estrous cycle, a daily 2-hour stress was applied over 21 days. Midmorning (6.5 hours after lights on), mice were removed from their home cage and singly housed in a new cage, then transported to a new room where they were immediately placed in a commercially available restraint tube (Braintree Scientific, Braintree, MA) within the new cage. Animals were able to turn around in the restraint device with minimal effort, but movements were restricted. After 2 hours, all stressors were removed and animals were placed back in their home cage.
Acute layered stress paradigm
For all other experiments, the above stress paradigm was modified in two ways. First, mice were exposed only once to the stress paradigm, and acute effects on the LH surge were examined. Second, the duration of stress was lengthened to better encompass the “critical period” of GnRH/LH surge induction (21) by sequentially layering multiple psychosocial stressors. First, a single mouse was removed from the home cage and placed in a new cage. The new cage containing the stress subject mouse was then immediately transported to a new environment. One hour later, mice were placed in the restraint device described above. Two hours after restraint was initiated, the predator odor 2,3,5-trimethyl-3-thiazoline (Contech Enterprises, Victoria, BC, Canada), a component of red fox (Vulpes vulpes) urine, was introduced for another 2 hours. This paradigm produced a 5-hour exposure to psychosocial stress.
We used the term mild to describe these stress paradigms to differentiate between the low level of discomfort and agitation observed in our animals vs potentially severe physical effects of stressors such as insulin-induced hypoglycemia or endotoxin. The effect of these stressors to independently increase corticosterone levels (pretreatment vs posttreatment) was examined. For the layered stress paradigm, tail blood was sampled at the outset of application of each stressor and at the conclusion of the stress period to test for corticosterone. Once the model was determined to be effective and consistent in activating the hypothalamo–pituitary–adrenal (HPA) axis (as determined by corticosterone levels), we simplified further experimental design by obtaining only “pretreatment” and “posttreatment” samples immediately before and after stress, respectively, for corticosterone determination. After the stress period, the odor source was removed from the cage and mice were released from the restraint device. Following stress, mice remained alone in the new cage until blood was sampled for LH measurement (2 to 2.5 hours later).
Blood sampling
LH was measured either as a single time point (sampled 20 to 40 minutes before lights out) in trunk blood or at multiple time points in tail blood. All samples for corticosterone measurement were obtained via tail blood. To minimize handling stress due to tail blood sampling, all samples were taken in a quiet, calm environment. Animals were gently removed from their cage and placed on a work surface. While lightly holding on to the tail, the tip of the tail (<2 mm) was removed with a sterile scalpel blade. Animals were allowed as much freedom of movement as possible while collecting tail blood via capillary tube (25 to 40 μL of blood per sample). This sampling procedure has been shown to elicit minimal stress responses in nonstressed animals [e.g., control animals in Fig. 1(a)]. For all blood samples, serum was separated by centrifugation and stored at −20°C until assay.
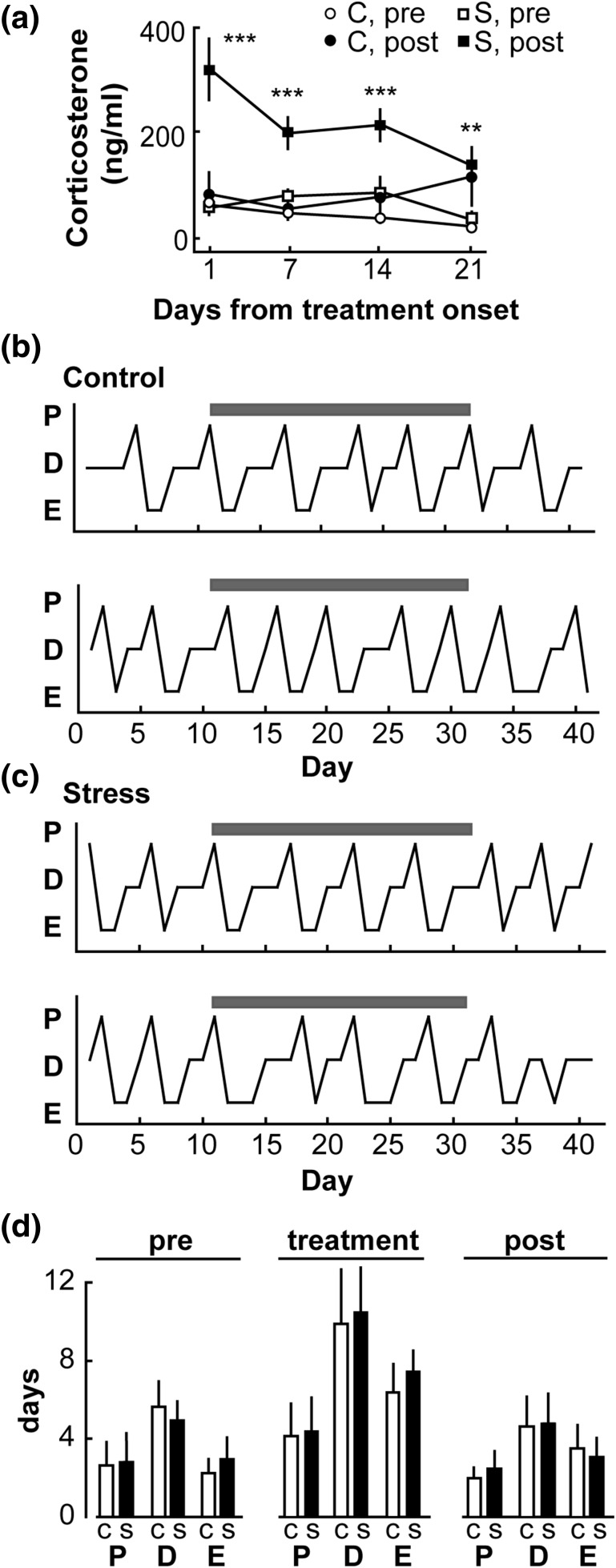
Figure 1.
Repeated, daily exposure to psychosocial stress does not alter the estrous cycle in the mouse. (a) Mean ± standard error of the mean (SEM) serum cortiocosterone in nonstressed controls (n = 6; C, circles) and stressed mice (n = 6; S, squares); samples taken immediately before treatment (open symbols) and after completion (closed symbols). Treatment period is denoted by the gray bars in panels (b) and (c). **P < 0.01, ***P < 0.001 stress before (pre) vs stress after (post) by two-way repeated measures ANOVA/Bonferroni. (b) Representative estrous cycle profiles for controls; proestrus (P), diestrus (D), and estrus (E). Gray bars denote days of treatment. (c) Representative estrous cycle profiles for stressed animals; gray bars denote days of treatment (daily stress). (d) Mean ± SEM number of days spent in each phase of the estrous cycle in control (C, open bar) and stressed animals (S, closed bar) during pretreatment (10 days, left panel), treatment (21 days, middle panel), and posttreatment (10 days, right panel) periods.
Experiment 1: Does a repeated, daily 2-hour psychosocial stress disrupt estrous cycles?
Estrous cycles were monitored under basal nonstressed conditions for 10 days to confirm normal cyclicity. Only mice with regular estrous cycles were used for further study. At the start of a 21-day treatment period, mice were assigned to either a nonstress control or stress group (n = 4 per group for each of two replicate studies, n = 8 per group total). Control mice were left undisturbed except for routine husbandry and veterinary observations, daily estrous cycle monitoring, and weekly tail blood samples to assess corticosterone. Mice in the stress group were stressed for 2 hours each morning beginning 6.5 hours after lights on for 3 weeks. In stressed mice, tail blood samples were taken immediately before and after daily stress exposure to measure corticosterone levels on days 1, 7, 14, and 21 relative to start of treatment period to assess efficacy of the stress to activate the HPA axis. Blood samples were taken at the same time points in control animals. After the 3-week exposure to daily psychosocial stress, estrous cycles were monitored for an additional 10 days under basal conditions to investigate any possible delayed effects of the repeated stress.
Experiment 2: Effect of acute, psychosocial stress on the proestrous LH surge
Based on a similar model of acute stress previously shown to suppress gonadotropin secretion in sheep (16, 17), we designed an acute paradigm of sequentially layered psychosocial stress as described above. To test effects of this stress on the proestrous LH surge, mice were either kept under nonstressed conditions (n = 11) or exposed to the layered stress (n = 24) on the midmorning of proestrus (6.5 hours after lights on). Tail blood was sampled immediately before and after stress for corticosterone measurement; samples were taken at the same time points in control mice. Trunk blood was collected 20 to 40 minutes before lights out in all animals for LH measurement. Uterine mass was measured and mice with uteri >100 mg were confirmed to be in proestrus and included in the analysis.
Experiment 3: Does psychosocial stress delay the proestrous LH surge in mice by 24 hours?
Previous studies in the Syrian hamster suggest a mild stress (e.g., novel object) can delay the LH surge by 24 hours (15). To determine whether our psychosocial stress paradigm caused a similar response, we applied the layered stress on the midmorning of proestrus (6.5 hours after lights on; n = 6 per group, stress and nonstressed controls). Tail blood was sampled before and after stress to measure corticosterone levels; samples were taken at the same time points from controls. To assess the LH surge, tail blood was sampled −1, 0, and +1 hour relative to lights out on both the day of stress (day 1; expected LH surge) and on the following day (day 2). To validate mice were in proestrus on day of treatment (day 1), only mice that exhibited estrous vaginal smears on day 2 were included in the analysis, as the estradiol rise during proestrus should produce vaginal cornification regardless of occurrence of an LH surge.
Experiment 4: Does psychosocial stress interfere with estradiol positive feedback needed for generation of the LH surge?
Adult female mice on a CBA/B6F1 background were used in this study (C57Bl6/J mice were used for all other experiments), as this hybrid strain has been shown to produce more consistent and robust estradiol-induced LH surges (OVX+E) than do 100% C57Bl6/J mice [LH surge amplitudes from previous studies: CBA/B6F1 (n = 38) 5.3 ± 0.6 ng/mL vs C57Bl6/J (n = 13) 1.2 ± 0.3 ng/mL]. Mice were randomly assigned to one of two treatment groups: nonstress control or stress; the experiment was conducted in four separate replicates (n = 3 per group per replicate; n = 12 per group total). All mice were handled daily for 7 days before surgery, as pilot studies showed that this reduced sensitivity to handling stress in mice postsurgery (E.R. Wagenmaker, unpublished data, May 2015). Midmorning (6.5 hours after lights on), 2 days after surgery, tail blood was sampled to assess basal corticosterone levels. The layered stress paradigm was then immediately applied to mice in the stress group; controls remained under nonstress conditions. After the completion of the stress, tail blood was sampled to measure corticosterone; samples were taken at the same time point in controls. All mice were euthanized and trunk blood collected 20 to 40 minutes before lights out for LH measurement.
Assays
LH was measured in serum from either trunk or tail blood, as described above. LH assays were conducted by the Ligand Assay and Analysis Core of the University of Virginia Center for Research and Reproduction. In serum from trunk blood samples, LH was measured in singlicate by a sensitive two-site sandwich immunoassay (22, 23) using monoclonal antibodies against bovine LH (no. 581B7) and against the human LH-β subunit (no. 5303: Medix, Kauniainen, Finland) as described (23). Intraassay and interassay coefficients of variation are 4.5% and 8.3%, respectively, with a functional assay sensitivity of 0.02 ng/mL. For LH measured in tail blood, a multiplex assay (EMD Millipore, Billerica, MA) was used that requires only 10 μL of serum. This assay has a functional sensitivity of 0.24 ng/mL, the intraassay coefficient of variation is 5.5%, and the interassay coefficient of variation is 11.5%. A proestrous LH surge was defined as a value ≥ 2 ng/mL. Surges in OVX+E mice are lower amplitude (24) and were defined as ≥0.5 ng/mL, which is 25-fold the assay sensitivity. Serum corticosterone was determined in duplicate aliquots (2 μL) using the DetectX corticosterone enzyme immunoassay kit (Arbor Assays, Ann Arbor, MI). Intraassay and interassay coefficients of variation for this assay are 5.2% and 7.9%, respectively. Assay sensitivity was 18.6 pg/mL.
Statistics
Data were tested for normal distribution using a Shapiro–Wilk test and analyzed using parametric or nonparametric tests as dictated by data distribution. Significance was set at P < 0.05. Details of specific tests and post hoc analyses are provided in the results.
Results
Experiment 1: Does a repeated, daily 2-hours psychosocial stress disrupt estrous cycles?
To address this question, mice were stressed daily or left untreated for 21 days after a 10-day pretreatment control period (used to establish estrous cycles were consistent between groups). In unstressed control mice, corticosterone levels remained at nonstressed, basal levels throughout the duration of the treatment period [Fig. 1(a)]. In contrast, after exposure to daily psychosocial stress, mice had increased corticosterone release on each day measured, demonstrating the efficacy of this stress to activate repeatedly the HPA axis [P < 0.01, two-way repeated measures analysis of variance (ANOVA)/Bonferroni; Fig. 1(a)]. The amplitude of these increases in corticosterone did decrease over the duration of the treatment period (P < 0.01, two-way repeated measures ANOVA/Bonferroni), but stress-induced elevations were maintained. Prestress corticosterone did not increase over time in the stress group, suggesting the HPA axis returned to basal levels after stress exposure. Fig. 1(b) and 1(c) show representative examples of estrous cycles from control and stress-exposed mice, respectively. Control mice had regular estrous cycles throughout the duration of the experiment [Fig. 1(b)]. Despite consistent elevations in corticosterone induced by stress throughout the treatment period, there were no effects on overall estrous cycle length (controls, 5.1 ± 0.4 vs 5.1 ± 0.3 vs 4.6 ± 0.2 days; stress, 5.3 ± 0.5 vs 5.8 ± 0.5 vs 4.6 ± 0.2 days; pretreatment vs treatment vs posttreatment periods, respectively; P > 0.05, two-way repeated measures ANOVA/Tukey). Additionally, there was no difference in the number of days spent in proestrus, estrus, or diestrus [P > 0.05, two-way repeated measures ANOVA/Tukey; Fig. 1(d)].
Experiment 2: Effect of acute, psychosocial stress on the proestrous LH surge
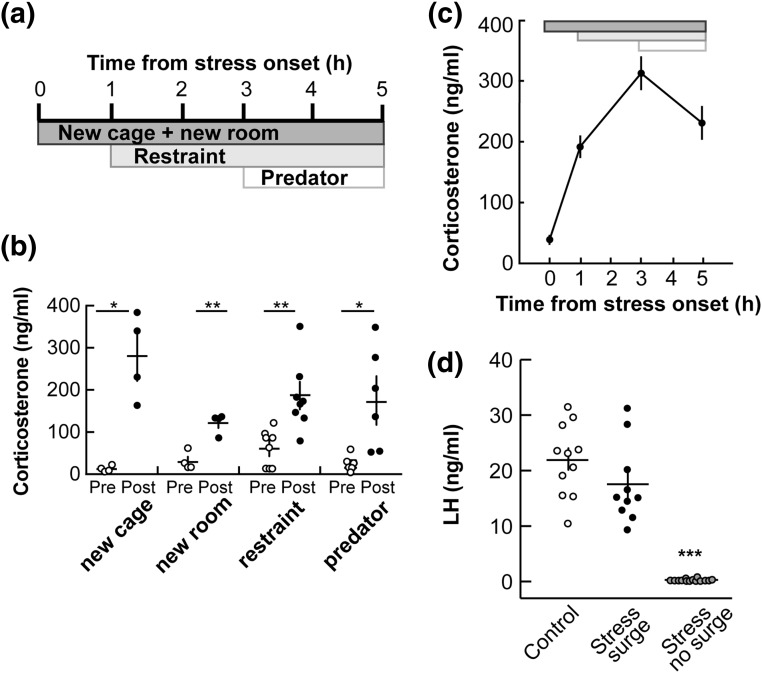
Results from the previous experiment suggest that the mouse estrous cycle is resistant to disruptions by this paradigm of psychosocial stress. It is also possible that there were effects of the repeated stress on particular aspects of the estrous cycle that were not investigated. We thus narrowed our focus to the effects of an acute stress on a specific component of the ovarian cycle: the proestrous LH surge. Because a daily 2-hour psychosocial stress did not detectably alter the estrous cycle, we expanded our stress paradigm to encompass a longer duration and increased the potency of the stress. To this end, we designed an “acute layered stress paradigm” (see Materials and Methods for details) similar to a stress paradigm that inhibited both GnRH and LH pulses in sheep (16, 17) [Fig. 2(a)]. Each individual stressor included in the layered stress induced an increase in corticosterone [Fig. 2(b)]. When applied sequentially in mice, this stress paradigm consistently elicited a robust and prolonged increase in corticosterone [Fig. 2(c)]. When the layered stress was applied midmorning of proestrus (6.5 hours after lights on), LH surges were not detected at the expected time in most mice (14 of 24; 58%) whereas 11 of 11 control mice had an LH surge at lights out [Fig. 2(d)]. When surges were observed in stressed mice (10 of 24), they were of similar amplitude to nonstress controls [P > 0.05, unpaired two-tailed Student t test; Fig. 2(d)], suggesting the effect of the layered stress is on surge timing/incidence rather than amplitude. Uterine mass was measured in all mice to confirm exposure to proestrous levels of estradiol (control, 124 ± 6 mg; stress no surge, 131 ± 8 mg; stress with surge, 114 ± 6 mg; P > 0.1, one-way ANOVA/Tukey), suggesting that the proestrous estradiol rise was similar in all groups.
Figure 2.
An acute model of layered, psychosocial stress increases corticosterone and disrupts the proestrous LH surge. (a) The layered stress paradigm consists of sequential application of new cage/transport to a new room, restraint, and exposure to predator odor. Time is depicted as hours relative to onset of stress. (b) Individual values and mean ± standard error of the mean (SEM) serum corticosterone; each individual stressor in the layered stress paradigm increases basal corticosterone [before (pre) ○ vs after (post) ●]: 20-minute new cage stress (n = 4), 20-minute transport to new room stress (n = 4), 20-minute restraint (n = 8), and 1-hour predator odor exposure (n = 6). *P < 0.05, **P < 0.01, paired Student t test. (c) Mean ± SEM corticosterone during the complete layered stress, as depicted at the top (n = 35; P < 0.0001, one-way repeated measures ANOVA/Tukey). (d) Individual values and mean ± SEM serum LH measured 20 to 40 minutes before lights out on the late afternoon of proestrus. All controls had an LH surge (n = 11; ○); some stressed animals had an LH surge (n = 10; ●), but most did not (58%, n = 14; gray circles). ***P < 0.0001, one-way ANOVA/Tukey.
Experiment 3: Does psychosocial stress delay the proestrous LH surge in mice by 24 hours?
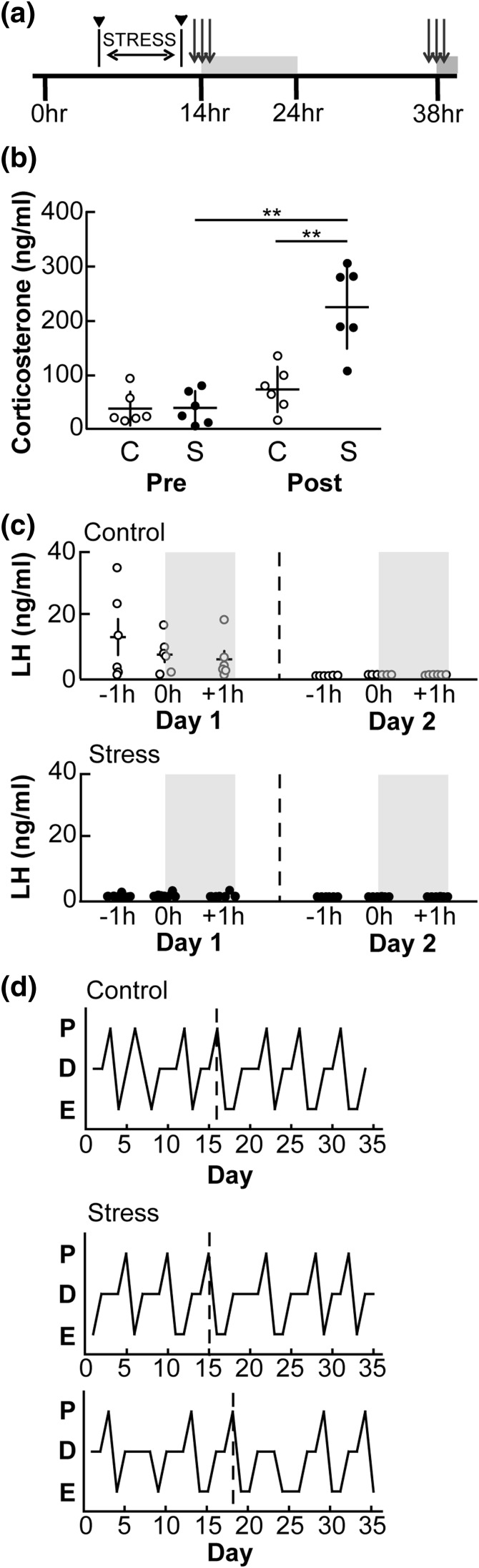
The LH surge in rodents can be delayed by a day by various treatments; this is likely attributable to the role the diurnal light cycle plays in the timing of the surge (15, 21, 25, 26). To determine whether proestrous LH surges were not observed in the above study because they were delayed by a day, we applied the layered stress on the midmorning of proestrus as previously noted [Fig. 3(a); n = 6 per group: nonstressed controls and stress). There was no difference in corticosterone between groups in the pretreatment sample [Fig. 3(b)]. Layered stress markedly increased corticosterone, whereas levels in controls were unchanged [P < 0.01, two-way repeated measures ANOVA/Sidak; Fig. 3(b)]. In control mice, five out of six had an LH surge during the sampling window on the evening of proestrus (day 1) [mean peak, 18.4 ± 5.4 ng/mL; Fig. 3(c), top]. No control mice showed surge-like levels of LH the following day (day 2). In contrast, only one out of six stressed mice had an LH surge on the day of proestrus (day 1); none had elevated LH levels the next day [Fig. 3(c), bottom]. Estrous cycles in all mice were further monitored for 10 days; all mice continued to cycle normally regardless of treatment [Fig. 3(d)].
Figure 3.
Exposure to the layered stress does not delay the proestrous LH surge by 24 hours. (a) Experimental timeline. Layered stress is applied midmorning on proestrus and blood samples for corticosterone taken immediately before and after stress (black, solid arrowhead). Blood samples for LH measurement were taken at −1, 0, and +1 hours relative to lights out and again at the same times 24 hours later (gray arrows). Gray boxes denote period of lights out. (b) Individual values and mean ± standard error of the mean (SEM) corticosterone in controls (C, ○) and stressed (S, ●) mice. **P < 0.01, two-way repeated measures ANOVA/Sidak. (c) Individual values and mean ± SEM serum LH values at the time of the expected surge (−1, 0, and +1 hours relative to lights out) on day 1 (day of proestrus, left panel) and the following day (day 2, right panel) in both control (top panel) and stressed animals (bottom panel); n = 6 per group. (d) Representative estrous cycle profiles for one control animal (top) and two stressed animals (bottom): days of proestrus (P), diestrus (D), and estrus (E) are indicated. Vertical dashed line in (d) denotes day of proestrus on which experiment was conducted.
Experiment 4: Does psychosocial stress interfere with estradiol positive feedback needed for generation of the LH surge?
In experiments involving the proestrous surge, estradiol levels were not measured directly due to insufficient serum. There is indirect evidence that suggests estradiol levels were elevated in these mice: vaginal smears and/or uterine mass provide a bioassay for circulating estradiol levels (27, 28). To test specifically whether stress interferes with estradiol positive feedback action required for surge generation, we used a model of exogenous estradiol administration that induces a daily LH surge in mice (OVX+E) (20).
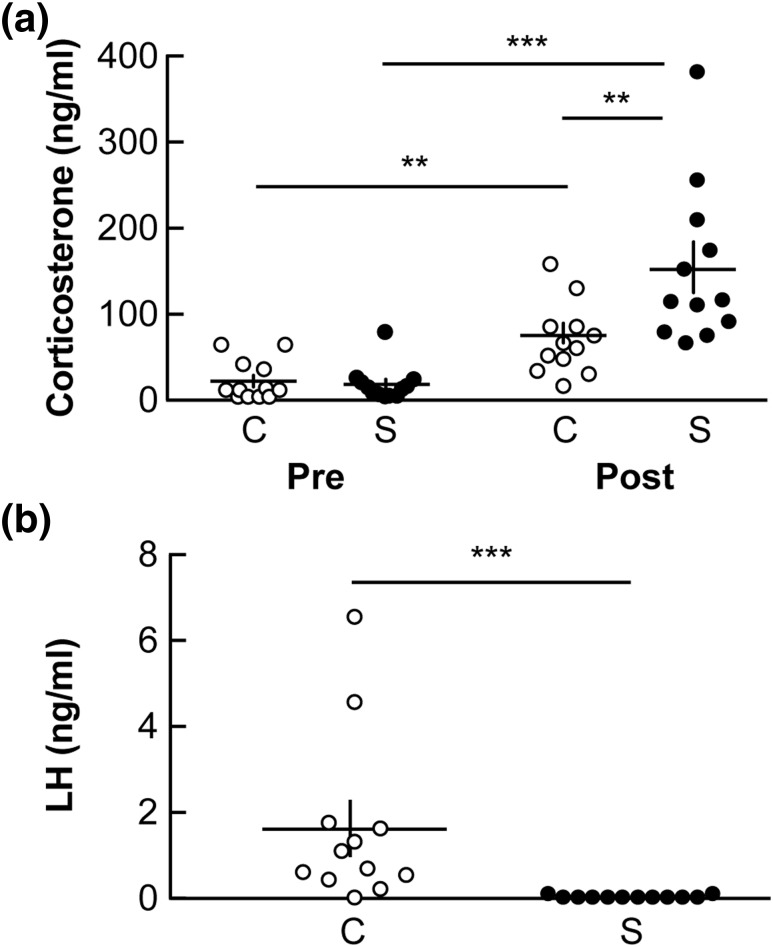
Mice were assigned to one of two treatment groups (n = 12 per group): nonstress control or stress. All mice exhibited basal levels of corticosterone before treatment [P > 0.05; Fig. 4(a)]. The layered stress increased corticosterone levels (P < 0.001, two-way repeated measures ANOVA/Sidak). In this experiment, a statistical increase in corticosterone in the control group in the posttreatment sample was also observed. This value is consistent with the expected diurnal pattern of corticosterone; of note, levels are near the higher end of the range of late-afternoon values we see (∼100 ng/mL; E.R. Wagenmaker, unpublished data, March 2011). Importantly, posttreatment corticosterone was higher in stressed than in nonstressed mice (P < 0.01, two-way repeated measures ANOVA/Sidak). In control mice, an LH surge was detected in nine of 12 mice (mean, 1.6 ± 0.6 ng/mL; Fig. 4(b)]. In contrast, LH surges were not observed in any stressed mice [mean, 0.04 ± 0.01 ng/mL; P < 0.001 control vs stress, Mann–Whitney U test, Fig. 4(b)].
Figure 4.
Psychosocial stress blocks the estradiol-induced LH surge in ovariectomized mice. (a) Stress was initiated midmorning on day 2 after ovariectomy. Individual values and mean ± standard error of the mean (SEM) serum corticosterone before (pre) and after (post) stress in control (n = 12; C, ○) and stressed animals (n = 12; S, ●). **P < 0.01, ***P < 0.001 by two-way repeated-measures ANOVA/Sidak. (b) Individual values and mean ± SEM serum LH measured 20 to 40 minutes before lights off in control (C, ○) and stressed (S, ●) animals. ***P < 0.001, Mann–Whitney U test.
Discussion
In the current study, we tested whether a repeated or single exposure to psychosocial stress affected the overall estrous cycle or either the proestrous or estradiol-induced LH surge in mice. Daily, repeated exposure to mild stress did not alter the length of the estrous cycle or the number of days spent in each phase of the cycle when compared with nonstressed controls. We thus developed a model of sequentially layered stress to test the effects of a single exposure to psychosocial stress on a specific aspect of the cycle: the proestrous LH surge. When this stress paradigm was applied on the morning of proestrus, it disrupted the surge in most mice. This disruption is likely due to interruption of the central mechanisms of estradiol positive feedback rather than production of the proestrous estradiol rise, as this stress paradigm also disrupted the estradiol-induced LH surge.
Exposure to psychosocial stress disrupts the estrous cycle in some species (11, 12) but not in others (29). Still, the lack of effect of psychosocial stress on the murine estrous cycle here was surprising, as a similar model (daily 3-hour restraint) was capable of lengthening the cycle in mice (13). This difference was not attributable to failure of our stress model to activate the HPA axis. Stress produced elevations in corticosterone, the major glucocorticoid in rodents, measured at weekly time points throughout the 3-week stress period, demonstrating the efficacy of this stress. Glucocorticoids can negatively affect both the estrous cycle and gonadotropin secretion in a variety of species (30–34). In many of these studies, however, disruptive effects of glucocorticoids were observed with more sustained elevations than those induced by stress in the current study. It is possible that mice were able to adapt to the mild stress used here, perhaps because of a shorter duration of the accompanying corticosterone elevation.
Although the results of experiment 1 suggest a lack of effect on the outward expression of the estrous cycle, it is still possible that stress had an impact on aspects of the cycle that were not examined. We thus focused on a specific feature of the cycle not previously monitored: the proestrous LH surge. We developed and validated a layered stress paradigm, with a longer duration (5 vs 2 hours) to better cover the “critical period” needed to generate the GnRH/LH surge (21). In proestrous mice, this stress disrupted the surge in most animals (63%; experiments 2 and 3 combined). The presence of the LH surge was not specifically tested in experiment 1, which used a repeated stress consisting of simultaneous application of three of the four stressors used in our layered stress (new cage, new room, restraint). Because exposure to a single bout of the layered stress (sequential application) disrupted the LH surge, yet did not alter the progression of the estrous cycle, speculation about possible disruption of the LH surge in experiment 1 is precluded.
Disruption of the LH surge by acute stress on the midmorning of proestrus raises several questions. First, did stress block the LH surge or alter its timing? In experiment 2, we measured LH at a single time point: 20 to 40 minutes before lights out; this sampling regimen was expanded in experiment 3 to encompass multiple time points around the time of lights out to increase the likelihood of observing a surge. Even with the more extensive blood sampling, LH surges were not detected in most of the stressed mice. Few studies demonstrate that stress exposure can affect the timing of the LH surge in rodents, which is tightly coupled to the light/dark cycle (21, 25, 26). Rather, studies have shown stress can delay the surge for a full day, with the LH surge still peaking near lights out. For example, in Syrian hamsters, a single exposure to mild stress can delay estrus onset and the LH surge by 24 hours (14, 15). Interestingly, the layered stress did not delay the surge by 1 day in our mice, suggesting either that stress completely blocked the surge or succeeded in altering the timing of the surge to outside our sampling window. If the timing of the surge was markedly altered, this raises the intriguing question of whether mice ovulated after stress exposure. If, however, the layered stress completely blocked the LH surge, and therefore ovulation, this indicates that the estrous cycle can progress in the absence of this hallmark of the ovarian cycle. Further studies will be needed to address these possibilities.
Because mice in the present studies did not display an LH surge near the time of lights out, a second question arises: does stress block the proestrous estradiol rise needed for estradiol positive feedback and LH surge induction (35)? In this regard, estradiol levels do not appear to be affected by psychosocial stress exposure. Increased uterine mass and vaginal cornification are indicative of elevated estradiol levels (27, 28). A uterine mass of >100 mg on the late afternoon of proestrus is highly correlated with incidence of the LH surge in our laboratory. All mice examined exhibited a uterine mass > 100 mg; furthermore, no difference in uterine mass was observed between mice that exhibited an LH surge vs those that did not (experiment 2). In studies that precluded removing the uterus on proestrus (experiment 3 in which the possibility of a 24-hour delay of the surge was studied), vaginal smears were used as a surrogate for estradiol levels, as cornification of the vaginal epithelium is also estradiol-dependent (28). These observations of the reproductive tract indicate a proestrous estradiol rise did occur in stressed mice, and they suggest disruption of the LH surge may be secondary to altering the neurobiological mechanisms underlying estradiol positive feedback.
To investigate directly whether estradiol positive feedback was compromised, we used a model of daily, estradiol-induced surges in ovariectomized mice (20). In this model, constant release estradiol implants are administered and reliably produce an LH surge peaking near the time of lights out. The layered stress paradigm blocked the surge in all stressed mice prepared in the daily surge model. Consistent with this observation, a recent preliminary report demonstrated that a short-term application of multiple mild stressors blocked estradiol-induced LH surges in mice (36). Two possible mediators of the stress-induced disruption of estradiol positive feedback mechanisms are glucocorticoids and corticotropin-releasing hormone, both potent suppressors of the HPG axis (30–34, 37–39). There are limited data on expression of receptors for either glucocorticoids (GRs) or corticotropin-releasing hormone (CRH-Rs) in mouse GnRH neurons, with colocalization observed only in a small subpopulation (40, 41). GnRH neurons, however, appear to lack detectable levels of the estradiol receptor (ERα) needed to convey estradiol-mediated feedback (42). A direct effect of stress mediators on GnRH neurons is thus not needed to impair this mechanism. Kisspeptin neurons in the anteroventral periventricular nucleus are postulated to mediate estradiol positive feedback signals to the GnRH neuron (43). Anteroventral periventricular kisspeptin neurons express ERα (44), and there is some evidence of both GR and CRH-R colocalization within these neurons (45). Another potential source of HPA/HPG integration is neurons producing gonadotropin-inhibitory hormone (GnIH). Both GR and CRH-R are expressed in a proportion of GnIH-expressing neurons (46), and GnIH neurons can directly interact with GnRH neurons (47, 48) to inhibit action potential firing (49). In mice, ERα is also expressed in a subset of GnIH neurons, but expression appears to be inversely correlated with estradiol levels (50, 51). Whether stress transmits effects directly or indirectly on the GnRH neuron requires additional investigation that could yield mechanistic information needed for potential treatments to reverse stress-induced disruptions on the HPG axis.
In summary, although repeated exposure to a short-duration psychosocial stress does not appear to have an effect on overall estrous cyclicity in the mouse, a single exposure to stress on the morning of proestrus can disrupt the LH surge. Acute layered psychosocial stress affected the timing and/or incidence of the LH surge in most mice tested. Additionally, the estradiol positive feedback mechanism needed for surge generation is disrupted by this stress. Further studies are needed to investigate potential mediators and mechanisms involved and whether the LH surge is fully blocked or whether the timing is altered.
Acknowledgments
We are grateful to Drs. Laura Burger and Veronica Otero-Corchon for their invaluable experimental assistance. We also thank Drs. Fred Karsch and Amy Oakley for their editorial comments.
Acknowledgments
This work was supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01 HD41469. The University of Virginia Center for Research and Reproduction Ligand Assay and Analysis Core (National Institutes of Health Grant P50HD28934) conducted LH assays.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- CRH-R
- corticotropin-releasing hormone receptor
- ERα
- estradiol receptor
- GnIH
- gonadotropin inhibitory hormone
- GnRH
- gonadotropin-releasing hormone
- GR
- glucocorticoid receptor
- HPA
- hypothalamo–pituitary–adrenal
- HPG
- hypothalamo–pituitary–gonadal
- LH
- luteinizing hormone.
References
- 1.Matthiesen SM, Frederiksen Y, Ingerslev HJ, Zachariae R. Stress, distress and outcome of assisted reproductive technology (ART): a meta-analysis. Hum Reprod. 2011;26(10):2763–2776. [DOI] [PubMed] [Google Scholar]
- 2.Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril. 2014;101(3):767–774. [DOI] [PubMed] [Google Scholar]
- 3.Nappi RE, Rivest S. Effect of immune and metabolic challenges on the luteinizing hormone-releasing hormone neuronal system in cycling female rats: an evaluation at the transcriptional level. Endocrinology. 1997;138(4):1374–1384. [DOI] [PubMed] [Google Scholar]
- 4.Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguié C, Karsch FJ. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology. 1997;138(10):4273–4281. [DOI] [PubMed] [Google Scholar]
- 5.Cagampang FR, Cates PS, Sandhu S, Strutton PH, McGarvey C, Coen CW, O’Byrne KT. Hypoglycaemia-induced inhibition of pulsatile luteinizing hormone secretion in female rats: role of oestradiol, endogenous opioids and the adrenal medulla. J Neuroendocrinol. 1997;9(11):867–872. [DOI] [PubMed] [Google Scholar]
- 6.Xiao E, Xia-Zhang L, Barth A, Zhu J, Ferin M. Stress and the menstrual cycle: relevance of cycle quality in the short- and long-term response to a 5-day endotoxin challenge during the follicular phase in the rhesus monkey. J Clin Endocrinol Metab. 1998;83(7):2454–2460. [DOI] [PubMed] [Google Scholar]
- 7.Xiao E, Xia-Zhang L, Ferin M. Stress and the menstrual cycle: short- and long-term response to a five-day endotoxin challenge during the luteal phase in the rhesus monkey. J Clin Endocrinol Metab. 1999;84(2):623–626. [DOI] [PubMed] [Google Scholar]
- 8.Reifenstein EC., Jr Psychogenic or hypothalamic amenorrhea. Med Clin North Am. 1946;30:1103–1114. [DOI] [PubMed] [Google Scholar]
- 9.Berga SL, Girton LG. The psychoneuroendocrinology of functional hypothalamic amenorrhea. Psychiatr Clin North Am. 1989;12(1):105–116. [PubMed] [Google Scholar]
- 10.Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76(2):310–316. [DOI] [PubMed] [Google Scholar]
- 11.Xiao E, Xia-Zhang L, Ferin M. Inadequate luteal function is the initial clinical cyclic defect in a 12-day stress model that includes a psychogenic component in the Rhesus monkey. J Clin Endocrinol Metab. 2002;87(5):2232–2237. [DOI] [PubMed] [Google Scholar]
- 12.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293(1):E270–E276. [DOI] [PubMed] [Google Scholar]
- 13.Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL. Stress levels of glucocorticoids inhibit LHβ-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26(10):1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young Janik L, Janik D. Nonphotic phase shifting in female Syrian hamsters: interactions with the estrous cycle. J Biol Rhythms. 2003;18(4):307–317. [DOI] [PubMed] [Google Scholar]
- 15.Legan SJ, Franklin KM, Peng XL, Duncan MJ. Novel wheel running blocks the preovulatory luteinizing hormone surge and advances the hamster circadian pacemaker. J Biol Rhythms. 2010;25(6):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ. Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology. 2007;148(4):1882–1890. [DOI] [PubMed] [Google Scholar]
- 17.Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology. 2009;150(2):762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobson H, Tebble JE, Phogat JB, Smith RF. Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil. 1999;116(1):1–8. [DOI] [PubMed] [Google Scholar]
- 19.Roozendaal MM, Swarts HJ, Wiegant VM, Mattheij JA. Effect of restraint stress on the preovulatory luteinizing hormone profile and ovulation in the rat. Eur J Endocrinol. 1995;133(3):347–353. [DOI] [PubMed] [Google Scholar]
- 20.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102(43):15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47(3):198–218. [DOI] [PubMed] [Google Scholar]
- 22.Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone β gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53(1):103–109. [DOI] [PubMed] [Google Scholar]
- 23.Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132(4):1687–1691. [DOI] [PubMed] [Google Scholar]
- 24.Silveira MA, Burger LL, DeFazio RA, Wagenmaker ER, Moenter SM. GnRH neuron activity and pituitary response in estradiol-induced vs proestrous luteinizing hormone surges in female mice. Endocrinology. 2017;158(2):356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawyer CH, Everett JW, Markee JE. A neural factor in the mechanism by which estrogen induces the release of luteinizing hormone in the rat. Endocrinology. 1949;44(3):218–233. [DOI] [PubMed] [Google Scholar]
- 26.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. [DOI] [PubMed] [Google Scholar]
- 27.Shim WS, Conaway M, Masamura S, Yue W, Wang JP, Kmar R, Santen RJ. Estradiol hypersensitivity and mitogen-activated protein kinase expression in long-term estrogen deprived human breast cancer cells in vivo. Endocrinology. 2000;141(1):396–405. [DOI] [PubMed] [Google Scholar]
- 28.Wood GA, Fata JE, Watson KL, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133(5):1035–1044. [DOI] [PubMed] [Google Scholar]
- 29.Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. The estrous cycle of the ewe is resistant to disruption by repeated, acute psychosocial stress. Biol Reprod. 2010;82(6):1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vreeburg JT, de Greef WJ, Ooms MP, van Wouw P, Weber RF. Effects of adrenocorticotropin and corticosterone on the negative feedback action of testosterone in the adult male rat. Endocrinology. 1984;115(3):977–983. [DOI] [PubMed] [Google Scholar]
- 31.Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod. 1985;33(2):423–431. [DOI] [PubMed] [Google Scholar]
- 32.Saketos M, Sharma N, Santoro NF. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993;49(6):1270–1276. [DOI] [PubMed] [Google Scholar]
- 33.Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ. Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology. 2005;146(4):2107–2115. [DOI] [PubMed] [Google Scholar]
- 34.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone blocks ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157(3):1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Döcke F, Dörner G. The mechanism of the induction of ovulation by oestrogens. J Endocrinol. 1965;33(3):491–499. [DOI] [PubMed] [Google Scholar]
- 36.Breen KM, Kreisman MJ, Chaing S, Song CI, Kauffman AS. Investigating the effect of psychosocial stress on the estradiol-induced LH surge in female mice. In: Program for the Endocrine Society’s 98th Annual Meeting; 2016; Boston, MA. Abstract SAT 581. [Google Scholar]
- 37.Rivier C, Vale W. Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology. 1984;114(3):914–921. [DOI] [PubMed] [Google Scholar]
- 38.Olster DH, Ferin M. Corticotropin-releasing hormone inhibits gonadotropin secretion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab. 1987;65(2):262–267. [DOI] [PubMed] [Google Scholar]
- 39.Williams CL, Nishihara M, Thalabard JC, Grosser PM, Hotchkiss J, Knobil E. Corticotropin-releasing factor and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey. Electrophysiological studies. Neuroendocrinology. 1990;52(2):133–137. [DOI] [PubMed] [Google Scholar]
- 40.Dondi D, Piccolella M, Messi E, Demissie M, Cariboni A, Selleri S, Piva F, Samara A, Consalez GG, Maggi R. Expression and differential effects of the activation of glucocorticoid receptors in mouse gonadotropin-releasing hormone neurons. Neuroendocrinology. 2005;82(3-4):151–163. [DOI] [PubMed] [Google Scholar]
- 41.Jasoni CL, Todman MG, Han SK, Herbison AE. Expression of mRNAs encoding receptors that mediate stress signals in gonadotropin-releasing hormone neurons of the mouse. Neuroendocrinology. 2005;82(5-6):320–328. [DOI] [PubMed] [Google Scholar]
- 42.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszán T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-β messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141(9):3506–3509. [DOI] [PubMed] [Google Scholar]
- 43.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 45.Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett. 2012;531(1):40–45. [DOI] [PubMed] [Google Scholar]
- 46.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA. 2009;106(27):11324–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol. 2003;15(8):794–802. [DOI] [PubMed] [Google Scholar]
- 48.Qi Y, Oldfield BJ, Clarke IJ. Projections of RFamide-related peptide-3 neurones in the ovine hypothalamus, with special reference to regions regulating energy balance and reproduction. J Neuroendocrinol. 2009;21(8):690–697. [DOI] [PubMed] [Google Scholar]
- 49.Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol. 2009;587(Pt 7):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA. 2006;103(7):2410–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molnár CS, Kalló I, Liposits Z, Hrabovszky E. Estradiol down-regulates RF-amide-related peptide (RFRP) expression in the mouse hypothalamus. Endocrinology. 2011;152(4):1684–1690. [DOI] [PubMed] [Google Scholar]