Abstract
Increasing evidence has demonstrated that exposure to endocrine-disrupting chemicals impacts maternal and fetal health, but the underlying mechanisms are still unclear. We previously showed that dietary exposure to 10 µg/kg body weight (bw)/d and 10 mg/kg bw/d of bisphenol A (BPA) during pregnancy induced metabolic abnormalities in F1 male offspring and gestational glucose intolerance in F0 pregnant mice. The aim of this study was to elucidate the underlying etiologies of BPA exposure−induced metabolic disease by analyzing the male fetal liver metabolome. Using the Metabolon Discover HD4 Platform, our laboratory identified metabolic pathways that were altered by BPA exposure, including biochemicals in lipid and amino acid metabolism. Specifically, primary and secondary bile acids were increased in liver from BPA-exposed embryonic day 18.5 male fetuses. We subsequently showed that increased bile acid was associated with a defective farnesoid X receptor−dependent negative feedback mechanism in BPA-exposed fetuses. In addition, through metabolomics, we observed that BPA-exposed fetuses had elevated tryptophan levels. Independent liquid chromatography and mass spectrometry measurement revealed that BPA-exposed dams also had increased tryptophan levels relative to those of controls. Because several key enzymes in tryptophan catabolism are vitamin B6 dependent and vitamin B6 deficiencies have been linked to gestational diabetes, we tested the impact of vitamin B6 supplementation and showed that it rescued gestational glucose intolerance in BPA-exposed pregnant mice. Our study has therefore identified two pathways (bile acid and tryptophan metabolism) that potentially underlie BPA-induced maternal and fetal metabolic disease.
Analysis of the male fetal liver metabolome revealed that bile acids and tryptophan are metabolites linked to increased risk for metabolic disease in BPA-exposed male offspring and pregnant mice.
Exposure to environmental chemicals during pregnancy can compromise maternal and fetal health. Endocrine-disrupting chemical (EDC) exposure is associated with adverse reproductive outcomes (1–3). In the fetus, in utero exposure to some EDCs alters susceptibility to metabolic disease (4, 5). Elucidating the mechanisms of environmental perturbations is essential for the prevention and treatment of associated maternal-fetal conditions.
Bisphenol A (BPA) is a highly produced EDC used in plastics and food and beverage packaging. Higher maternal BPA blood levels are associated with recurrent miscarriages, premature labor, preeclampsia, and gestational diabetes in pregnant women (2, 6–8). In children, elevated BPA levels during prenatal development and early childhood are linked to obesity (9–14). Recently, our laboratory reported that gestational exposure to physiological doses of BPA (10 µg/kg body weight (bw)/d, or lower dose, and 10 mg/kg bw/d, or upper dose) resulted in adult obesity, glucose intolerance, and insulin resistance in F1 and F2 male offspring and gestational glucose intolerance in F0 pregnant mice (15). The molecular mechanisms underlying these changes are unknown; however, it is most likely that in utero BPA exposure is linked to fetal reprogramming of metabolic pathways in the F1 male offspring. Because liver metabolism is key to metabolic health and changes in liver metabolism may underlie the etiology of disease, studying liver from BPA-exposed male fetuses and pregnant mice may elucidate the underlying mechanisms of environment-induced metabolic disease.
In this study, we hypothesized that BPA exposure substantially altered the metabolome of the liver from F1 male fetuses. To test this hypothesis, we compared the metabolome of livers from control and BPA-exposed F1 male fetuses and found that exposure was linked to differences in the bile acid pool. In addition, through liquid chromatography–tandem mass spectrometry (LC-MS) analysis, we found that livers from BPA-exposed pregnant mice had elevated tryptophan levels relative to those of controls. Tryptophan is a precursor of many biologically important metabolites, with approximately 95% of tryptophan used in kynurenine catabolism (16). Disruption of this pathway produces xanthurenic acid (XA), which can impair insulin function and has been linked to diabetes (17). Several rate-limiting enzymes in tryptophan catabolism use pyridoxal 5′-phosphate (PLP), the active form of vitamin B6, as a cofactor, and vitamin B6 insufficiency has been linked to increased risks for gestational glucose intolerance (18–22). We hypothesized that vitamin B6 supplementation in BPA-exposed pregnant mice can rescue the gestational glucose intolerance phenotype and tested the hypothesis using the glucose tolerance test (GTT). We report that bile acid and tryptophan metabolism are candidate mechanisms underlying exposure-induced fetal and maternal metabolic disease and that vitamin B6 supplementation can improve maternal glucose homeostasis.
Materials and Methods
Overview of the studies
The studies were conducted between November 2013 and January 2016 and comprised three independent studies (metabolomics, GTT, and measurement of tryptophan catabolites) using separate cohorts of mice. A schematic of the exposure groups, timeline, and sample sizes of dams/fetuses is shown in Supplemental Fig. 1 (5.4MB, eps) . The metabolomics was a follow-up of a previous study in which we analyzed only control, lower-dose, and upper-dose BPA (15). We had no prior knowledge of vitamin B6 and tryptophan relevance at the time the metabolomics was performed. Analysis of the metabolomics resulted in our hypothesis that the gestational diabetes phenotype in upper-dose BPA−exposed pregnant mice was linked to vitamin B6 deficiency and elevated tryptophan level. We subsequently tested this hypothesis by performing GTTs on new control, lower-dose BPA, upper-dose BPA, upper-dose BPA plus vitamin B6, and vitamin B6 alone cohorts (see our methods in the following sections). The tryptophan catabolite measurement included the same categories of exposure as the GTT.
Mouse information
Six-week-old virgin C57BL/6J female mice were exposed to a modified AIN 93G diet or control diet (TD 95092 with 7% corn oil substituted for 7% soybean oil), 50 µg/kg BPA (lower-dose diet; TD 110337), and 50 mg/kg of BPA (upper-dose diet; TD 06156) purchased from Envigo (Madison, WI) 2 weeks before mating, and exposure continued during mating until gestation day 18.5 (for metabolomics and tryptophan catabolite measurements) or 16.5 (for the GTT). Previously, we had determined that these exposures resulted in maternal serum BPA levels within the range of human exposure (23).
For the rescue experiment, we included a diet of upper-dose BPA supplemented with 24 mg/kg of vitamin B6 (TD14336). The dose was selected because vitamin B6 supplementation in laboratory mice typically ranged from 24 to 35 mg/kg (24–26). As a reference, our control diet was supplemented with 6 mg/kg of vitamin B6. A diet with 24 mg/kg of vitamin B6 alone (TD14335) was also used to control for potential vitamin B6−related effects. All animal studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
For metabolomics, pregnant mice were euthanized on gestation day 18.5, and the liver was isolated from the fetuses. Fetal sex was determined by physical appearance of the gonads and was confirmed by sex-specific polymerase chain reaction. Liver tissues from both males and females were weighed, snap frozen, and stored at −80°C until analysis. Because we previously reported male-specific metabolic phenotypes (15), only male fetuses were included in the metabolomics. For tryptophan metabolite measurements, we collected maternal livers.
Metabolomics
A total of 30 liver samples (≥50 mg/sample) were analyzed in the study, representing 10 male fetuses from each exposure group (Supplemental Fig. 1 (5.4MB, eps) ). Global metabolomic profiling was conducted in collaboration with Metabolon, Inc. (Durham, NC) following previously described methods (27). Briefly, samples underwent a series of organic and aqueous extractions optimized for small molecule recovery and were then split into equal parts for gas chromatography-mass spectrometry and LC-MS analyses. For the latter platform, samples were again divided for profiling in both positive (acidic) and negative (basic) ionization modes.
Raw data were extracted, peak-identified, and quality control processed using Metabolon proprietary hardware and software. Peaks were called against a library of 3300 named biochemicals composed of amino acids, lipids, carbohydrates, nucleotides, peptides, vitamins, cofactors, and xenobiotics. A total of 335 biochemicals were identified in this study (Supplemental Tables 1 (172.9KB, xlsx) and 2 (159.8KB, xlsx) ). Using hierarchical clustering, heat maps were generated with GENE-E software developed by the Broad Institute (available at http://www.broadinstitute.org/cancer/software/GENE-E/). Heat maps depict the fold-change difference in metabolite concentration across exposure groups (i.e., control vs lower, control vs upper, and lower vs upper) or the z score comparing the metabolite concentrations between controls and BPA-exposed mice with their respective population means. Principal components analysis was used to determine separation among replicates within and between exposure groups.
To gain insights into BPA exposure−induced metabolic phenotypes, we searched PubMed for published works using the keywords obesity, diabetes, and BPA and each of our differentially regulated metabolites of interest. We focused on the categories of metabolites showing the most prominent changes in our analysis, including glucose, γ-glutamyl amino acid, phenylalanine, tyrosine, tryptophan, bile acids, and BPA metabolites (Supplemental Table 3 (33.3KB, xlsx) ). The resulting numbers of publications are listed in Supplemental Table 4 (53.7KB, docx) , showing that γ-glutamyl, phenylalanine, tryptophan, and bile acids have major relevance to metabolic disease but with a limited link to BPA. We subsequently pursued the analysis of bile acids and tryptophan, which showed the greatest magnitude of change among our exposure groups.
Real-time quantitative polymerase chain reaction analysis
For analysis of genes related to bile acid metabolism, total RNA was extracted from a subset of the embryonic day 18.5 fetal livers used in the previously described metabolomics analysis (n = 6 to 9) using the TRIzol reagent (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s guidelines and quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific). Using Invitrogen SuperScript Transcriptase III (Carlsbad, CA) and random hexamers, we subsequently generated complementary DNA. Real-time quantitative polymerase chain reaction (qRT-PCR) analysis was conducted using an Applied Biosystems 7900HT Fast Real-Time PCR system (Foster City, CA) with the following protocol: 5 μL of Master Mix, 0.2 μL of reverse and forward primers (10 μM), and 4.6 μL of complementary DNA (5 ng). The following primers were used: 5′ GCTTGATGTGCTACAAAAGCTG 3′ (forward) and 5′ CGTGGTGATGGTTGAATGTCC 3′ (reverse) for Fxr; 5′ GTCTTTCTGGAGCCTTGAGCTG 3′ (forward) and 5′ TCCTGTTGCAGGTGTGCGA 3′(reverse) for Shp; 5′ GGGATTGCTGTGGTAGTGAGC 3′ (forward) and 5′ GGTATGGAATCAACCCGTTGTC 3′ (reverse) for Cyp7a1; and 5′ GACAAGGGTTTTGTGCCCTG 3′ (forward) and 5′ GTGAAGACATCCCCGTGCTT 3′ (reverse) for Cyp8b1. Samples were set up in triplicate and analyzed using Applied Biosystem design and analysis software. Total expression of genes of interest in exposed groups was calculated relative to the control group using the comparative Ct method and was analyzed using the statistical methods described in a following section. We used Arppo, Gapdh, and Nono as the reference genes.
GTT
At gestation day 16.5, pregnant mice were fasted overnight and subsequently injected with 2 g/kg bw of glucose intraperitoneally. At 0, 15, 30, 60, and 120 minutes, blood was sampled from the tail vein and glucose was measured with a handheld glucometer. To measure total glucose concentration for each mouse, we calculated the area under the curve and compared mean areas under the curve among the different exposure groups using the statistical analysis described in the following section.
LC-MS analysis
Tryptophan metabolites in maternal liver were measured using a modification of a previously established method (28). Briefly, the analytes were separated on a C18 silica column by reverse-phase LC and detected by electrospray ionization tandem MS in positive ion multiple-reaction monitoring mode. The limits of detection and the lower limits of quantification were in the range of 0.1 to 50 nM and 0.5 to 100 nM, respectively. 13C isotope−labeled and deuterated internal standards were used to achieve accurate quantification of metabolites. Mean levels among groups were compared using the statistical analysis described in the next section.
Statistical analysis
For metabolomics data, after log transformation, Welch two-sample t test was used for pairwise comparison and assessment of whether two unknown means were different from two independent populations. In addition, a random effects model was used to take into account that the fetuses were derived from different dams. For this analysis, we used a mixed analysis of variance model to identify biochemicals that differed significantly between the exposure groups using the following model: Log (Metabolite) = Exposure + Litter (Exposure) [Random]. We used random forest analysis (29) to segregate the various exposure groups and provide an “importance” rank ordering of metabolites. The analysis resulted in a predictive accuracy of 77%. To determine which metabolites make the largest contribution to the classification, a “variable importance” measure was computed and expressed as “mean decrease accuracy.” For all comparisons, P < 0.05 and a false discovery rate (q-value) <0.05 were considered significant. For qRT-PCR, GTT, and tryptophan catabolite measurements, we performed the Student t test or analysis of variance followed by Tukey post hoc test using the Prism software program. P < 0.05 was considered significant, and all values were expressed as mean ± standard error of the mean.
Results
In utero BPA exposure significantly altered fetal liver metabolome
To determine whether BPA-induced adult disease was linked to early developmental perturbations of metabolic pathways, we exposed pregnant F0 mice to control, lower-dose BPA, and upper-dose BPA and studied the metabolic profiles of the embryonic day 18.5 fetuses through metabolomics. By analyzing the fetus as the unit of measurement, we found levels of 13 metabolites that significantly differed between control and lower-dose BPA, 39 metabolites between control and upper-dose BPA, and 37 metabolites between lower-dose and upper-dose BPA [Fig. 1(a); Supplemental Tables 1 (172.9KB, xlsx) –3a (33.3KB, xlsx) ]. These biochemicals can be segregated into pathways related to carbohydrates, peptides, amino acids, lipids, purines, cofactors and vitamins, and xenobiotic metabolism (Supplemental Table 3 (33.3KB, xlsx) ). Because of the small sample sizes and heterogeneity among the replicates, principal components analysis did not reveal significant separation among groups or clustering among replicates within the same group (Supplemental Fig. 2 (1.9MB, eps) ). The subtle differences among groups, however, could be distinguished well when analyzed by random forest analysis. Random forest analysis accurately clustered the samples on the basis of their exposure groups and identified the top 30 biochemicals responsible for the segregation (Supplemental Fig. 3 (2.3MB, eps) ).
Figure 1.
Metabolomic analysis of BPA-exposed embryonic day 18.5 fetal liver shows dose-sensitive changes in the metabolome of BPA-exposed mice relative to that of controls. (a) Venn diagrams show the number of metabolites that are increased (top) or decreased (bottom) in lower-dose BPA vs control groups and upper-dose BPA vs control groups. (b) Hierarchically clustered heat map depicts fold-change differences in the mean concentration of metabolites in control (C), lower-dose BPA (L), and upper-dose BPA (U) exposure groups. The abundance of each metabolite was standardized to mean 0 and a standard deviation of 1. Unchanged metabolites are displayed as zero and are colored black. Metabolites with increased abundance have positive values and are displayed as red, and decreased metabolites have negative values and are displayed as green. The fetus was used as the unit of measurement.
When the lower dose was compared with control, we found that lipid metabolites (e.g., squalene, carnitine, 3-dehydrocarnitine, 3-hydroxybutyrate, propionylcarnitine, and hydroxybutyrylcartinine) were increased (Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ). Biochemicals in the carbohydrate, peptide, and amino acid metabolism classes (e.g., glycerate, glycylleucine and tyrosine, and proline, respectively) were decreased, whereas p-cresol sulfate and 2-hydroxybutyrate (i.e., amino acid metabolites) and pyridoxate (i.e., vitamin B6 metabolism) were significantly increased relative to controls (Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ).
In contrast to lower-dose BPA exposure, upper-dose BPA exposure produced more substantial metabolomic changes [Fig. 1(b); Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ]. Upper-dose BPA exposure significantly elevated levels of BPA glucuronide and BPA monosulfate relative to controls [Fig. 1(b); Supplemental Fig. 4 (1.5MB, eps) ; Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ]; these changes were unique to the upper dose and were not induced by lower-dose exposure. We also observed that upper-dose BPA altered lipid metabolism and that bile acid levels significantly differed between the experimental groups [Fig. 2(a); Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ]. Upper-dose BPA−exposed fetal livers showed increases in primary [e.g., taurocholate, β-muricholate, tauro (α + β) muricholate, and taurochenodeoxycholate] and secondary bile acids (e.g., taurohyodeoxycholic acid) relative to controls [Fig. 2(a); Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ]. Other biochemicals that were increased in upper-dose BPA fetuses were aromatic amino acid catabolites (e.g., phenylalanine, tyrosine, and tryptophan) and peptide metabolites (e.g., γ-glutamylphenylalanine, γ-glutamyltryptophan, γ-glutamyltyrosine, and phenylalanylaspartate). In general, upper-dose BPA increased levels of metabolites relative to controls [Fig. 1(a) and 1(b); Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ], except for some glucose metabolites (e.g., glucose-6-phosphate and fructose-6-phosphate) that were decreased [Fig. 1(b); Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ].
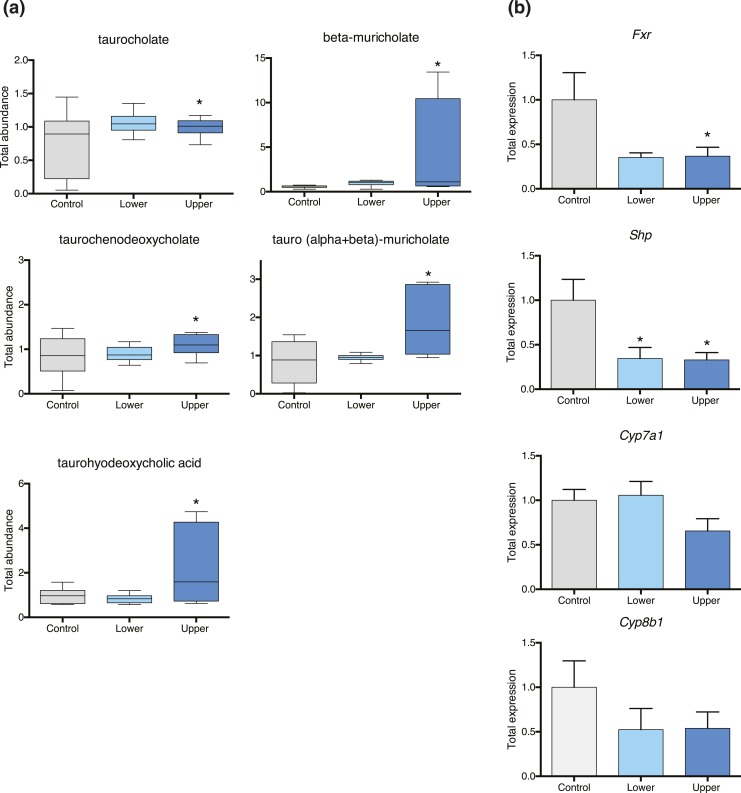
Figure 2.
Bile acid metabolism is perturbed in upper-dose BPA−exposed fetal liver. (a) Elevated levels of taurocholate, β-muricholate, taurochenodeoxycholate, tauro (α + β) muricholate, and taurohyodeoxycholate were observed in upper-dose BPA−exposed fetal liver relative to controls. (b) qRT-PCR analysis shows that fetal liver from the upper-dose BPA exposure group had significantly reduced Fxr and Shp messenger RNA expression relative to that of controls. No changes were detected with Cyp7a1 and Cyp8b1 messenger RNA expression. *P < 0.05 for all statistical analyses; the fetus was used as the unit of measurement. Error bars represent standard error of the mean.
When the metabolomes of the upper-dose BPA vs lower-dose BPA groups were compared, we observed a significant increase in the levels of bile acid metabolites, except for primary bile acids β-muricholate and taurocholate (Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ). Several amino acid and peptide metabolites were also increased, including glutamate, tyrosine, tryptophan, γ-glutamyltyrosine, and γ-glutamyltryptophan (Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ). No significant alteration in carbohydrate metabolism was detected, except for reduced ribulose/xylulose 5-phosphate and increased glycerate (Supplemental Tables 2 (159.8KB, xlsx) and 3a (33.3KB, xlsx) ). Overall, our results demonstrated that the effects of BPA exposure on the metabolome were dose sensitive and that upper-dose BPA produced markedly more changes than the lower dose.
When we reanalyzed the data using the litter as the unit of measurement, we found that levels of five metabolites significantly differed between control and lower-dose BPA, 17 metabolites between control and upper-dose BPA, and 14 metabolites between lower-dose BPA and-upper dose BPA (Supplemental Table 3b (33.3KB, xlsx) ). When lower-dose BPA was compared with control, we found increased levels of amino acid (e.g., betaine, p-cresol sulfate, and 2-hydroxybutyrate) and lipid (e.g., squalene) metabolites and decreased levels of glycerate (Supplemental Table 3b (33.3KB, xlsx) ). Except for betaine, differences in the abundance of these metabolites were also significant when we analyzed the data using the fetus as the unit of measurement (Supplemental Table 3a (33.3KB, xlsx) ).
In the reanalysis of the upper-dose BPA vs control groups, we consistently observed that peptide (e.g., γ-glutamylmethionine and γ-glutamyltryptophan) and amino acid (e.g., phenylalanine, p-cresol sulfate, and tryptophan) metabolites and BPA monosulfate were more abundant and that carbohydrate metabolites (e.g., glucose-6-phosphate, fructose, ribose 5-phosphate, and mannose-6-phosphate) were significantly reduced (Supplemental Table 3b (33.3KB, xlsx) ). No changes were detected in bile acids, except for primary bile acid cholate, which increased in the upper-dose BPA group (Supplemental Table 3b (33.3KB, xlsx) ). Using this alternative statistical method, we also observed that levels of betaine, alanylalanine, lysyl-leucine, phenylalanylalanine, phenylalanylaspartate, and serylphenyalanine were significantly higher; these changes were not detected when the analysis was done using the fetus as the unit of measurement. Furthermore, comparison between upper-dose BPA and lower-dose BPA revealed an increased abundance of glycerate, peptides (e.g., γ-glutamylmethionine, alanylalanine, lysyl-leucine, phenylalanylaspartate, and serylphenyalanine), amino acids (e.g., phenylalanine, tyrosine, and tryptophan), lipids (cholate and pentadecanoate), 2′-deoxyuridine, and BPA monosulfate. Ribulose was the only metabolite that showed decreased abundance in this comparison (Supplemental Table 3b (33.3KB, xlsx) ). Differences in alanylalanine, lysyl-leucine, phenylalanylaspartate, serylphenyalanine, and cholate were not previously detected when the fetus was used as the unit of measurement (Supplemental Table 3a (33.3KB, xlsx) ).
In summary, fewer metabolites were significantly different in abundance when the litter vs the fetus was used as the unit of measurement in the analysis (i.e., a total of 24 vs 65 metabolites, respectively, across exposure groups). However, both methods similarly showed that BPA affected the metabolome in a dose-sensitive manner, with upper-dose BPA producing more effects than lower-dose BPA.
Increased bile acids in liver from the upper-dose BPA−exposed fetus were linked to lack of negative feedback regulation
One of the top metabolic pathways that was altered in upper-dose BPA−exposed fetal livers was bile acid metabolism. Bile acid synthesis occurs exclusively in the liver through the conversion of cholesterol into more water-soluble amphiphatic compounds by the action of the cytochrome P450 superfamily of enzymes, CYP7A1 and CYP8B1. The size of the bile acid pool is tightly regulated by a farnesoid X receptor (Fxr)−dependent negative feedback mechanism in which increased bile acids activate Fxr gene expression, which subsequently inhibits transcription of Cyp7a1 and Cyp8b1 (30).
Because upper-dose BPA exposure resulted in increased bile acids in the liver [Fig. 2(a)], we analyzed expression of genes involved in bile acid regulation. Under normal conditions, increased bile acid levels activate Fxr messenger RNA (mRNA) expression (30). However, our qRT-PCR analysis revealed that Fxr mRNA expression was significantly reduced in upper-dose BPA−exposed livers [Fig. 2(b); P = 0.02]. Consistently, Shp mRNA levels were also decreased in the upper-dose BPA group relative to the control group [Fig. 2(b); P = 0.01]. However, reduced Fxr and Shp mRNA expression was not associated with increased transcription of Cyp7a1 and Cyp8b1, as we observed no significant difference in mRNA expression between control and upper-dose groups [Fig. 2(b)]. These data suggest that elevated bile acid levels in upper-dose BPA−exposed fetal liver may be linked to defective Fxr-dependent negative feedback regulation of bile acid synthesis.
Elevated tryptophan level was linked to gestational glucose intolerance
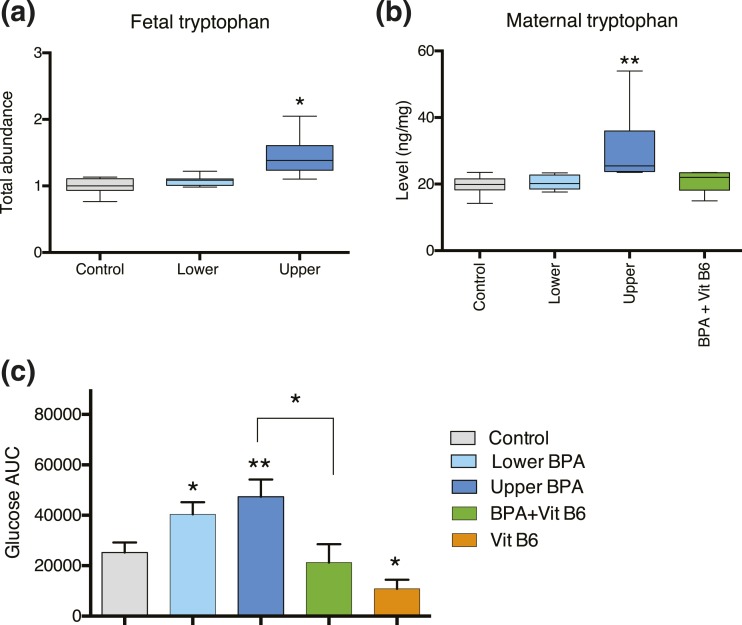
We and others have reported that BPA exposure in pregnant mice resulted in gestational glucose intolerance (15, 31). Although various mechanisms have been proposed (31), the exact link remains unclear. In the current study, we observed that upper-dose BPA exposure increased fetal tryptophan levels [Fig. 3(a); Supplemental Table 3] (33.3KB, xlsx) . We next investigated whether fetal tryptophan levels reflected levels in the pregnant dams and whether tryptophan catabolism was a potential mechanism underlying BPA-induced gestational glucose intolerance in pregnant mice. Using LC-MS, we independently measured tryptophan in the maternal liver and found that upper-dose BPA−exposed pregnant mice had elevated tryptophan levels relative to those of controls [Fig. 3(b); P = 0.0002]. We also found a trend of increased levels of maternal XA in the upper dose−exposed mice relative to levels in controls (P = 0.07).
Figure 3.
Tryptophan metabolism and vitamin B6 deficiency are linked to BPA-induced gestational glucose intolerance. (a) Metabolomic analysis revealed that liver from upper-dose BPA−exposed fetuses had increased tryptophan levels relative to that of controls. (b) LC-MS assay shows that liver from upper-dose BPA−exposed pregnant mice (dark blue) had elevated tryptophan levels compared with levels in control pregnant mice (gray); however, the level decreased to control-like values when the mice were supplemented with vitamin B6 (green). (c) GTTs show that upper-dose BPA−exposed pregnant mice (dark blue) had a significantly increased glucose concentration relative to that of controls (gray). In contrast, vitamin B6 supplementation in upper-dose BPA−exposed pregnant mice (green) normalized the glucose level to a control-like value. Our data also show that pregnant mice supplemented with vitamin B6 alone (orange) had a significantly lower glucose concentration compared with that of controls. *P < 0.05; **P < 0.001; the pregnant mouse was used as the unit of measurement.
One condition that may result in elevated tryptophan and XA levels is vitamin B6 deficiency; studies have suggested that vitamin B6 supplementation can partially rescue gestational diabetes in women (21, 22). We tested whether gestational glucose intolerance in BPA-exposed mice could be alleviated with vitamin B6 supplementation by conducting a GTT in pregnant mice exposed to control, upper-dose BPA, and upper-dose BPA supplemented with 24 mg/kg of vitamin B6. Consistent with previous findings, upper-dose BPA−exposed pregnant mice had increased glucose levels relative to those of controls [P = 0.01 (15)], suggesting a phenotype consistent with gestational glucose intolerance [Fig. 3(c)]. Interestingly, BPA-exposed mice supplemented with vitamin B6 had glucose levels similar to those of controls [Fig. 3(c)]; tryptophan levels in these mice were also similar to those of controls [Fig. 3(b)]. Our results demonstrate that vitamin B6 supplementation rescued gestational glucose intolerance and suggest that the BPA-induced metabolic phenotype is, at least in part, linked to vitamin B6−dependent tryptophan catabolism.
Discussion
Studies have demonstrated that some health effects related to BPA exposure are linked to its estrogenlike properties (32). Previously, we reported that early developmental BPA exposure is linked to male-specific glucose intolerance, higher body fat, and insulin resistance during adulthood (15). Through profiling of the fetal liver metabolome, we identified bile acid metabolism as a pathway that was perturbed in BPA-exposed fetuses, providing a candidate mechanism for adult metabolic disease susceptibility. Elevated fetal bile acid levels have been linked to adverse pregnancy outcomes (33). In adults, bile acids have been shown to regulate lipid, glucose, and energy metabolism, and abnormal levels contributed to the development of adult obesity and diabetes (34). Our studies demonstrated that BPA exposure altered bile acid metabolism in the fetus. Whether this contributed to the development of abnormal glucose metabolism that we observed in BPA-exposed mice remains to be determined.
Bile acids are synthesized from cholesterol through the classic pathway in which CYP7A1 and CYP8B1 convert cholesterol to 7 α-hydroxycholesterol and subsequently to 7 α-hydroxyl 4 cholesten, which is the precursor of the primary bile acids cholate and chenodeoxycholic acid. In the mouse, most chenodeoxycholic acid is converted to α- and β-muricholate, and cholate and α- and α-muricholate are the major primary bile acids in the mouse bile acid pool. Some primary bile acids subsequently undergo conjugation with taurine or glycine and are converted to secondary bile acids, although it is unclear how much of the conjugation process occurs in the fetus and whether most conjugates are derived maternally.
Levels of primary bile acids and their conjugates are relatively low in the fetal liver 2 days before birth but markedly increase at parturition, consistent with patterns of Cyp7a1 and Cyp8b1 mRNA expression (35). Cyp7a1 transcription and bile acid synthesis are under negative feedback regulation by Fxr-dependent signaling. An increased bile acid pool induces Fxr gene activity and activates transcription of Shp. Shp is a corepressor that inhibits Lrh1 or Hnf4-α, which subsequently represses Cyp7a1 and inhibits bile acid synthesis (30). However, elevated bile acids in our upper-dose BPA−exposed fetal liver did not result in the expected response; we observed reduced transcription of Fxr and Shp, suggesting that the negative feedback regulation was aberrant. We also noted that although upper-dose BPA−exposed fetal liver had reduced Fxr and Shp gene expression, gene expression of Cyp7a1 and Cyp8b1 was unchanged [Fig. 2(b)], which suggests that the increased bile acid pool was not due to elevated synthesis per se. This observation implies that an alternative mechanism must exist to increase the bile acid pool in the upper-dose BPA−exposed fetal liver.
Although detailed exploration of the bile acid signaling pathway is beyond the scope of this study, future analyses that include studies of fetal import and/or transport of bile acids are potentially important in elucidating mechanisms underlying a BPA-induced increase in the bile acid pool. In newborn mice, Fxr-dependent signaling initiates the expression of major transporters involved in enterohepatic circulation of bile acids (35). Reduced Fxr mRNA expression in upper-dose BPA−exposed mice may be associated with impaired transport and increased accumulation of bile acids in fetal liver. In addition, BPA may impact placental and maternal circulation of bile acids (36). How these processes contribute to the later development of increased fat deposition in BPA-exposed mice (15) remains to be determined, and future studies will be important to elucidate more mechanistic details of BPA-induced alterations in fetal bile acid metabolism.
This study reported a link between abnormal tryptophan catabolism and EDC exposure. In normal human pregnancy, maternal levels of tryptophan typically decrease by ∼40% from the first to the third trimester (37). In addition, increased tryptophan is more frequently observed in miscarriages and pregnancies with complications, including preeclampsia (38, 39). Furthermore, abnormal tryptophan catabolism has been implicated in gestational diabetes (21, 22). We tested the relevance of tryptophan to the development of gestational glucose intolerance in this mouse model (15). One potential mechanism that disrupts the tryptophan catabolic pathway is cellular insufficiencies of PLP, the active form of vitamin B6 (20). Tryptophan catabolism is sensitive to reduced vitamin B6 bioavailability because various enzymes in the pathway (e.g., kynureninase and kynurenine aminotransferase) use PLP as a cofactor (20). In both humans and mice, decreased PLP level can alter activities of these key enzymes, thereby increasing levels of tryptophan catabolites (18, 19). In mice, kynureninase activity decreases during pregnancy, and this is exacerbated by gestational vitamin B6 deficiency (20). One prevailing hypothesis is that estrogen conjugates, which are typically elevated during pregnancy, inhibit binding of PLP to kynureninase (40, 41). Further studies have suggested that compounds comparable in structure to estradiol sulfate, such as diethylstilbestrol disulfate, act similarly (41).
We postulate that the increased level of BPA monosulfate in upper-dose BPA−exposed mice (Supplemental Fig. 4 (1.5MB, eps) ) inhibited tryptophan catabolism by perturbing binding of PLP to PLP-dependent enzymes and that these defects were linked to gestational glucose intolerance, which we have observed in our BPA-exposed pregnant mice. Supplementing BPA-exposed pregnant mice with vitamin B6 rescued the gestational diabetes phenotype. Because sulfate conjugation is a common step in phase II metabolism of xenobiotics, elucidating the mechanisms using these mouse models may contribute to understanding of how BPA or other estrogenic chemicals interfere with the tryptophan-kynurenine catabolic pathway. In humans, although glucuronide is the major BPA metabolite, BPA sulfate represents about 15% of total BPA (42). In addition, tryptophan is the precursor of serotonin, and modulation of this pathway has also been linked to gestational diabetes (43). Although mechanisms underlying gestational glucose intolerance are complex (i.e., lower-dose BPA−exposed pregnant mice had elevated glucose level but normal tryptophan level [Fig. 3(b) and 3(c)], future studies of tryptophan catabolism and the effects of environmental exposures on the regulation of this pathway may illuminate some etiologies of environment-induced human pregnancy complications.
Despite these findings, our studies have some limitations. First, our initial analysis used the fetus, as opposed to the litter, as the unit of measurement. This statistical approach may not have controlled for potential litter effects (i.e., if exposure differentially affected litters, such that fetuses from one litter had different values of the outcome variable than fetuses from the other litter). Reanalysis of our metabolomics data using a random effects model, which took into account the contribution of each litter, revealed that tryptophan was still significantly more abundant in the upper-dose group (Supplemental Tables 2 (159.8KB, xlsx) and 3 (33.3KB, xlsx) ). We also detected similar trends for taurocholate and tauro (α + β) muricholate (Supplemental Tables 2 (159.8KB, xlsx) and 3 (33.3KB, xlsx) ). Second, we tested only males because no metabolic phenotypes were observed in F1 female offspring (15). Lastly, we used independent cohorts of mice for the different components of this study, which may have limited integration of biological interpretations of the results.
In conclusion, our study has identified two metabolic pathways that are abnormal in BPA-exposed mice and could be responsible for the observed metabolic phenotypes: bile acid metabolism and tryptophan catabolism. In-depth studies of various components of each pathway will provide more mechanistic insights into how gestational exposure to BPA or other EDCs disrupts maternal and fetal health.
Acknowledgments
We acknowledge the Penn Diabetes Endocrine Research Center (supported by Grant P30-DK19525) and the services of the Mouse Phenotyping, Physiology, and Metabolism Core. We also acknowledge the Center of Excellence in Environmental Toxicology (supported by Grant P30-ES013508). We thank David Condon for assistance in data analysis and Aimee Juan for technical assistance in qPCR.
Current Affiliation: M. Susiarjo’s current affiliation is the Department of Environmental Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York 14642.
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences Grants K99ES022244 and R00ES022244 (to M. Susiarjo), T32ES019851 (to F.X.), and ES023284 and ES013508 (to M.S.B. and R.A.S.) and March of Dimes Foundation Research Grant FY12-509 (to M.S.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- bw
- body weight
- EDC
- endocrine-disrupting chemical
- Fxr
- farnesoid X receptor
- GTT
- glucose tolerance test
- LC-MS
- liquid chromatography–tandem mass spectrometry
- mRNA
- messenger RNA
- PLP
- pyridoxal 5′-phosphate
- qRT-PCR
- real-time quantitative polymerase chain reaction
- XA
- xanthurenic acid.
References
- 1.Krieg SA, Shahine LK, Lathi RB. Environmental exposure to endocrine-disrupting chemicals and miscarriage. Fertil Steril. 2016;106(4):941–947. [DOI] [PubMed] [Google Scholar]
- 2.Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF. Urinary concentrations of bisphenol A and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ Health Perspect. 2016;124(10):1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leclerc F, Dubois M-F, Aris A. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertens Pregnancy. 2014;33(3):341–348. [DOI] [PubMed] [Google Scholar]
- 4.Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson KK, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD. Phthalate metabolites and bisphenol-A in association with circulating angiogenic biomarkers across pregnancy. Placenta. 2015;36(6):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlich S, Lambers D, Baccarelli A, Khoury J, Macaluso M, Ho S-M. Endocrine disruptors: a potential risk factor for gestational diabetes mellitus. Am J Perinatol. 2016;33(13):1313–1318. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20(8):2325–2329. [DOI] [PubMed] [Google Scholar]
- 9.Vafeiadi M, Roumeliotaki T, Myridakis A, Chalkiadaki G, Fthenou E, Dermitzaki E, Karachaliou M, Sarri K, Vassilaki M, Stephanou EG, Kogevinas M, Chatzi L. Association of early life exposure to bisphenol A with obesity and cardiometabolic traits in childhood. Environ Res. 2016;146:379–387. [DOI] [PubMed] [Google Scholar]
- 10.Veiga-Lopez A, Kannan K, Liao C, Ye W, Domino SE, Padmanabhan V. Gender-specific effects on gestational length and birth weight by early pregnancy BPA exposure. J Clin Endocrinol Metab. 2015;100(11):E1394–E1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huo W, Xia W, Wan Y, Zhang B, Zhou A, Zhang Y, Huang K, Zhu Y, Wu C, Peng Y, Jiang M, Hu J, Chang H, Xu B, Li Y, Xu S. Maternal urinary bisphenol A levels and infant low birth weight: a nested case-control study of the Health Baby Cohort in China. Environ Int. 2015;85:96–103. [DOI] [PubMed] [Google Scholar]
- 12.Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, Morisset A-S, Taback S, Bouchard MF, Monnier P, Dallaire R, Fraser WD. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health. 2014;13(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troisi J, Mikelson C, Richards S, Symes S, Adair D, Zullo F, Guida M. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta. 2014;35(11):947–952. [DOI] [PubMed] [Google Scholar]
- 14.Braun JM, Lanphear BP, Calafat AM, Deria S, Khoury J, Howe CJ, Venners SA. Early-life bisphenol A exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122(11):1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Susiarjo M, Xin F, Bansal A, Stefaniak M, Li C, Simmons RA, Bartolomei MS. Bisphenol A exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology. 2015;156(6):2049–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. Tryptophan-catabolizing enzymes - party of three. Front Immunol. 2014;5:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oxenkrug GF. Increased plasma levels of xanthurenic and kynurenic acids in type 2 diabetes. Mol Neurobiol. 2015;52(2):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender DA, Njagi EN, Danielian PS. Tryptophan metabolism in vitamin B6-deficient mice. Br J Nutr. 1990;63(1):27–36. [DOI] [PubMed] [Google Scholar]
- 19.da Silva VR, Rios-Avila L, Lamers Y, Ralat MA, Midttun Ø, Quinlivan EP, Garrett TJ, Coats B, Shankar MN, Percival SS, Chi Y-Y, Muller KE, Ueland PM, Stacpoole PW, Gregory JF III. Metabolite profile analysis reveals functional effects of 28-day vitamin B-6 restriction on one-carbon metabolism and tryptophan catabolic pathways in healthy men and women. J Nutr. 2013;143(11):1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Kamp JL, Smolen A. Response of kynurenine pathway enzymes to pregnancy and dietary level of vitamin B-6. Pharmacol Biochem Behav. 1995;51(4):753–758. [DOI] [PubMed] [Google Scholar]
- 21.Spellacy WN, Buhi WC, Birk SA. Vitamin B6 treatment of gestational diabetes mellitus: studies of blood glucose and plasma insulin. Am J Obstet Gynecol. 1977;127(6):599–602. [DOI] [PubMed] [Google Scholar]
- 22.Bennink HJ, Schreurs WH. Improvement of oral glucose tolerance in gestational diabetes by pyridoxine. BMJ. 1975;3(5974):13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol A exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9(4):e1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeve VE, Bosnic M, Boehm-Wilcox C, Cope RB. Pyridoxine supplementation protects mice from suppression of contact hypersensitivity induced by 2-acetyl-4-tetrahydroxybutylimidazole (THI), ultraviolet B radiation (280-320 nm), or cis-urocanic acid. Am J Clin Nutr. 1995;61(3):571–576. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu S, Watanabe H, Oka T, Tsuge H, Kat N. Dietary vitamin B6 suppresses colon tumorigenesis, 8-hydroxyguanosine, 4-hydroxynonenal, and inducible nitric oxide synthase protein in azoxymethane-treated mice. J Nutr Sci Vitaminol (Tokyo). 2002;48(1):65–68. [DOI] [PubMed] [Google Scholar]
- 26.Selhub J, Byun A, Liu Z, Mason JB, Bronson RT, Crott JW. Dietary vitamin B6 intake modulates colonic inflammation in the IL10-/- model of inflammatory bowel disease. J Nutr Biochem. 2013;24(12):2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudonck KJ, Mitchell MW, Német L, Keresztes L, Nyska A, Shinar D, Rosenstock M. Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol Pathol. 2009;37(3):280–292. [DOI] [PubMed] [Google Scholar]
- 28.Zhu W, Stevens AP, Dettmer K, Gottfried E, Hoves S, Kreutz M, Holler E, Canelas AB, Kema I, Oefner PJ. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(10):3249–3261. [DOI] [PubMed] [Google Scholar]
- 29.Breiman L. Random forests. Mach Learn. 2001;45(1):5–32. [Google Scholar]
- 30.Chiang JYL. Bile acid metabolism and signaling. Compr Physiol. 2013;3(3):1191–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113(8):926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouwers L, Koster MPH, Page-Christiaens GCML, Kemperman H, Boon J, Evers IM, Bogte A, Oudijk MA. Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. Am J Obstet Gynecol. 2015;212(1):100.e1–100.e7. [DOI] [PubMed] [Google Scholar]
- 34.Taoka H, Yokoyama Y, Morimoto K, Kitamura N, Tanigaki T, Takashina Y, Tsubota K, Watanabe M. Role of bile acids in the regulation of the metabolic pathways. World J Diabetes. 2016;7(13):260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui JY, Aleksunes LM, Tanaka Y, Fu ZD, Guo Y, Guo GL, Lu H, Zhong X-B, Klaassen CD. Bile acids via FXR initiate the expression of major transporters involved in the enterohepatic circulation of bile acids in newborn mice. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G979–G996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marín JJG, Macías RIR, Briz O, Pérez MJ, Serrano MA. Molecular bases of the excretion of fetal bile acids and pigments through the fetal liver-placenta-maternal liver pathway. Ann Hepatol. 2005;4(2):70–76. [PubMed] [Google Scholar]
- 37.Schröcksnadel H, Baier-Bitterlich G, Dapunt O, Wachter H, Fuchs D. Decreased plasma tryptophan in pregnancy. Obstet Gynecol. 1996;88(1):47–50. [DOI] [PubMed] [Google Scholar]
- 38.Kudo Y, Boyd CAR, Sargent IL, Redman CWG. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. 2003;188(3):719–726. [DOI] [PubMed] [Google Scholar]
- 39.Obayashi Y, Ozaki Y, Goto S, Obayashi S, Suzumori N, Ohyama F, Tone S, Sugiura-Ogasawara M. Role of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in patients with recurrent miscarriage. Am J Reprod Immunol. 2016;75(1):69–77. [DOI] [PubMed] [Google Scholar]
- 40.Mason M, Manning B. Effects of steroid conjugates on availability of pyridoxal phosphate for kynureninase and kynurenine aminotransferase activity. Am J Clin Nutr. 1971;24(7):786–791. [DOI] [PubMed] [Google Scholar]
- 41.Mason M, Gullekson EH. Estrogen-enzyme interactions: inhibition and protection of kynurenine transaminase by the sulfate esters of diethylstilbestrol, estradiol, and estrone. J Biol Chem. 1960;235:1312–1316. [PubMed] [Google Scholar]
- 42.Gerona RR, Pan J, Zota AR, Schwartz JM, Friesen M, Taylor JA, Hunt PA, Woodruff TJ. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: a cross-sectional study. Environ Health. 2016;15(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16(7):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]