Abstract
Fasting evokes a homeostatic response that maintains circulating levels of energy-rich metabolites and increases the drive to eat. Centrally, this reflex activates a small population of hypothalamic neurons that are characterized by the expression of AgRP, a neuropeptide with an extremely restricted distribution. Apart from the hypothalamus, the only other site with substantial expression is the adrenal gland, but there is disagreement about which cells synthesize AgRP. Using immunohistochemistry, flow cytometry, and reverse transcription-polymerase chain reaction, we show AgRP is present in the mouse adrenal medulla and is expressed by neuroendocrine chromaffin cells that also synthesize the catecholamines and neuropeptide Y. Short-term fasting led to an increase in adrenal AgRP expression. Because AgRP can act as an antagonist at MC3/4 receptors, we tested whether melanotan II, an MC3/4 receptor agonist, could regulate pre- and postsynaptic signaling within the adrenal medulla. Melanotan II decreased the paired-pulse ratio of evoked synaptic currents recorded in chromaffin cells; this effect was blocked by exogenous AgRP. In contrast, neither melanotan II nor AgRP altered the optogenetically evoked release of catecholamines from isolated chromaffin cells. These results are consistent with the idea that AgRP regulates the strength of the sympathetic input by modulation of presynaptic MC3/4 receptors located on preganglionic neurons. We conclude that a small population of neuroendocrine cells in the adrenal medulla, and the arcuate nucleus of the hypothalamus, express AgRP and neuropeptide Y and are functionally involved in the systemic response to fasting.
Adrenal chromaffin cells synthesize AgRP and this neuropeptide modulates sympathoadrenal synaptic transmission.
Food deprivation and other metabolic stressors that can result in hypoglycemia evoke a counter-regulatory response (1, 2). In the periphery, this includes an increase in the circulating level of hormones that elevate glucose availability, whereas centrally the response to fasting involves a modulation of the circuitry that controls food intake, detects changes in blood glucose and coordinates the systemic response. These central and peripheral components interact. Critical to the central response to fasting are hypothalamic arcuate neurons that synthesize both neuropeptide Y (NPY) and AgRP. These interoceptive neurons are metabolic sensors that monitor the levels of blood-borne factors involved in the peripheral response (3). Optogenetic or pharmacological activation of these cells increases food intake, whereas their inhibition reduces food consumption (4, 5). The depletion of AgRP neurons in adult mice leads to a loss of feeding and rapid starvation (6), whereas fasting is associated with a change in their activity (7–9). By modulating the activity of the arcuate AgRP/NPY neurons, peripheral signals are thus thought to increase (ghrelin) or decrease (insulin, leptin) the systemic response to a metabolic stressor (10, 11).
An unusual feature of these hypothalamic arcuate neurons that has facilitated the study of their functional role is their distinctive neurochemical phenotype (12, 13). Although NPY (and GABA, their classical transmitter) are ubiquitous in the nervous system, AgRP expression is highly restricted; the only other site of substantial expression appears to be the adrenal gland (13, 14).
This peripheral source of AgRP is intriguing given that the hypothalamic, pituitary, and adrenal axis is also involved in metabolic regulation. Food deprivation evokes glucocorticoid release from the adrenal cortex and epinephrine from the adrenal medulla, and both hormones increase plasma glucose levels (15, 16), contributing to the restoration of euglycemia. Fasting also increases both arcuate and adrenal expression of AgRP messenger RNA (mRNA) (12, 17), suggesting a conservation of function. However, the identity of the adrenal cells that express AgRP has been controversial. Although exogenous AgRP can inhibit glucocorticoid secretion from bovine (18, 19) and rat cortical cells (20), initial in situ hybridization studies in rodents localized the peptide to the adrenal medulla, which is part of the sympathetic nervous system (13). In contrast, a later report argued that this arose from a misidentification of adrenal zones and concluded that AgRP expression was restricted to cells in the adrenal cortex (17). In AgRP knockout mice, expression of the lacZ reporter was observed in cells in the medulla (21).
Given the widespread use of AgRP transgenic lines to study the control of metabolism, we decided to reexamine which adrenal cells expressed AgRP. The adrenal contains a diverse array of steroidal, neuroendocrine, and immune cells, not all of which are likely to be involved in the response to fasting (22–24). Using a variety of approaches, we find that this peptide is expressed by chromaffin cells, which also synthesize NPY. We confirmed that fasting led to an increase in the adrenal levels of AgRP and found that this involves a change in expression in chromaffin cells. Because AgRP can act as an antagonist at MC3/4 (melanocortin) receptors, we tested whether an agonist of these receptors could modulate the function of the adrenal medulla. Melanotan II did not alter catecholamine secretion from isolated chromaffin cells, but increased the strength of the preganglionic → chromaffin cells synapse; this effect was prevented by AgRP. Thus we find that an autonomic component of the fasting pathway unexpectedly uses the same peptide neurotransmitters as the small hypothalamic circuit that is central to the systemic response to metabolic stress.
Materials and Methods
Animals and food deprivation paradigm
C57BL/6J wild-type mice, ChR-tdTomato(B6.Cg-Gt(ROSA)26Sortm27.1(CAG-COP4*H134R/tdTomato)Hze/J (stock no. 012567); Ai27D (25), ChR-EYFP (B6;129S-Gt(ROSA)26Sortm32(CAG-COP4*H134R/EYFP)Hze/J (stock no. 012569, Ai32) (25), and NPY[green fluorescent protein (GFP)] mice (B6.FVB-Tg(Npy-hrGFP)1Lowl/J (stock no. 00641) (26) were purchased from The Jackson Laboratory. TH-cre (B6.FVB(Cg)-Tg(Th-cre)FI172Gsat/Mmucd (stock no. 031029-UCD) (27) was purchased from the Mutant Mouse Resource and Research Centers. Paired littermates were food-deprived for 24 hours from the start of the dark cycle; water was available ad libitum. In experiments involving insulin-induced hypoglycemia, paired littermates were food-deprived for 3 hours and then injected intraperitoneally with 2.5 U/kg insulin (Sigma, no. I2643) or vehicle as described (28). Food was returned 1 hour after injection and mice were euthanized 1 day later. Blood glucose was quantified using a handheld monitor (OptiumEZ, Abbott Diabetes Care Inc.). Experiments used both male and female animals that were euthanized either by decapitation or pentobarbital (150 mg/kg). All experiments involving animals were approved by the Animal Care and Use Committee at Louisiana State University Health Sciences Center.
Cell culture
Chromaffin cells were dissociated from the adrenal medulla of P24-60 mice as described (29). In brief, the medulla was separated from the cortex and incubated in HEPES-buffered salt solution (in millimolar: 138 NaCl, 5.3 KCl, 0.44 KH2PO4, 4 NaHCO3, 0.3 Na2HPO4, 20 HEPES, and 5.5 glucose, pH 7.25, with NaOH) containing 1 mg/mL collagenase type IA and 6 mg/mL bovine serum albumin (BSA) for 15 minutes at 37°C followed by incubation in 1 mg/mL trypsin and 6 mg/mL BSA for 30 minutes at 37°C. After digestion, the medulla was triturated using a fire-polished Pasteur pipette, and cells were plated on poly-d-lysine–coated coverslips in culture medium [Dulbecco’s modified Eagle medium (DMEM)/10% fetal calf serum (FCS)]. Superior cervical ganglia were obtained from P31 mice. Ganglia were desheathed using forceps and then digested and triturated as described previously for the adrenal medulla. Cells were plated in DMEM/10% FCS and then fixed within 6 hours after dissociation.
Fluorescence-activated cell sorting
Adrenal glands were isolated from a female P47 NPY(GFP) mouse and the medulla was digested as described previously. Cells were collected by centrifugation (200g for 3.5 minutes) and resuspended in 0.5 mL DMEM/1% FCS at a density of ∼2.5 × 104 cells/mL. Cells were subsequently sorted using a FACSAria and collected in DMEM/1% FCS. Sorted GFP cells were collected by centrifugation (2300g for 3 minutes) and resuspended in 100 µL Hanks balanced salt solution. TRIzol (200 µL; Invitrogen) was added to the suspension, which was then incubated at room temperature for 5 minutes before storage at −20°C for further RNA extraction.
Reverse transcription polymerase chain reaction and molecular biology
mRNA was extracted from adrenal medulla and brain using TRIzol following the manufacturer’s instruction. Samples were treated with DNase (30) and purified using an RNeasy purification kit (Qiagen). Complementary DNA was synthesized by reverse transcription of 200 ng of mRNA and used in polymerase chain reactions (PCRs). Primers and PCR protocols were as follows: AgRP: forward, 5′ CTGACTGCAATGTTGCTGAG 3′; reverse, 5′ CAACATCCATTGGCTAGGTG 3′ (406 bp, annealing temperature 55°C, 35 cycles); tyrosine hydroxylase (TH): forward, 5′ CCCCACCTGGAGTACTTTGTGC 3′; reverse, 5′ TGTGCACTGAAACACACGGAAG 3′ (556 bp, annealing temperature 56°C, 35 cycles); actin: forward, 5′ GCCAACCGTGAAAAGATGAC 3′; reverse, 5′ CAACGTCACACTTCATGATG 3′ (523 bp, annealing temperature 52°C, 35 cycles). DNA bands were excised from the agarose gel, purified using a Geneclean kit (Bio 101), and sequenced.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 20 minutes at room temperature and then stained as described previously (31). In brief, cells were permeabilized for 15 minutes in 0.3% Triton in phosphate-buffered saline (PBS), blocked in 0.25% immunoglobulin G (IgG)-free BSA in PBS for 30 minutes and incubated overnight at 4°C in primary antibody. After washing in PBS, the cells were incubated in secondary antibody for 90 minutes at room temperature, then washed with PBS and mounted in Vectashield. Primary antibodies were goat anti-AgRP [1:100; Santa Cruz Biotechnology; sc-18634; research resource identifier (RRID): AB_2258141], rabbit anti-NPY (1:40,000; Peninsula Laboratories; T4070; RRID: AB_518504), rabbit anti-TH (1:10,000; Millipore; ab152; RRID: AB_390204) and guinea pig anti-phenylethanolamine N-methyltransferase (PNMT) (1:100; Acris Antibodies; EUD 7001; RRID: AB_1006533). Secondary antibodies were donkey anti-goat DyLight 549 (1:100; Jackson ImmunoResearch), donkey antirabbit DyLight 488 (1:100; Jackson ImmunoResearch), donkey antirabbit Alexa Fluor 488 (1:200; Invitrogen), and donkey anti-guinea pig FITC (1:50; Jackson ImmunoResearch). All antibodies used are listed in Table 1.
Table 1.
Antibodies Used
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody, RRID | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| AgRP | Goat anti-AgRP | Santa Cruz Biotechnology, sc-18634, RRID: AB_2258141 | Goat; polyclonal | 1:100 (ICC); 1:200 (IHC fluorescence); 1:2000 (IHC peroxidase and TSA methods) |
| Neuropeptide Y | Rabbit anti-NPY | Peninsula Laboratories, T4070, RRID: AB_518504 | Rabbit; polyclonal | 1:40,000 (ICC); 1:10,000 (IHC) |
| Tyrosine hydroxylase | Rabbit anti-TH | Millipore, ab152, RRID: AB_390204 | Rabbit; polyclonal | 1:10,000 (ICC) |
| Tyrosine hydroxylase | Rabbit anti-TH | Cell Signaling Technology, #2792, RRID: AB_2303165 | Rabbit; polyclonal | 1:200 (IHC) |
| Phenylethanolamine N-methyltransferase | Guinea pig anti-PNMT | Acris Antibodies, EUD 7001, RRID: AB_1006533 | Guinea pig; polyclonal | 1:100 (ICC); 1:200 (IHC) |
| Aldo-keto reductase family 1, member C1 | Rabbit anti-AKR1C1 | GeneTex, GTX105620, RRID: AB_1949621 | Rabbit; polyclonal | 1:1000 (IHC) |
| Biotin | DTAF-streptavidin | Jackson ImmunoResearch, 016-010-084, RRID: AB_2337236 | na | 1:5000 (IHC) |
Abbreviations: ICC, immunocytochemistry; IHC, immunohistochemistry; na, not applicable; TSA, tyramide signal amplification.
Immunohistochemistry
Adrenal glands were dissected free of fat, fixed in 4% paraformaldehyde in PBS overnight at room temperature or 1 hour at 4°C, and then transferred to PBS/30% sucrose for 24 to 48 hours. To make frozen sections, the glands were snap frozen in 2-methylbutane on dry ice, embedded in CryoMatrix medium, sectioned (30 µm), and then mounted on Superfrost slides or stained as floating sections. For immunofluorescence staining, sections were permeabilized and blocked (5% IgG-free BSA + 0.3% Triton in PBS) for 2 hours at room temperature. After washing in PBS, sections were incubated in 50 mM glycine or 2 mg/mL NaBH4 in PBS for 30 minutes, then reblocked (5% IgG-free BSA + 0.05% Triton in PBS) for 30 minutes. Sections were subsequently incubated overnight at 4°C in primary antibody. After washing, sections were incubated in secondary antibody for 90 minutes at room temperature, then washed and mounted in Vectashield. To colocalize AgRP-immunoreactive (ir) with adrenal zone markers in fed mice [Fig 1(b)], most sections were first stained for zonal markers and then incubated overnight at room temperature in goat anti-AgRP (1:2000). After washing, sections were incubated in donkey anti-goat horseradish peroxidase (1:250; Jackson ImmunoResearch) and staining was subsequently revealed using TSA-Cy3 reagent (1:50, PerkinElmer). Sections were then stained with 4′,6-diamidino-2-phenylindole for 15 minutes (1 µg/mL) and mounted in Vectashield. Primary antibodies were goat anti-AgRP (1:200; direct immunofluorescence), rabbit anti-TH (1:200; Cell Signaling Technology; #2792), rabbit anti-NPY (1:10,000), rabbit anti-AKR1C1 (1:1000; GeneTex; GTX105620; RRID: AB_1949621), and guinea pig anti-PNMT (1:200) and DTAF-streptavidin (1:5000; Jackson ImmunoResearch; 016-010-084; RRID: AB_2337236).
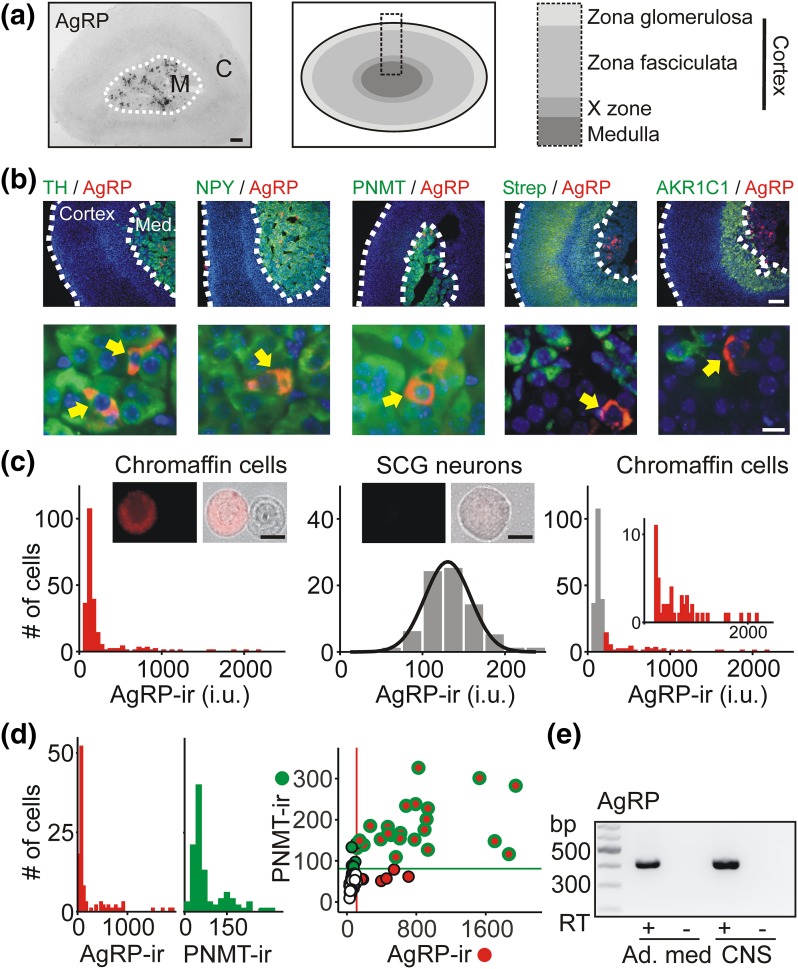
Figure 1.
Adrenal chromaffin cells express the AgRP neuropeptide. (a) Adrenal cryosection stained for AgRP using immunoperoxidase shows labeled cells in the medulla but not in the cortex. Schematic illustrates the zonal arrangement of the adrenal gland in mice (not to scale). (b). Adrenal cryosections showing that AgRP-ir cells colocalize with markers of chromaffin cells (TH, NPY, and PNMT), but not with AKR1C1 (X-zone marker) or streptavidin (pan cortical marker). (Lower panels) Representative AgRP-ir cells at higher resolution. (c) Frequency histogram of AgRP-ir [measured as intensity units (i.u.)] in chromaffin cells in vitro in a typical experiment (left panel; 233 cells) has a wide distribution, suggesting two populations of cells. (Middle panel ) The intensity distribution (n = 77 cells) of AgRP-ir in sympathetic superior cervical ganglion neurons, which are not thought to express AgRP. Overlaid is a fitted single Gaussian distribution that was used to estimate background levels of AgRP-ir. (Right panel) After background subtraction (gray bars), ∼20% of chromaffin cells are genuinely AgRP-ir (red bars). Inset is an enlargement showing the distribution of AgRP-ir. (d) Frequency distribution of the levels of AgRP- and PNMT-ir in chromaffin cells in vitro in a typical experiment (n = 105 cells). (Right panel) Quantification of the levels of PNMT- and AgRP-ir in chromaffin cells. Horizontal and vertical lines show background levels of PNMT-ir and AgRP-ir, respectively. Most AgRP-ir chromaffin cells costained for PNMT-ir (red/green symbols in upper right quadrant; 23 of 30 cells). (e) Reverse transcription polymerase chain reaction showing that adrenal medulla contains AgRP mRNA. CNS tissue was used as a positive control. Amplicons were only generated in the presence of reverse transcriptase (RT+). Scale bars: 100 µm (a, b upper); 10 µm (b lower, c). SCG, superior cervical ganglion.
For immunoperoxidase staining, adrenal sections were washed in PBS/0.05% Triton and then incubated overnight at 4°C in 1:2000 goat anti-AgRP in PBS/1% BSA/0.3% Triton. Sections were washed, and endogenous peroxidase activity was quenched in 3% H2O2 for 40 minutes. Sections were then incubated in donkey anti-goat horseradish peroxidase (1:250; Jackson ImmunoResearch) in PBS/1% BSA/0.05% Triton for 90 minutes at room temperature. Staining was subsequently revealed using diaminobenzidine as a chromogen (ImmPACT DAB kit; Vector Laboratories), and sections were mounted in a glycerol-based mounting medium.
Primary antibody specificities
We have previously shown the AgRP antibody stains INS-1 endocrine cells following transfection with the mouse AgRP complementary DNA clone and the antibody does not cross-react with NPY (30). The NPY antibody recognizes NPY-expressing adrenal chromaffin cells identified in an NPY(GFP) BAC transgenic mouse, and staining is absent in chromaffin cells from NPY knockout animals (29). Both TH antibodies selectively stain the adrenal medulla consistent with the known expression of the catecholamines and both label a ∼50 kDa band in Western blots from PC12 cells (manufacturer’s data) consistent with the recognition of rodent TH. The PNMT antibody labels a subpopulation of chromaffin cells (29, 32) consistent with the known expression of this enzyme (33). The AKR1C1 antibody recognizes an ∼37 kDa band in HepG2 cells consistent with the size of this enzyme and its expression in liver (manufacturer’s data). In adrenal sections, AKR1C1-ir was localized to a supramedullary zone that was sex-specific consistent with the absence of the X-zone (and thus AKR1C1 expression) in adult male mice (34).
Image analysis
Images were obtained with a Nikon TE2000U microscope, ×4, ×10, and ×60 oil-immersion (1.4 numerical aperture) objectives, a Lambda DG-4 wavelength switcher (Sutter Instruments), and a Retiga 1300 monochrome camera. Images were captured and deconvolved (×60 images) using NIS-Elements (Nikon) software. Origin Pro7, Excel, and ImageJ (Rasband WS, ImageJ, National Institutes of Health; http://imagej.nih.gov/ij/, 1997–2016) were used for data analysis. When quantitative comparisons were made between treatments, the same microscope settings and acquisition parameters were used. Biological replications (each using paired littermates) were performed close in time and quantitative measurements were always made on raw (not processed) data and were not thresholded. For quantification of immunoreactivity in tissue sections, areas of interest that corresponded to individual cells were selected (usually from four sections per animal) and mean pixel intensity per cell was calculated. For quantification of cells in vitro, images of single chromaffin cells were taken at the level of the nucleus to maintain a consistent plane of focus between experiments. In most experiments, 50 to 80 cells were photographed per treatment. The mean pixel intensity was then calculated from an area of interest (the entire cell) without background subtraction (except in the case that follows). Background levels of AgRP- and PNMT-ir in Fig. 1(c) and 1(d) were calculated by fitting each frequency-intensity histogram with two Gaussian distributions and using the [mean + (2 × standard deviation)] of the first distribution to set the threshold level of staining. In all experiments in which the level of immunoreactivity was quantified, control and experimental slides were stained and analyzed in parallel and slides were blinded until the analysis was complete. The Kolmogorov-Smirnov test was used to calculate statistical significance in the cumulative frequency distributions.
Amperometry
Chromaffin cells were isolated as described previously and plated on poly-d-lysine–coated dishes. Amperometric recordings were made from single chromaffin cells after 1 to 9 days in culture as described (29, 31). Recordings were made with carbon fiber electrodes that were coated with Sylgard and mounted on the head stage of a Multiclamp 700A amplifier (Molecular Devices). Electrodes were held at 700 mV and current was sampled at 5 kHz and filtered at 2 kHz. During an experiment, cells were superfused with extracellular solution (in millimolar: 135 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 5.5 glucose, pH 7.3, with NaOH) at room temperature. To examine the effect of melanotan II and AgRP on catecholamine release, secretion was evoked optogenetically or by the puffed application of nicotine. For optogenetically evoked secretion, chromaffin cells were isolated from TH-cre × ChR-tdTomato or TH-cre × ChR-EYFP mice and plated in vitro as described previously. Secretion was evoked by brief exposure to 470-nm light from a fiber-coupled laser-emitting diode (Thorlabs). Light exposure was triggered by transistor-transistor logic pulses from a Digidata 1322A (in most cases using two 20-ms flashes separated by 50 ms repeated with a 1-second interval). For agonist-evoked release, secretion was elicited from single cells from wild-type animals by focal application of 100 µM nicotine from a patch pipette placed close to the recorded cell. Each application was controlled using a Picospritzer (1-second application with a 30-second interapplication interval).
Slice electrophysiology
Adrenal glands were isolated from P30-40 male mice and embedded in 3.5% low-melting-point agarose, and 350-µm slices were prepared in ice-cold slicing solution (in millimolar: 81.2 NaCl, 2.4 KCl, 0.5 CaCl2, 6.7 MgCl2, 1.4 NaH2PO4, 23.4 NaHCO3, 23.3 glucose, and 69.9 sucrose, pH 7.4) using a Leica VT1000 Vibratome. Slices were transferred to artificial cerebrospinal fluid (ACSF; in millimolar: 125 NaCl, 2.5 KCl, 0.5 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 25 glucose, pH 7.4) for 100 minutes at 37°C, then held at room temperature until recordings were made as described (28). All solutions were saturated with 95% O2/5% CO2. Slices were superfused with ACSF containing 0.5 mM Ca2+ and recordings were made at 31°C to 33°C. Chromaffin cells were voltage clamped using patch electrodes (5-7 MΩ) that were filled with intracellular solution (in millimolar: 120 Cs acetate, 10 EGTA-Cs, 0.4 MgCl2, 1 CaCl2, 1.5 Na2ATP, 0.4 Na2GTP, 2.5 MgATP, 1 lidocaine N-ethyl bromide (QX-314), 5 tetraethylammonium chloride, and 10 HEPES, pH 7.25, with CsOH). Synaptic currents were recorded in chromaffin cells voltage clamped at −60 mV and were evoked by stimulating the preganglionic axon with a focal electrode (3-6 MΩ) that was filled with ACSF and placed close (20 to 100 μm) to the postsynaptic chromaffin cell. Series resistance was compensated by 80% and monitored throughout the experiment. If the value changed >20%, the recorded cell was excluded from analysis. To examine the effect of melanotan II and AgRP on synaptic efficacy, slices were incubated in ACSF (containing 0.5 mM Ca2+) with 1 µM melanotan II or 1 µM melanotan II plus 200 nM AgRP for 3 to 6 hours and then recordings were made in agonist-free ACSF. To quantify synaptic plasticity, the paired pulse ratio (PPR) of evoked excitatory postsynaptic currents (EPSCs; 10 to 90 events per cell) was monitored by stimulating the presynaptic input with two depolarizations (each 1 ms) separated by a 50-ms interval; the protocol was repeated every 10 seconds. The PPR of excitatory postsynaptic currents was calculated by dividing the amplitude of the second EPSC by that of the first (PPR = EPSC2/EPSC1).
Statistical tests
Comparisons between groups that contained normally distributed data (assessed using the Shapiro-Wilk test) were made using analysis of variance (Tukey post hoc). The Wilcoxon rank sum test was used for non-normal data, and the Kolmogorov-Smirnov test was used to analyze cumulative distributions. R (3.3.1; GUI Rcmdr 2.2-5), Clampfit, and OriginPro7 were used for analysis. All values are mean ± standard error of the mean (SEM) and P < 0.05 was considered significant.
Results
AgRP is expressed by adrenal medullary chromaffin cells
The distribution of AgRP in rodents is highly restricted. Apart from a population of AgRP/NPY neurons in the arcuate nucleus, the other main site of mRNA expression is the adrenal (13, 14). However, which adrenal cells contain AgRP is unclear (13, 17). The adrenal medulla is part of the sympathetic nervous system and contains neuroendocrine chromaffin cells that secrete the catecholamines epinephrine and norepinephrine. In contrast, the adrenal cortex has a different developmental origin, and cells in this area synthesize steroid hormones including corticosterone and aldosterone. To reexamine the question of localization, we used an AgRP-specific antibody (30) to stain sections of the mouse adrenal gland. Using immunoperoxidase staining, AgRP-ir cells were found scattered throughout the inner region of the gland but were not observed in the cortex [Fig. 1(a)]. To confirm the AgRP-ir cells were located in the medulla, we used a dual immunofluorescence approach and costained adrenal sections for AgRP and TH, a marker of catecholamine synthesis and NPY, a cotransmitter in mouse chromaffin cells (29). The AgRP-ir cells colocalized with both of these markers of the adrenal medulla [Fig. 1(b)]. Some AgRP-ir cells were also PNMT-ir, indicating that they synthesized epinephrine. In contrast, AgRP-ir did not overlap with AKR1C1-ir [Fig. 1(b)], which selectively labels the X-zone in mice (34) and did not colocalize with cells identified with fluorescently labeled streptavidin [Fig. 1(b)], which reveals all steroidogenic cells in the adrenal gland (35). When chromaffin cells were stained in vitro, the same colocalization of AgRP-ir with TH-, NPY-, and PNMT-ir was observed. Furthermore the AgRP-ir was punctate as expected for a neuropeptide that enters the regulated secretory pathway (Supplemental Fig. 1 (1.8MB, tif) ).
These results indicate that the AgRP-ir cells were chromaffin cells located within the adrenal medulla. However, when we quantified the levels of AgRP-ir in individual chromaffin cells in vitro, the intensity distribution was skewed, with a large fraction of cells having low levels of staining [Fig. 1(c), left]. To clarify what fraction was genuinely AgRP-ir, we determined background staining using superior cervical ganglion neurons, which have not been reported to express AgRP. The distribution of AgRP-ir intensity in superior cervical ganglion neurons was well-fit with a single Gaussian distribution [Fig. 1(c), middle]. Background subtraction then revealed the population of stained chromaffin cells [Fig. 1(c), right]. Group data indicated that a subset of chromaffin cells were authentically AgRP-ir (21 ± 0.6%; mean ± SEM, n = 3, ≥111 cells per experiment).
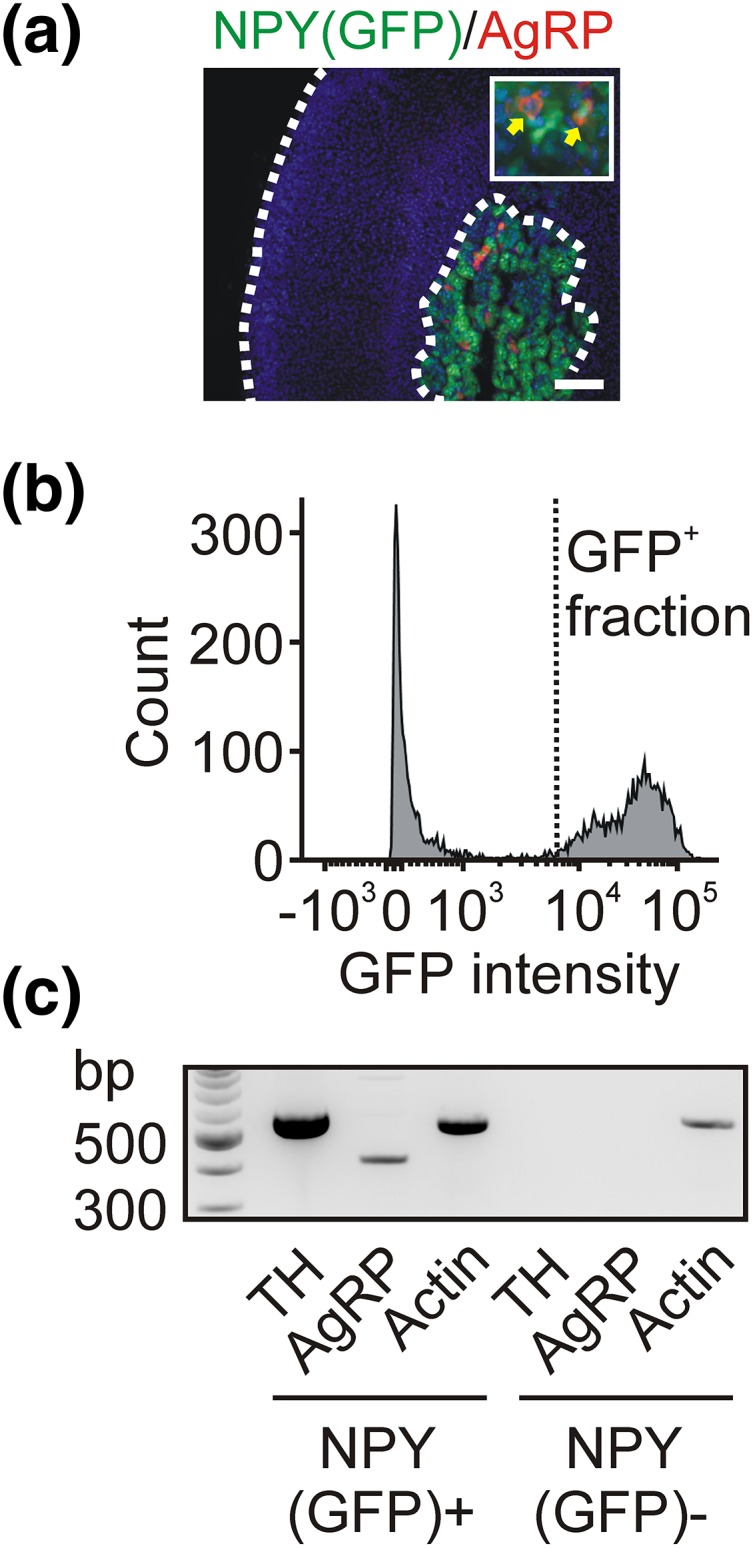
Because chromaffin cells synthesize either epinephrine or norepinephrine and are activated under different physiological conditions, we next determined which chromaffin cells contained AgRP. PNMT-ir was used to identify epinephrine-synthesizing chromaffin cells [Fig. 1(d)]. In the experiment shown in Fig. 1(d), most AgRP-ir chromaffin cells were PNMT positive; this was consistent with the group data (63 ± 7%, mean ± SEM, n = 3, ≥105 cells per experiment). Using reverse transcription PCR (RT-PCR), we confirmed that the adrenal medulla contains AgRP mRNA [Fig. 1(e)]. Next, because all mouse chromaffin cells synthesize NPY as a cotransmitter with the catecholamines (29), we dissociated the adrenal medulla from an NPY(GFP) BAC transgenic mouse (26) and used flow cytometry to isolate a pure population of GFP-expressing cells. We confirmed that, as in wild-type mice, the adrenal AgRP-ir cells in NPY(GFP) mice were confined to the medulla, which was identified by the expression of GFP [Fig. 2(a)]. The fluorescence-activated cell-sorted GFP-expressing cells [Fig. 2(b)] were used in an RT-PCR reaction, which showed that the NPY(GFP)-positive population contained both TH and AgRP mRNA. In contrast, the GFP-negative cells lacked both of these markers as expected [Fig. 2(c)]. From these experiments we conclude that AgRP is present in chromaffin cells in the adrenal medulla.
Figure 2.
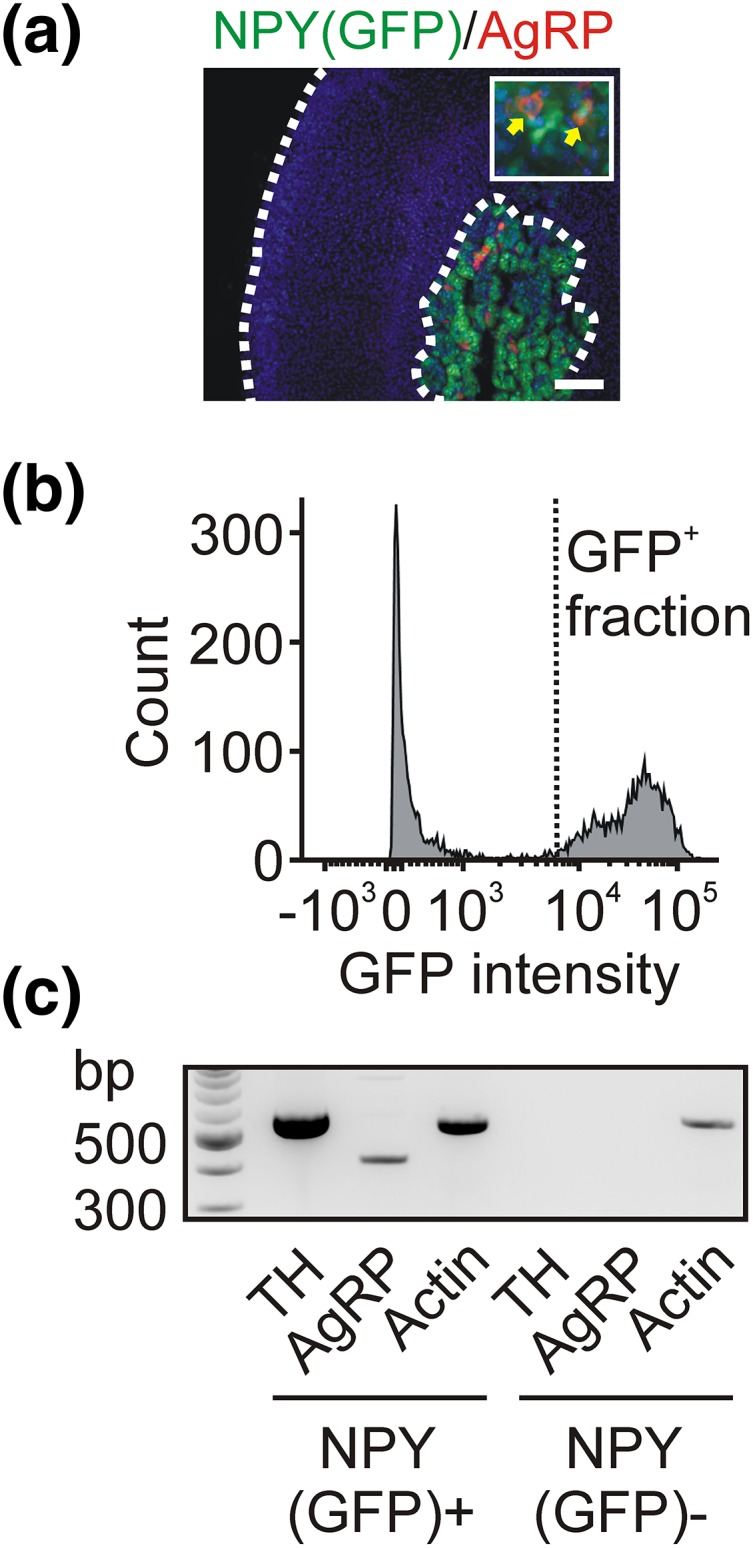
Fluorescence-activated cell-sorted adrenal chromaffin cells express AgRP. (a) Immunofluorescence staining of AgRP in an adrenal cryosection from an NPY(GFP) BAC transgenic mouse shows that AgRP-ir cells in the medulla also express GFP. (b) Fluorescence-activated cell-sorting analysis of adrenal cells isolated from an NPY(GFP) mouse. (c) RT-PCR showing that cells in the GFP-positive fraction contain TH and AgRP mRNA, whereas no TH or AgRP mRNA was detected in the GFP-negative fraction. Actin was used as a positive control. Scale bar, 100 µm.
Adrenal AgRP expression is regulated by metabolic state
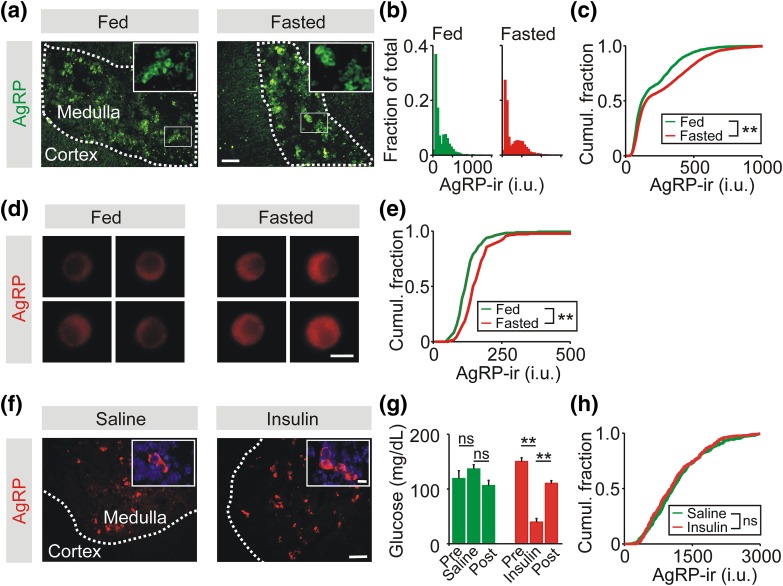
During fasting, there is a decrease in the sympathetic output to many tissues, presumably to conserve energy reserves. However, two important exceptions are a fasting-induced increase in sympathetic traffic to some white adipose tissue depots and to the adrenal medulla (36, 37). The latter leads to elevated levels of circulating epinephrine during food deprivation (37) and contributes to the fasting-induced increase in hepatic gluconeogenesis (38). We next determined whether food deprivation altered the adrenal expression of AgRP. Mice were food-deprived for 24 hours and the levels of AgRP-ir were quantified in cells in the medulla in adrenal sections from fed and fasted littermates [Fig. 3(a)]. The AgRP-ir intensity distribution from fed and fasted animals was bimodal [Fig. 3(b)]. Fasting resulted in a significant increase in AgRP-ir, as shown by the rightward shift in the cumulative intensity distribution [Fig. 3(c)]. A fasting-induced increase in AgRP expression was also observed when the levels of AgRP-ir were quantified in vitro from chromaffin cells dissociated from fed and fasted littermates [Fig. 3(d) and 3(e)]. These results are consistent with previous reports that showed that food deprivation increased the adrenal level of AgRP mRNA (17, 39).
Figure 3.
Food deprivation increases AgRP-ir in adrenal chromaffin cells. (a) Adrenal cryosections stained for AgRP-ir from fed and fasted littermates. AgRP-ir cells are confined to the adrenal medulla. (b) Frequency histograms of the levels of AgRP-ir in chromaffin cells from fed and fasted animals normalized to the total cell number (fed, 1887 cells; fasted, 4133 cells; n = 3 experiments). (c) Cumulative frequency distribution shows that food deprivation led to a significant increase in the level of AgRP-ir in chromaffin cells in adrenal sections. (d) Examples of chromaffin cells in vitro from fed and fasted littermates. (e) Cumulative frequency distribution showing that food deprivation led to a significant increase in the levels of AgRP-ir quantified from single cells in vitro (182 cells per distribution, n = 3 experiments). (f) Adrenal cryosections stained for AgRP-ir from control mice or 1 day after insulin-induced hypoglycemia. (g) Blood glucose levels from saline- or insulin-injected mice (mean ± SEM, n = 4) Bars show measurements before, 1 hour after, and 24 hours after injection, respectively. (h) Cumulative frequency distribution showing that insulin-induced hypoglycemia did not significantly alter the levels of AgRP-ir quantified from chromaffin cells in adrenal sections (saline, 373 cells; insulin, 387 cells; n = 4 experiments). **P < 0.01. Scale bars 100 µm (a, f) and 10 µm (d, f inset). ns, not signficiant.
To examine whether a metabolic challenge other than fasting could alter the expression of AgRP, the adrenal levels of AgRP-ir were quantified from mice after exposure to insulin-induced hypoglycemia [Fig. 3(f)]. The dose of insulin (2.5 U/kg) was chosen to ensure that blood glucose levels fell below ∼60 mg/dL, the threshold for evoking epinephrine release (15). AgRP-ir was quantified 1 day after insulin injection because previous work has shown that insulin-induced hypoglycemia produces a delayed rise in the level of other adrenal neuropeptide cotransmitters (40, 41). Insulin treatment produced a marked hypoglycemia when measured 1 hour after injection, but had returned close to basal levels when measured 1 day later [Fig. 3(g)]. However, there was no difference in the levels of adrenal AgRP-ir between control and insulin-injected animals when measured 1 day after insulin injection [Fig. 3(h)], perhaps because insulin-induced hypoglycemia is a relatively acute metabolic stress (or that any change is below the limit of detection).
Melanocortin receptors regulate signaling within the adrenal medulla
Because AgRP is expressed in chromaffin cells, what is the function of the secreted peptide? Although AgRP is found in the systemic circulation, adrenalectomy does not alter the plasma levels of AgRP (42); thus, an autocrine or paracrine site of action for AgRP within the adrenal is more likely than action at a distant target. AgRP can act as an endogenous antagonist at melanocortin 3 and 4 receptors (14) and mRNA for both receptors have been reported in the adrenal (18, 20, 43), but whether they directly regulate the function of the medulla is not clear.
We tested whether activation of melanocortin receptors could alter catecholamine secretion postsynaptically by acting directly on the chromaffin cells. Secretion was quantified from single cells in vitro using carbon fiber amperometry in the absence and presence of melanotan II, a nonselective melanocortin agonist of MC3/4 receptors. Because these G-protein–coupled receptors can signal via a cyclic adenosine monophosphate–mediated pathway, we evoked secretion optogenetically rather than by voltage clamp depolarizations (29) to avoid cell dialysis. Channelrhodopsin was expressed in chromaffin cells by crossing TH-cre and ChR-tdTomato mouse lines. Control experiments indicated that ChR-tdTomato expression was limited to chromaffin cells, and light-evoked catecholamine secretion did not occur in the absence of channelrhodopsin expression (Y. Ma, Q. Wang, and M. Whim, unpublished data). A double flash protocol repeated at 1 Hz was used to evoke catecholamine secretion and 100 nM melanotan II was superfused over the recorded cell. In the sample recording in Fig. 4(a), melanotan II did not result in an obvious change in release. Secretion was then quantified as a cumulative amperometric amplitude distribution [Fig. 4(b)]. The averaged release curve from multiple cells [white line in Fig. 4(b)] appeared linear with no change in the gradient following melanotan II application. Catecholamine secretion before and after melanotan II application was not significantly different [Fig. 4(b), right]. To confirm that secretion could be modulated, 10 µM nicotine was subsequently bath applied to each recorded cell. This agonist evoked a large increase in the frequency of amperometric events, as seen in the sample recording in Fig. 4(c). The change in release was also evident in the cumulative amplitude distributions [Fig. 4(d), left]. Group data showed that nicotine led to a significant increase in catecholamine release [Fig. 4(d), right].
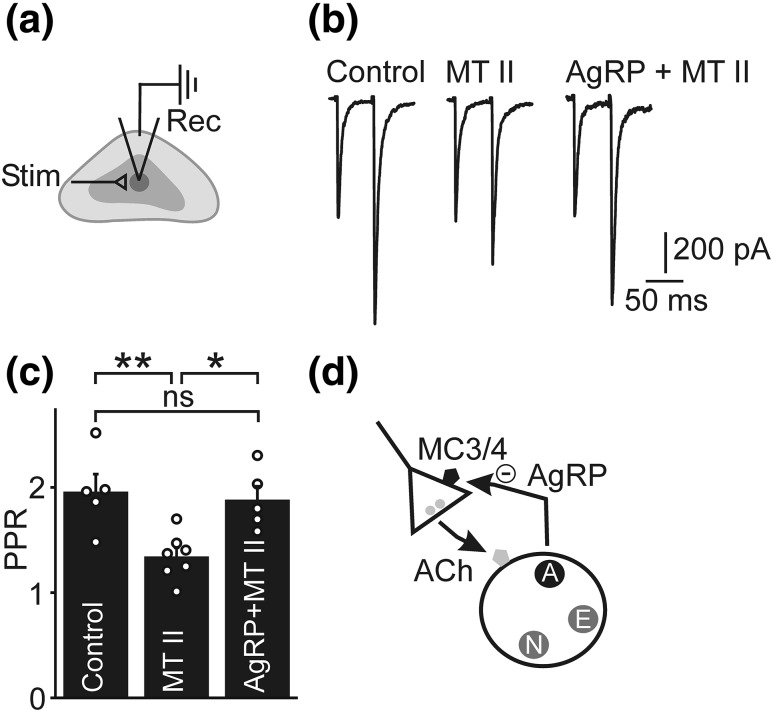
Figure 4.
MC3/4 receptor agonist and AgRP do not modulate catecholamine secretion from chromaffin cells in vitro. (a) Amperometric recording of optogenetically evoked catecholamine secretion from a chromaffin cell in vitro. Application of 100 nM melanotan II (MT II), an MC3/4 receptor agonist, is shown by the gray bar (left). Lower bars indicate optogenetic activation (not to scale). Five consecutive sweeps taken at the end of MT II application (right). The timing of optical stimulation is shown by the lower bars. Note that although secretion was not phase locked to stimulation, amperometric events were not detected in the absence of light flashes (not shown). (b) Group data showing cumulative amplitude distribution of amperometric events from multiple cells (left; n = 7 cells from three animals). White line shows the averaged response. Catecholamine secretion was not significantly different in the presence and absence of MT II (right). Secretion was quantified as the summed amplitude of amperometric events in a 100-second window before [control (cntl)] and at the termination of agonist application (MT II). Histogram shows mean ± SEM (n = 7 cells). (c) Example recording showing that application of 10 µM nicotine increased the frequency of amperometric events (left). Five consecutive sweeps taken at the end of nicotine application (right). (d) Change in the gradient of the cumulative amplitude distribution indicates that nicotine increased the frequency of amperometric events (left; n = 7 cells from three animals). Nicotine (nic.) led to a substantial increase in catecholamine secretion (right). (e) Example recording showing application of 100 nM AgRP. (f) Group data showing cumulative amplitude distribution of amperometric events (n = 5 cells from three animals). White line shows the averaged response. (g) Catecholamine secretion was not significantly altered by 10 or 100 nM AgRP (n = 5 cells from three animals for each treatment). (h) Catecholamine secretion in response to a 1-second application of 100 µM nicotine in the presence and absence of 100 nM AgRP. Three consecutive sweeps are shown, from before and after AgRP application. (i) Group data showing cumulative amplitude distribution of amperometric events (n = 11 cells from three animals). White line shows the averaged response. (j) Change in catecholamine secretion in after treatment with 10 or 100 nM AgRP (n = 10-11 cells from three animals for each treatment). *P < 0.05; ns, not significant.
These results suggest that functional MC3/4 receptors are not expressed by chromaffin cells. However, a recent study has shown that AgRP can regulate excitability via modulation of an inwardly rectifying potassium channel and that this action is independent of α–melanocyte-stimulating hormone (α-MSH) (44). We therefore examined whether AgRP alone could regulate catecholamine release. As shown in Fig. 4(e) and 4(f), bath application of 100 nM AgRP did not alter optogenetically evoked catecholamine release. Group data confirmed that neither 10 nor 100 nM AgRP significantly altered catecholamine release [Fig. 4(g)].
These experiments measured catecholamine secretion evoked by direct depolarization; however, in chicken sympathetic neurons, substance P is reported to suppress norepinephrine release from postganglionic neurons by increasing the desensitization rate of nicotinic acetylcholine receptors (45). We considered the possibility that AgRP might regulate secretion from chromaffin cells in a similar manner. To test this idea, we evoked catecholamine release from isolated chromaffin cells using the puffed application of nicotine and quantified catecholamine secretion using amperometry in the presence and absence of exogenous AgRP. As shown in Fig. 4(h) and 4(i), the bath application of 100 nM AgRP did not markedly alter catecholamine release. Group data [Fig. 4(j)] showed that release before and after treatment with 100 nM AgRP was not significantly different (control 1216 ± 541 pA vs AgRP 361 ± 103 pA, mean ± SEM summed event amplitude, n = 11, P = 0.065, Wilcoxon rank sum test). Similar experiments using 10 nM AgRP resulted in a small inhibition of release although the effect barely reached significance (control 501 ± 148 pA vs AgRP 213 ± 79 pA, n = 10, P = 0.043). Thus, although there is a trend toward inhibition of nicotine-evoked catecholamine secretion by exogenous AgRP, the effect is quantitatively small.
Because AgRP does not appear to act postsynaptically, we next examined whether melanocortin receptors could regulate the strength of the preganglionic → chromaffin cell synapse. Chromaffin cells were voltage clamped in adrenal slices and excitatory synaptic transmission was evoked by stimulating cholinergic preganglionic axons with a focal electrode [Fig. 5(a)]. Transmission was quantified by measuring the PPR of synaptic currents in response to two presynaptic depolarizations separated by 50 ms [Fig. 5(b). The PPR was significantly lower after application of 1 µM melanotan II indicating an increase in synaptic strength [Fig. 5(c)]. Because a change in PPR is usually interpreted as occurring via a presynaptic mechanism (46, 47), these results are consistent with the idea that activation of melanocortin receptors leads to a presynaptic increase in the strength of the preganglionic → chromaffin cell synapse. To determine whether this effect was modulated by AgRP, the PPR was quantified after the coapplication of melanotan II and AgRP. As shown in Fig. 5(b) and 5(c), this treatment prevented the melanotan II–evoked decrease in PPR. We conclude that one of the roles of AgRP is to regulate the strength of the preganglionic → chromaffin cell synapse.
Figure 5.
MC3/4 receptor agonist and AgRP modulate preganglionic → chromaffin cell synaptic strength. (a) Schematic of experiment. EPSCs were evoked in an adrenal slice preparation by focal stimulation (stim) of preganglionic axons and recorded (rec) in chromaffin cells voltage clamped at −60 mV. (b) Representative recording of evoked EPSCs. Two presynaptic depolarizations evoked paired pulse facilitation that was reduced after exposure to 1 µM melanotan (MT) II; this effect was absent in the presence of 200 nM AgRP. (c) Group data showing that the reduction in PPR evoked by MT II was prevented by coincubation with 200 nM AgRP. Histogram shows mean ± SEM (control, n = 5 cells from 3 animals; MT II, n = 7 cells from 3 animals, MT II + AgRP, n = 5 cells from 3 animals). (d) Working model of the experimental results. Activation of presynaptic MC3/4 receptors increases the strength of the cholinergic preganglionic → chromaffin cell synapse; exogenously applied AgRP prevents this effect. AgRP is expressed by a subset of chromaffin cells, suggesting that it acts as a cotransmitter that locally regulates catecholamine secretion. Because fasting increases both epinephrine release and AgRP expression, the role of the AgRP-mediated negative feedback loop may be to prevent excessive catecholamine release during periods of high sympathoadrenal activity. For simplicity, the adrenal cotransmitters [epinephrine (E), NPY (N), and AgRP (A)] are shown contained in separate populations of dense core granules. **P < 0.01, *P < 0.05. ns, not significant.
Discussion
Neuropeptides play important and diverse roles as cotransmitters and are found in most, probably all, neurons. Consistent with their role as neuromodulators, many neuropeptides have a widespread distribution and thus their functions are context dependent (48). NPY exemplifies this diversity. NPY is found throughout the central nervous system (49), where its roles include the regulation of synaptic transmission, food intake, and anxiety (50–52). In the periphery, NPY is released from postganglionic sympathetic neurons and regulates vasoconstriction (53).
If NPY is a neuropeptide with a wide anatomical distribution, AgRP occupies the opposite extreme. In the central nervous system apart from a population of NPY/AgRP/GABAergic cells in the arcuate nucleus, AgRP is found in very few neurons (30, 54). In the periphery, the primary site of expression is the adrenal, where its precise location and function has been mysterious (13, 17). Using multiple approaches, we find this neuropeptide is predominantly synthesized by a population of chromaffin cells in the adrenal medulla that coexpress NPY and release the catecholamines. Although this is in contrast to a previous report (17), it accords with the idea that adrenal cortical cells are largely steroidogenic. Although AgRP is present in the medulla, we do not exclude the possibility that cortical cells can also express AgRP under some circumstances. However, with rare exceptions, such as the opioid peptides found in subcapsular cell hyperplasia (55), neuropeptidelike signaling molecules have not been identified in the adrenal cortex. The cortex is also thought to lack the prohormone convertases that are required for the processing of neuropeptide precursors such as AgRP (56, 57), consistent with a restriction of AgRP expression to the medulla.
The function of AgRP in chromaffin cells is not known, but because adrenalectomy does not alter plasma levels of AgRP (42), it is likely to act in an autocrine or paracrine manner. One possible function is the modulation of steroid hormone release because exogenous AgRP can inhibit α-MSH–mediated glucocorticoid release from cortical cells in vitro (18–20). However, adrenal blood flow is directed centripetally and the gland lacks a portal system (58), making it unlikely that the AgRP released from chromaffin cells is responsible for this action. Because we found that application of a high-affinity melanocortin receptor agonist resulted in a reduction in PPR at the preganglionic → chromaffin cell synapse and that this effect was prevented by a low concentration of AgRP, we favor the idea that the function of medulla AgRP is to presynaptically regulate transmission at this sympathetic synapse [Fig. 5(d)]. Although synaptic strengthening and epinephrine release are required for effective glycemic regulation during fasting (28), epinephrine modulates blood pressure and immune function, among many other physiological variables. By acting as a brake on synaptic strength, an inhibitory cotransmitter such as AgRP may reduce the possibility of an unrestrained increase in sympathetic activity.
AgRP can act as an endogenous antagonist at MC3/4 receptors (14) and MC3 and MC4 receptor mRNA has been detected in the rat adrenal gland (18, 20, 43). These receptors are presumably postsynaptic, but whether they are also expressed by the preganglionic neurons that innervate the adrenal is not known. Sympathetic preganglionic neurons in the intermediolateral cell column in the thoracic spinal cord express MC4R mRNA (59), and this location corresponds to the site of the preganglionic neurons that innervate the adrenal (60). MC4 receptors have also been functionally identified in sympathetic preganglionic neurons (61). Patch clamp recordings from spinal preganglionic sympathetic neurons has shown that a subset are depolarized by THIQ, a selective MC4R agonist (62); because a similar effect was seen in the presence of TTX, it is likely that the action of THIQ was direct (62). MC4R activation also increases sympathetic outflow (63, 64). The increase in the preganglionic → chromaffin cell synaptic efficacy we observed is thus consistent with the reported expression of MC4 receptors in other autonomic neurons and the excitatory effect of MC4R activation on sympathetic output in general.
In addition to acting as an MC3/4 receptor antagonist, AgRP can regulate neuronal activity independently of the actions of α-MSH (the endogenous agonist of these receptors). For example, in ventromedial hypothalamic neurons, α-MSH and AgRP both inhibit neuronal activity but through independent mechanisms. These actions of AgRP are mediated through a presynaptic inhibition of excitatory transmission via Gi/o (65). This is inconsistent with the involvement of melanocortin receptors, which act through Gs signaling (66). As shown recently in the paraventricular nucleus, AgRP can also inhibit neuronal excitability by a G protein–independent mechanism that involves the opening of Kir7.1 inward rectifier channels (44). Thus, depending upon the tissue, AgRP-dependent signaling can extend beyond the regulation of MC3/4 receptor activity.
What are the implications for metabolic regulation given the expression of AgRP in adrenergic chromaffin cells? Hypoglycemia and food deprivation are potent stimulators of epinephrine secretion (37, 67, 68). Fasting also increases the activity of the NPY/AgRP-containing arcuate neurons that control feeding (4, 5, 7, 12, 51). Although the sympathetic nervous system does not control food intake per se, plasma epinephrine levels also rise sufficiently to contribute to lipolysis during food deprivation (69, 70); thus, the AgRP-expressing hypothalamic and adrenal neuroendocrine cells are likely to be coactivated during the counterregulatory response to fasting.
Here we report that activation of MC3/4 receptors (perhaps by α-MSH from the pituitary) (71, 72) leads to an increase in synaptic strength that is blocked by AgRP. We have recently shown that fasting also leads to an NPY-dependent strengthening of the preganglionic → chromaffin cell synapse (28). Because AgRP and NPY are cotransmitters in a subset of chromaffin cells, these results suggest that one of the roles of AgRP is to activate a negative feedback loop that limits the fasting-induced synaptic strengthening and epinephrine release. Finally, transgenic AgRP mouse lines have been widely used in studies investigating the role of hypothalamic AgRP/NPY neurons in metabolism (3, 73, 74). Our observation that chromaffin cells can also synthesize this peptide is a reminder that an autonomic contribution to a transgenic phenotype is worth considering.
Acknowledgments
We thank Ms. Constance Porretta (Louisiana State University Health Sciences Center, Comprehensive Alcohol Research Center, Analytical Core Laboratory) for help with fluorescence-activated cell sorting and Dr. June Liu for critically reading the manuscript.
Acknowledgments
This work was supported by National Institutes of Health Grants DK080441 and DK098134 (to M.D.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- BSA
- bovine serum albumin
- DMEM
- Dulbecco’s modified Eagle medium
- EPSC
- excitatory postsynaptic current
- FCS
- fetal calf serum
- GFP
- green fluorescent protein
- IgG
- immunoglobulin G
- ir
- immunoreactive
- mRNA
- messenger RNA
- NPY
- neuropeptide Y
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PPR
- paired pulse ratio
- PNMT
- phenylethanolamine N-methyltransferase
- RRID
- research resource identifier
- RT-PCR
- reverse transcription polymerase chain reaction
- SEM
- standard error of the mean
- TH
- tyrosine hydroxylase.
References
- 1.Watts AG, Donovan CM. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front Neuroendocrinol. 2010;31(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter S, Li A-J, Wang Q, Dinh TT. Minireview: the value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit. Endocrinology. 2011;152(11):4019–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77(5):810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121(4):1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146(3):1043–1047. [DOI] [PubMed] [Google Scholar]
- 8.Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci. 2011;31(33):11825–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146(6):992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–449. [DOI] [PubMed] [Google Scholar]
- 11.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7(5):493–494. [DOI] [PubMed] [Google Scholar]
- 12.Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1(4):271–272. [DOI] [PubMed] [Google Scholar]
- 13.Shutter JR, Graham M, Kinsey AC, Scully S, Lüthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev. 1997;11(5):593–602. [DOI] [PubMed] [Google Scholar]
- 14.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–138. [DOI] [PubMed] [Google Scholar]
- 15.Cryer PE. Glucose counterregulation: prevention and correction of hypoglycemia in humans. Am J Physiol. 1993;264(2 Pt 1):E149–E155. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DG. Organismal carbohydrate and lipid homeostasis. Cold Spring Harb Perspect Biol. 2012;4(5):a006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bicknell AB, Lomthaisong K, Gladwell RT, Lowry PJ. Agouti related protein in the rat adrenal cortex: implications for novel autocrine mechanisms modulating the actions of pro-opiomelanocortin peptides. J Neuroendocrinol. 2000;12(10):977–982. [DOI] [PubMed] [Google Scholar]
- 18.Doghman M, Delagrange P, Blondet A, Berthelon M-C, Durand P, Naville D, Bégeot M. Agouti-related protein antagonizes glucocorticoid production induced through melanocortin 4 receptor activation in bovine adrenal cells: a possible autocrine control. Endocrinology. 2004;145(2):541–547. [DOI] [PubMed] [Google Scholar]
- 19.Doghman M, Delagrange P, Berthelon M-C, Durand P, Naville D, Bégeot M. Sustained inhibitory effect of Agouti related protein on the ACTH-induced cortisol production by bovine cultured adrenal cells. Regul Pept. 2005;124(1-3):215–219. [DOI] [PubMed] [Google Scholar]
- 20.Dhillo WS, Small CJ, Gardiner JV, Bewick GA, Whitworth EJ, Jethwa PH, Seal LJ, Ghatei MA, Hinson JP, Bloom SR. Agouti-related protein has an inhibitory paracrine role in the rat adrenal gland. Biochem Biophys Res Commun. 2003;301(1):102–107. [DOI] [PubMed] [Google Scholar]
- 21.Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW. Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab. 2005;2(6):421–427. [DOI] [PubMed] [Google Scholar]
- 22.Coupland RE. Electron microscopic observations on the structure of the rat adrenal medulla: II. Normal innervation. J Anat. 1965;99(Pt 2):255–272. [PMC free article] [PubMed] [Google Scholar]
- 23.Engström L, Rosén K, Angel A, Fyrberg A, Mackerlova L, Konsman JP, Engblom D, Blomqvist A. Systemic immune challenge activates an intrinsically regulated local inflammatory circuit in the adrenal gland. Endocrinology. 2008;149(4):1436–1450. [DOI] [PubMed] [Google Scholar]
- 24.Xing Y, Lerario AM, Rainey W, Hammer GD. Development of adrenal cortex zonation. Endocrinol Metab Clin North Am. 2015;44(2):243–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu Y-WA, Garcia AJ III, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Pol AN, Yao Y, Fu L-Y, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29(14):4622–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27(37):9817–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Wang Q, Whim MD. Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia. Proc Natl Acad Sci USA. 2016;113(21):E3029–E3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Wang M, Whim MD. Neuropeptide Y gates a stress-induced, long-lasting plasticity in the sympathetic nervous system. J Neurosci. 2013;33(31):12705–12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramamoorthy P, Wang Q, Whim MD. Cell type-dependent trafficking of neuropeptide Y-containing dense core granules in CNS neurons. J Neurosci. 2011;31(41):14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whim MD. Near simultaneous release of classical and peptide cotransmitters from chromaffin cells. J Neurosci. 2006;26(24):6637–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Whim MD. Stress-induced changes in adrenal neuropeptide Y expression are regulated by a negative feedback loop. J Neurochem. 2013;125(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, Pfeifer K. Targeted insertion of the Cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: a new model for studying the developmental distribution of adrenergic cells. Dev Dyn. 2004;231(4):849–858. [DOI] [PubMed] [Google Scholar]
- 34.Hershkovitz L, Beuschlein F, Klammer S, Krup M, Weinstein Y. Adrenal 20alpha-hydroxysteroid dehydrogenase in the mouse catabolizes progesterone and 11-deoxycorticosterone and is restricted to the X-zone. Endocrinology. 2007;148(3):976–988. [DOI] [PubMed] [Google Scholar]
- 35.Paul A, Laufer E. Endogenous biotin as a marker of adrenocortical cells with steroidogenic potential. Mol Cell Endocrinol. 2011;336(1-2):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol. 2008;294(5):R1445–R1452. [DOI] [PubMed] [Google Scholar]
- 37.Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247(1 Pt 1):E35–E40. [DOI] [PubMed] [Google Scholar]
- 38.Chu CA, Sindelar DK, Neal DW, Allen EJ, Donahue EP, Cherrington AD. Comparison of the direct and indirect effects of epinephrine on hepatic glucose production. J Clin Invest. 1997;99(5):1044–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KLJ, Cone RD. Melanocortin-3 receptor regulates the normal fasting response. Proc Natl Acad Sci USA. 2012;109(23):E1489–E1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanamatsu T, Unsworth CD, Diliberto EJ Jr, Viveros OH, Hong JS. Reflex splanchnic nerve stimulation increases levels of proenkephalin A mRNA and proenkephalin A-related peptides in the rat adrenal medulla. Proc Natl Acad Sci USA. 1986;83(23):9245–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han S, Chen X, Wu YM, Naes L, Westfall T. Elevated neuropeptide Y gene expression and release during hypoglycemic stress. Peptides. 1997;18(9):1335–1340. [DOI] [PubMed] [Google Scholar]
- 42.Li JY, Finniss S, Yang YK, Zeng Q, Qu SY, Barsh G, Dickinson C, Gantz I. Agouti-related protein-like immunoreactivity: characterization of release from hypothalamic tissue and presence in serum. Endocrinology. 2000;141(6):1942–1950. [DOI] [PubMed] [Google Scholar]
- 43.Dhillo WS, Gardiner JV, Castle L, Bewick GA, Smith KL, Meeran K, Todd JF, Ghatei MA, Bloom SR. Agouti related protein (AgRP) is upregulated in Cushing’s syndrome. Exp Clin Endocrinol Diabetes. 2005;113(10):602–606. [DOI] [PubMed] [Google Scholar]
- 44.Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, Denton JS, Cone RD. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520(7545):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valenta DC, Downing JE, Role LW. Peptide modulation of ACh receptor desensitization controls neurotransmitter release from chicken sympathetic neurons. J Neurophysiol. 1993;69(3):928–942. [DOI] [PubMed] [Google Scholar]
- 46.Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophys J. 1991;60(5):1288–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9(11):813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76(1):98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221(4613):877–879. [DOI] [PubMed] [Google Scholar]
- 50.Acuna-Goycolea C, Tamamaki N, Yanagawa Y, Obata K, van den Pol AN. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J Neurosci. 2005;25(32):7406–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krashes MJ, Shah BP, Koda S, Lowell BB. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell Metab. 2013;18(4):588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, Herzog H, Sperk G. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 2010;30(18):6282–6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pernow J, Kahan T, Lundberg JM. Neuropeptide Y and reserpine-resistant vasoconstriction evoked by sympathetic nerve stimulation in the dog skeletal muscle. Br J Pharmacol. 1988;94(3):952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA. 1998;95(25):15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeda Y, Kumagai H, Skach A, Sato M, Yanagisawa M. Modulation of circadian glucocorticoid oscillation via adrenal opioid-CXCR7 signaling alters emotional behavior. Cell. 2013;155(6):1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scopsi L, Gullo M, Rilke F, Martin S, Steiner DF. Proprotein convertases (PC1/PC3 and PC2) in normal and neoplastic human tissues: their use as markers of neuroendocrine differentiation. J Clin Endocrinol Metab. 1995;80(1):294–301. [DOI] [PubMed] [Google Scholar]
- 57.Creemers JWM, Pritchard LE, Gyte A, Le Rouzic P, Meulemans S, Wardlaw SL, Zhu X, Steiner DF, Davies N, Armstrong D, Lawrence CB, Luckman SM, Schmitz CA, Davies RA, Brennand JC, White A. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)-83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology. 2006;147(4):1621–1631. [DOI] [PubMed] [Google Scholar]
- 58.Coupland RE, Selby JE. The blood supply of the mammalian adrenal medulla: a comparative study. J Anat. 1976;122(Pt 3):539–551. [PMC free article] [PubMed] [Google Scholar]
- 59.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457(3):213–235. [DOI] [PubMed] [Google Scholar]
- 60.Kesse WK, Parker TL, Coupland RE. The innervation of the adrenal gland. I. The source of pre- and postganglionic nerve fibres to the rat adrenal gland. J Anat. 1988;157:33–41. [PMC free article] [PubMed] [Google Scholar]
- 61.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13(2):195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sohn J-W, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152(3):612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148(11):5339–5347. [DOI] [PubMed] [Google Scholar]
- 64.Morgan DA, McDaniel LN, Yin T, Khan M, Jiang J, Acevedo MR, Walsh SA, Ponto LLB, Norris AW, Lutter M, Rahmouni K, Cui H. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes. 2015;64(6):1976–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu L-Y, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28(21):5433–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodrigues AR, Almeida H, Gouveia AM. Intracellular signaling mechanisms of the melanocortin receptors: current state of the art. Cell Mol Life Sci. 2015;72(7):1331–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vollmer RR, Balcita JJ, Sved AF, Edwards DJ. Adrenal epinephrine and norepinephrine release to hypoglycemia measured by microdialysis in conscious rats. Am J Physiol. 1997;273(5 Pt 2):R1758–R1763. [DOI] [PubMed] [Google Scholar]
- 68.Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1763–R1775. [DOI] [PubMed] [Google Scholar]
- 69.Galster AD, Clutter WE, Cryer PE, Collins JA, Bier DM. Epinephrine plasma thresholds for lipolytic effects in man: measurements of fatty acid transport with [l-13C]palmitic acid. J Clin Invest. 1981;67(6):1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79(1):207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clark AJL. 60 years of POMC: the proopiomelanocortin gene: discovery, deletion and disease. J Mol Endocrinol. 2016;56(4):T27–T37. [DOI] [PubMed] [Google Scholar]
- 72.Enriori PJ, Chen W, Garcia-Rudaz MC, Grayson BE, Evans AE, Comstock SM, Gebhardt U, Müller HL, Reinehr T, Henry BA, Brown RD, Bruce CR, Simonds SE, Litwak SA, McGee SL, Luquet S, Martinez S, Jastroch M, Tschöp MH, Watt MJ, Clarke IJ, Roth CL, Grove KL, Cowley MA. α-Melanocyte stimulating hormone promotes muscle glucose uptake via melanocortin 5 receptors. Mol Metab. 2016;5(10):807–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren H, Orozco IJ, Su Y, Suyama S, Gutiérrez-Juárez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, Accili D. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake [published correction appears in Cell. 2013;153(5):1166]. Cell. 2012;149(6):1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruan H-B, Dietrich MO, Liu Z-W, Zimmer MR, Li M-D, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, Yang X. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell. 2014;159(2):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]