Abstract
Obese women, on average, give birth to babies with high fat mass. Placental lipid metabolism alters fetal lipid delivery, potentially moderating neonatal adiposity, yet how it is affected by maternal obesity is poorly understood. We hypothesized that fatty acid (FA) accumulation (esterification) is higher and FA β-oxidation (FAO) is lower in placentas from obese, compared with lean women. We assessed acylcarnitine profiles (lipid oxidation intermediates) in mother–baby–placenta triads, in addition to lipid content, and messenger RNA (mRNA)/protein expression of key regulators of FA metabolism pathways in placentas of lean and obese women with normal glucose tolerance recruited at scheduled term Cesarean delivery. In isolated trophoblasts, we measured [3H]-palmitate metabolism. Placentas of obese women had 17.5% (95% confidence interval: 6.1, 28.7%) more lipid than placentas of lean women, and higher mRNA and protein expression of FA esterification regulators (e.g., peroxisome proliferator-activated receptor γ, acetyl-CoA carboxylase, steroyl-CoA desaturase 1, and diacylglycerol O-acyltransferase-1). [3H]-palmitate esterification rates were increased in trophoblasts from obese compared with lean women. Placentas of obese women had fewer mitochondria and a lower concentration of acylcarnitines, suggesting a decrease in mitochondrial FAO capacity. Conversely, peroxisomal FAO was greater in placentas of obese women. Altogether, these changes in placental lipid metabolism may serve to limit the amount of maternal lipid transferred to the fetus, restraining excess fetal adiposity in this population of glucose-tolerant women.
Maternal obesity alters placental lipid metabolism pathways by increasing lipid storage and impairing mitochondrial function. These adaptations potentially modulate fetal adiposity.
Sixty percent of women who become pregnant in the United States are overweight or obese [body mass index (BMI) >25 kg/m2] (1). Offspring of obese women have a greater risk of developing obesity and cardiovascular disease in later life (2, 3). Impaired placental function of obese women may mediate these poor outcomes by altering the supply of key nutrients, such as long-chain fatty acids (FA) to the fetus (4, 5). Placentas of obese women are characterized by lipid accumulation, inflammation, and oxidative stress (6, 7). The driver of this lipotoxic environment, high placenta lipid content, is not explained by enhanced uptake, as long-chain FA uptake is generally decreased in placentas of obese women (8, 9). However, changes in placental FA metabolism, such as decreased FA β-oxidation (FAO) and/or increased esterification, determine the fate of intracellular FA, ultimately affecting fetal FA delivery, and altering growth, fat accretion, and development (5, 10).
Our group and others have reported reduced mitochondrial function and/or number in placentas of obese, compared with lean women (11–13). As mitochondria are the major site of FAO (14), these findings suggest that placental FAO may be impaired in obese women. Long-chain FA (>14 carbons) must be esterified to carnitine prior to transport across the inner mitochondrial membrane for subsequent β-oxidation. Activity of mitochondrial enzymes [e.g., carnitine palmitoyltransferase (CPT)1] that synthesize these acylcarnitines (ACs) regulates β-oxidation rates (15, 16). Ryckman et al. (17) recently reported that obese women have higher plasma long-chain AC concentrations compared with lean women during pregnancy; however, a larger study by Hellmuth et al. (18) found no association between maternal obesity and AC concentrations. Placental AC content, reflecting local FAO capacity/activity, was not assessed in either study. Under conditions of lipid oversupply (e.g., obesity), peroxisomes enhance cellular FAO capacity by shortening long-chain FA, allowing them to bypass CPT1 and enter mitochondria for complete oxidation (19). Peroxisomal proliferation is stimulated by high FA concentrations (20), suggesting that, in the setting of obesity and hyperlipidemia, these organelles may play an important role in cellular metabolism, although their placental activity in obese women is unknown.
To test the hypothesis that placental FAO capacity is impaired and FA esterification is enhanced in obese women, we assessed the following: (1) AC profiles, lipid content, and expression of key components of FA esterification and oxidation pathways in placentas of lean and obese women, and (2) [3H]-palmitate oxidation and esterification in trophoblasts isolated from placentas of lean and obese women.
Materials and Methods
Study design
We performed a cross-sectional study of healthy women recruited at 38 to 40 weeks of pregnancy who delivered by scheduled cesarean section at MetroHealth Medical Center (Cleveland, OH). Women were grouped by prepregnancy BMI (lean: <25 kg/m2, n = 40 and obese >30 kg/m2, n = 40). Group size was based upon our previous study (10) of placental metabolism in fish oil–supplemented patients at MetroHealth Medical Center. Subjects with fetal anomalies, multiple gestations, pre-eclampsia, diabetes (pre-existing and gestational), or other comorbid disease were excluded. Placental tissue was collected at the time of delivery from the maternal face of the placenta avoiding calcified or underperfused cotyledons. Several full-depth samples were collected randomly across the surface of the placenta from multiple cotyledons. Chorionic membranes and maternal decidua layers were removed, and large villous samples were further dissected into small pieces that were blotted for removal of blood and separately snap frozen in liquid nitrogen within 5 minutes of biopsy. Maternal and cord blood were collected and processed, as previously described (11). Blood glucose, insulin, and free FA concentrations were measured, and homeostasis model assessment–estimated insulin resistance (HOMA-IR) was calculated, as described previously (11). Neonatal anthropometrics (birth weight, length, skin folds) were collected within 48 hours of birth. Neonatal lean body mass, fat mass, and percentage of fat were calculated from skin fold data, as previously described (21). The study was conducted according to the guidelines in the Declaration of Helsinki. Written and informed consent was obtained prior to participation, and the study was approved by the Institutional Review Board of MetroHealth Medical Center/Case Western Reserve University (IRB 1300650).
Placental lipid analysis
Total lipids were extracted from 80 to 100 mg frozen placental tissue with chloroform:methanol [2:1 volume-to-volume ratio (v/v)], as previously described (10, 22), and normalized to tissue weight. Data were expressed as total extractable lipids/g tissue.
Separation of phospholipids (PL) and neutral lipid species was performed on thin-layer chromatography (TLC) plates (TLC-precoated silica gel 60 F254 plates; Millipore, Billerica, MA). TLC plates were prewashed by an ascending development up to 1 cm from the top in a clean tank containing a mixture of chloroform:methanol (1:1, v/v). Plates were air dried in a fume hood for 30 minutes to remove any material interfering with further quantitative and qualitative analyses. Before use, plates were completely wetted with 2.3% boric acid, drained for 5 minutes in a fume hood, and dried at l00°C for 15 minutes. A total of 2 µl each sample and 1 µl standards in chloroform was spotted 1 cm from the edge of the plate in 1-cm bands. Plates were developed in a stepwise fashion in chambers saturated with the following: 1) chloroform-methanol-water 60:30:5 (v/v/v) up to the middle of the plate; and 2) hexane-diethyl ether-acetic acid 80:20:1.5 (v/v) up to 1 cm from the top. After separation, lipids were charred by spraying the plate with phosphomolybdic acid solution (Sigma-Aldrich, St. Louis, MO), thoroughly air dried in a fume hood, and immediately heated at 200°C for 2 to 4 minutes. An image of the plate was acquired with ChemiDoc-It TS2 810 imager (UVP, Upland, CA). Lipid spots were quantified using UVP VisionWorksLS software. Total of lipid fractions per sample was considered 100%, and each lipid fraction was calculated based on amount of lipid applied and normalized to grams of starting tissue. Standards for each of the lipid classes were applied to every plate [cholesteryl oleate, triolein, oleic acid, free cholesterol, and 1,2 distearolyl, 18-5a, Nu-chek Prep, Elysian, MN; phosphatidylethanolamine (PE), phosphatidylcholine (PC), lysophosphatidylcholine, all from egg yolk; phosphatidylinositol from soybean, and cardiolipin from bovine heart, Sigma-Aldrich].
Immunohistochemical staining for peripilin 2
The placenta samples from both lean and obese patients that had been flash frozen in liquid nitrogen and stored at −80°C were used in this study. The samples were cryosectioned and mounted on Superfrost Plus Slides (Fisher Scientific, Waltham, MA) and, after air drying at room temperature, were placed in ice-cold 4% paraformaldehyde/0.1 M phosphate buffer (Electron Microscopy Sciences, Hatfield, PA) for 30 minutes. After rinsing in phosphate-buffered saline (PBS), the slides were blocked for nonspecific staining with 10 mM phosphate buffer containing 1% bovine serum albumin (BSA; Jackson ImmunoResearch, West Grove, PA), 0.3% Triton X-100, and 5% normal donkey serum (Jackson ImmunoResearch) for 30 minutes. Then the slides were incubated overnight at 4°C in a humidified chamber with the primary antibody solution, made up of PBS with 1% BSA, 0.3% Triton X-100, and 1:200 anti-peripilin 2 [LS-B4850, Research Resource Identifier (RRID): AB_10801440; LSBio, Seattle, WA) and 1:50 cytokeratin 7 (Dako, Santa Clara, CA; M7018, RRID: AB_2134589). After rinsing in PBS, the slides were incubated at room temperature with 1:500 donkey anti-rabbit AlexaFluor488 (Jackson ImmunoResearch; catalog 711-545-152, RRID: AB_2313584) and 1:500 donkey anti-mouse AlexaFluor594 (Jackson ImmunoResearch; catalog 715-585-151, RRID: AB_2340854) in PBS, 0.3% Triton X-100, and 5% normal donkey serum for 90 minutes. Following rinsing in PBS, the slides were coverslipped with Diamond Prolong (Invitrogen, Carlsbad, CA). The images were acquired with Leica Microsystem SPE Confocal Microscope.
Placental gene expression analysis by quantitative polymerase chain reaction
Total RNA was obtained following homogenization of ∼50 mg placental tissue in TRIzol reagent (Invitrogen) following the manufacturer’s guidelines. RNA integrity was assessed for each sample by visualizing ribosomal RNA via gel electrophoresis. Reverse transcription of 1 μg RNA to complementary DNA was performed using MultiScribe reverse transcription with random primers following manufacturer’s guidelines and cycling conditions (high-capacity complementary DNA reverse-transcription kit; Applied Biosystems, Carlsbad, CA). Gene expression was monitored by real-time polymerase chain reaction (PCR) using a Roche thermal cycler (Roche Applied Science, Indianapolis, IN) with Lightcycler Fast-Start DNA Sybr Green 1 master mix (Roche). Gene-specific primers were designed to analyze the expression of genes involved in FA accumulation/esterification: monoacyglycerol acyltransferase 1 and 2, FA synthase, acetyl-CoA carboxylase (ACC), peroxisome proliferator-activated receptor (PPAR) γ, steroyl-CoA desaturase (SCD)1, diacylglycerol O-acyltransferase-1 (DGAT1), and sterol regulatory element-binding transcription factor 1; FA oxidation (mitochondrial): CPT1b, PPARα, acyl-coenzyme A dehydrogenase, very long chain, carnitine/AC translocase (CACT), carnitine O-palmitoyltransferase 2, and organic cation/carnitine transporter 2; FA oxidation (peroxisomal): peroxisomal biogenesis factor 3, D-bifunctional protein (DBP), and peroxisomal carnitine O-octanoyltransferase (COT). Placental mitochondrial biogenesis and number were assessed by messenger RNA (mRNA) expression of PPARγ coactivator 1α (PGC1-α, a transcriptional coactivator required for mitochondrial biogenesis) for the quantitative determination of mitochondrial DNA (mtDNA) content of cytochrome B (CytB) relative to nuclear (β-actin) DNA, as described previously (11).
Primer sequences are shown in Supplemental Table 1 (33.3KB, docx) . The cycling conditions for the real-time PCR were the same for all primer pairs: 1) 95°C for 10 minutes; 2) 40 cycles of 95°C 20 seconds, 55°C for 30 seconds, and 72°C for 30 seconds; and 3) final elongation at 72°C for 10 minutes. For each primer pair, a standard curve including no template control and unknowns was run in triplicate. The melt curve of the resulting amplicon was analyzed to ensure that a single product was detected for each replicate, and data were analyzed using Roche LightCycler 480 software (v 1.5.1), as described previously (10). L19 was used as a reference gene because no association between maternal obesity and L19 expression within the placenta was observed. Values were expressed as a ratio of the gene of interest:reference in each sample.
For determination of mtDNA number, genomic DNA was isolated from frozen placental tissue (20 mg) using a Qiagen DNeasy kit. Amplifications were performed in duplicate using 200 ng genomic DNA. Standard curves for mitochondrial CytB and nuclear β-actin were generated using female genomic DNA. For the determination of mtDNA content relative to nuclear DNA, mitochondrial CytB in each sample was normalized against nuclear β-actin.
Western blotting
To further confirm the association between maternal obesity and regulators of lipid metabolism, Western blotting was performed in a subset of samples (n = 7 lean and n = 7 obese). Briefly, 20 μg total protein was separated on a 10% sodium dodecyl sulfate–polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. After blocking the membrane with 5% milk PBS-T buffer, it was incubated with primary antibody rabbit anti-PPARγ (1:2000, catalog sc-7196, RRID: AB_654710; Santa Cruz, Santa Cruz, CA) or anti-PPARα (1:2000, catalog sc-9000, RRID: AB_2165737; Santa Cruz) for 1 hour at room temperature or anti-SCD1 (1:1000, catalog 2438, RRID: AB_823634; Cell Signaling Technology, Danvers, MA) or anti-DGAT1 (1:500, catalog 54037, RRID: AB_869453; Abcam, Cambridge, MA) or anti-CPT1b (1:500, catalog 134988, RRID: AB_2650476; Abcam) overnight. After washing with PBS-T, the membrane was exposed to the anti-rabbit secondary antibody (1:2000 to 1:7000, catalog sc-2004, RRID: AB_631746; Cell Signaling) for 1 hour at room temperature. Protein expression of CPT1b was also measured in isolated mitochondrial extracts under similar conditions. β-actin and voltage-dependent anion channel were the housekeeping proteins used to normalize the data for placenta and mitochondria, respectively. Values were expressed as the ratio between protein of interest and housekeeping protein expression. Image was detected using enhanced chemiluminescence (Amersham, Pittsburg, PA) and edited using NIH ImageJ software (v 1.48).
Carnitine and AC analysis
Carnitine and AC (lipid oxidation intermediates) were quantified in plasma and tissue in mother–baby–placenta triads by mass spectrometry, as described previously (23, 24), in a subset of nine lean (pregravid BMI = 21.8 ± 3.1 kg/m2) and nine obese (39.6 ± 7.2 kg/m2) women.
Isolation of human trophoblast cells
To determine the effect of maternal obesity on lipid metabolism activity at the cellular level in placental tissue, human trophoblast cells were freshly isolated from n = 12 lean and n = 13 obese women with a singleton pregnancy recruited at term (38 to 40 weeks) prior to an elective cesarean section. This separate study was approved by the Institutional Review Board of MetroHealth Medical Center, Case Western Reserve University (IRB 1300650), and written informed consent was obtained prior to collecting placental tissue. Maternal and cord blood and neonatal anthropometrics were collected, as described above. Trophoblasts were isolated by sequential trypsin and DNase digestion, followed by gradient centrifugation, as described previously (10, 25). Cells were seeded into six-well plates at a density of 3 × 106 cells/well and cultured overnight in Iscove’s modified Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin and maintained at 37°C under 5% CO2. Each assay was performed in triplicate.
FA esterification into total lipids in trophoblast cells
FA esterification in isolated placental trophoblast cells was determined, as described previously (10). Briefly, trophoblast cells were incubated in culture medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in the presence of 1.25% BSA, 0.1 mmol/L unlabeled palmitate, and 18,500 Bq/mL [3H]-palmitate (100 μM) for 18 hours at 37°C under 5% CO2. At the end of the treatment period, trophoblast cells were washed with ice-cold PBS and homogenized in 200 μL high-performance liquid chromatography–grade acetone. Following lipid extraction, radioactivity in a 100 μL aliquot, representing esterified palmitate, was counted on a Beckman LS3801 liquid scintillation counter (Beckman Coulter, Brea, CA). An additional aliquot was used to determine total proteins using the bicinchoninic acid method (Sigma-Aldrich). Esterification was calculated as nmol palmitate/mg protein/h. The [9,10-[3H]]-almitic acid was from Movarek Biochemicals (Brea, CA), and FA-free BSA was from Sigma-Aldrich.
FA oxidation assay in human trophoblast cells
FAO assays were performed in vitro in isolated placental trophoblast cells, as described previously, with some modifications (10). After 18 hours incubation with [3H]-palmitate, as described above, the medium was collected, and tritiated water ([3H]2O), representing the oxidized palmitate, was determined by the phase equilibration method (26). Data were calculated as nmol palmitate/mg protein/h.
Placentas from five lean and four obese women were used to assess mitochondrial vs peroxisomal FAO capacity because both organelles play an important role in FAO. Placental trophoblast cells were incubated in the conditions described for esterification and FAO in the absence or presence of 200 µM Etomoxir (a specific and irreversible inhibitor of CPT1), therefore limiting the entry of FA to the mitochondria for oxidation. FA esterification and oxidation were assessed, as described above.
Statistics
All data in tables and text are presented as means ± standard deviation, and in figures as mean ± standard error of the mean, unless noted otherwise. Results of the mRNA and protein quantification were expressed in arbitrary units and normalized (by natural log transformation) before analysis. Differences between groups were analyzed using the nonparametric Mann-Whitney U test. Correlations between lipid content, mRNA expression, and carnitine concentration were assessed using Spearman’s correlation. For multivariate regression analyses, all data were first tested for normality via Shapiro–Wilk’s test and log transformed if necessary. Etomoxir data were analyzed via one-sample t test. Statistical analysis was performed using GraphPad Prism (version 6; La Jolla, CA). P values <0.05 were considered statistically significant.
Results
Demographic data of the cohort are summarized in Table 1. There were no significant differences in parity, maternal, or gestational age at delivery. HOMA-IR, glucose, and insulin concentrations in maternal blood were significantly higher in the obese than in the lean group (P < 0.05). Neonatal birth weight, fat, lean body mass, and placental weights were not significantly different between groups in this cohort of women. As groups differed by maternal race and gestational weight gain, which may impact fetal growth, we used multiple linear regression to adjust for these and other relevant factors. Following adjustment for these potential confounding variables (maternal age, parity, race, gestational weight gain, and fetal sex), placental weight, birth weight, and fat-free mass were significantly higher in obese vs lean women (Table 1). HOMA-IR in cord blood was significantly higher in the offspring of obese compared with lean women (P < 0.05). Neither cord nor maternal free FA was different between groups. We did not detect any gender effect on any of these outcomes (see Supplemental Table 2 (33.3KB, docx) for breakdown of groups by fetal sex).
Table 1.
Maternal and Neonatal Characteristics of Study Population
| Lean (n = 40) | Obese (n = 40) | Maternal Adiposity Effect (P Value)a | Adjusted Maternal Adiposity Effect (P Value)b | |
|---|---|---|---|---|
| Maternal | ||||
| Maternal age (y) | 28 ± 6 | 28 ± 6 | 0.918 | |
| Race (AA/Cauc/Hisp) | 7/28/5 | 18/20/2 | 0.024c | |
| Parity (previous live births) | 1.5 | 1.5 | 0.359 | |
| Prepregnancy BMI (kg/m2) | 21.9 ± 2.2 | 39.0 ± 6.8 | <0.0001 | |
| Gestational weight gain (kg) | 18.5 ± 7.1 | 13.2 ± 9.2 | 0.002 | |
| Net weight gain (kg) | 13.9 ± 6.3 | 9.1 ± 8.9 | 0.002 | |
| Glucose (mg/dL) | 73.1 ± 9.6 | 79.8 ± 9.2 | 0.001 | 0.004 |
| Insulin (μU/mL) | 13.3 ± 6.5 | 24.1 ± 9.1 | <0.0001 | <0.0001 |
| HOMA-IR | 2.6 ± 1.5 | 4.5 ± 2.2 | <0.0001 | <0.0001 |
| Free fatty acids (mEq/L) | 0.84 ± 0.34 | 0.78 ± 0.26 | 0.388 | 0.319 |
| Placental weight (g) | 647 ± 192 | 682 ± 207 | 0.419 | 0.013 |
| Neonatal | ||||
| Gestational age at delivery (wk) | 38.9 ± 0.5 | 38.7 ± 0.6 | 0.147 | 0.365 |
| Sex (M/F) | 20/20 | 20/20 | 1 | |
| Birth weight (kg) | 3.28 ± 0.62 | 3.37 ± 0.65 | 0.462 | 0.016 |
| Length (cm) | 48.9 ± 1.9 | 49.3 ± 1.7 | 0.282 | 0.126 |
| Fat mass (kg) | 0.43 ± 0.23 | 0.46 ± 0.28 | 0.736 | 0.104 |
| Fat-free mass (kg) | 2.85 ± 0.41 | 2.91 ± 0.39 | 0.462 | 0.011 |
| % Body fat | 12.2 ± 4.5 | 12.8 ± 5.8 | 0.624 | 0.207 |
| Cord glucose (mg/dL) | 63.8 ± 15.3 | 68.5 ± 12.2 | 0.06 | 0.254 |
| Cord insulin (μU/mL) | 7.1 ± 3.7 | 8.9 ± 5.4 | 0.201 | 0.082 |
| HOMA-IR | 1.1 ± 0.7 | 1.5 ± 0.9 | 0.034 | 0.036 |
| Cord-free fatty acids (mEq/L) | 0.27 ± 0.11 | 0.24 ± 0.14 | 0.181 | 0.283 |
Data are mean ± standard deviation.
Abbreviations: AA/Cauc/Hisp, African American, Caucasian, Hispanic.
P value calculated by nonparametric Mann-Whitney U test, unless otherwise noted.
P value calculated after adjustment for maternal age, parity, race, gestational weight gain, and fetal sex.
P value calculated via χ2 test.
Maternal obesity is associated with increased placental lipid content
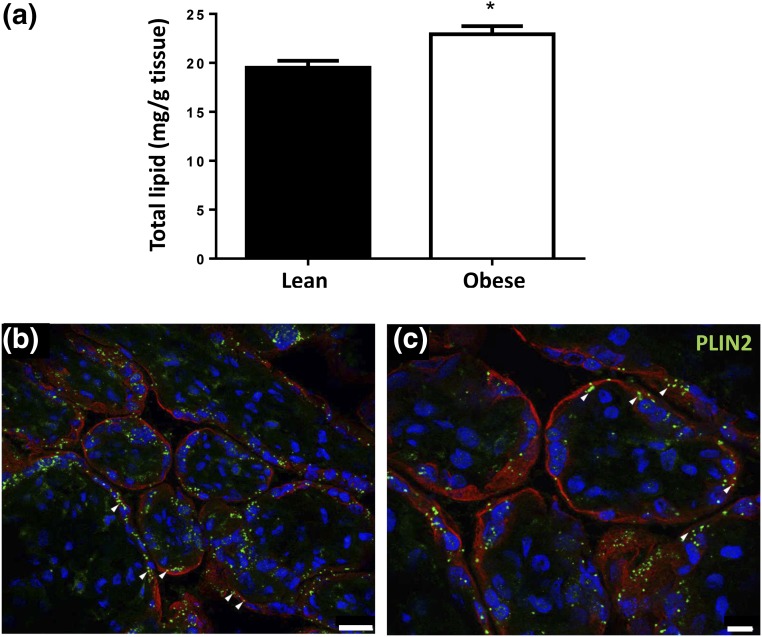
To determine the effect of maternal obesity on placental lipid content, total lipids were extracted from placentas of lean (n = 34) and obese (n = 39) women. The placentas from obese women had 17% more total lipid than placentas from lean women (22.9 ± 5.2 vs 19.5 ± 4.1 mg lipid/g tissue; P = 0.002) [Fig. 1(a)]. Adjustment for maternal age, parity, gestational weight gain, race, or fetal sex did not affect this association. Total placental lipid content was weakly correlated with neonatal fat mass (B = −0.66 [95% confidence interval (CI): −1.31, −0.003], P = 0.049; adjusted R2 = 0.19) and percentage of body fat [B= −0.50 (95% CI: −0.98, −0.02), P = 0.043; adjusted R2 = 0.16] after adjustment for the above variables and maternal obesity. Placental lipid content was not associated with birth weight or fat-free mass. Adjusted R2 values of the above models were calculated with and without inclusion of placental lipid content, the difference in the R2 representing the percentage of neonatal adiposity explained by placental lipid content. Placental lipid content explained 8% of both neonatal fat mass and percent body fat.
Figure 1.
Placental lipid content in lean and obese women: (a) Quantification of total lipid in placentas of lean (n = 34) and obese (n = 39) women. Total lipid was extracted using the Folch method and normalized to tissue weight. Data are means ± standard error of the mean. *P < 0.05 compared with lean by Mann-Whitney U test. (b and c) Representative images from n = 4 obese placental sections stained for peripilin 2 (PLIN2), a lipid-droplet membrane component (white arrows), and cytokeratin 7, a marker of trophoblasts. Scale bars represent (b) 25 µm and (c) 10 µm.
TLC was used to separate placental total lipid into PL and neutral lipid components (Supplemental Fig. 1 (15.3MB, tiff) ). Placentas from obese women had 20% more esterified lipids (cholesterol esters, triglycerides, PE, and PC) than placentas of lean women (Table 2). Free FA content was not statistically different between groups. Lipid droplets, visualized via perilipin 2 [a component of lipid droplet membranes (27)] staining, were localized largely to the syncytiotrophoblast layer of the term placenta [Fig. 1b)].
Table 2.
Placental Lipid Profile
| µg Lipid/g Tissue | Lean (n = 32) | Obese (n = 37) | Maternal Adiposity Effect (P Value) |
|---|---|---|---|
| Free cholesterol | 55.9 ± 13.7 | 71.0 ± 20.1 | 0.0004 |
| Cholesterol esters | 44.4 ± 14.2 | 57.0 ± 15.5 | 0.0005 |
| Free fatty acid | 36.1 ± 8.8 | 40.7 ± 12.4 | 0.157 |
| Triglyceride | 39.3 ± 10.5 | 46.1 ± 11.2 | 0.002 |
| Cardiolipin | 32.8 ± 7.6 | 32.6 ± 13.0 | 0.473 |
| Phosphatidylethanolamine | 45.6 ± 10.1 | 53.0 ± 10.5 | 0.017 |
| Phosphatidylinositol | 36.0 ± 7.5 | 38.6 ± 11.0 | 0.424 |
| Phosphatidylcholine | 41.9 ± 8.8 | 49.6 ± 12.3 | 0.008 |
Data are mean ± standard deviation. P value calculated via Mann-Whitney U test.
Maternal obesity is associated with increased placental lipid esterification gene and protein expression
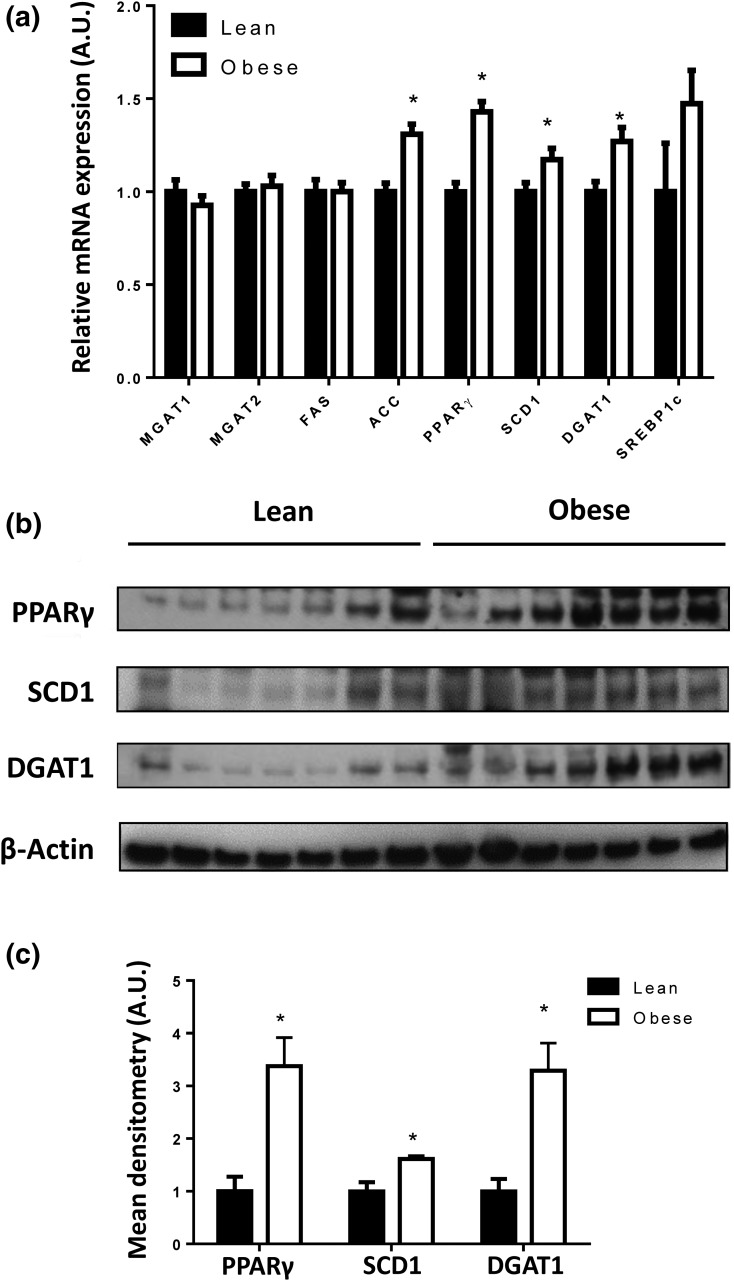
To gain further insight into the molecular mechanisms underlying lipid accumulation in placentas of obese women, we measured the expression of placental genes and proteins involved in FA esterification. As shown in Fig. 2(a), placental mRNA expression of ACC, PPARγ, SCD1, and DGAT1 was significantly higher in placentas from obese women compared with lean women (P < 0.05), and gene expression was positively correlated with placental lipid content (ACC: Spearman r = 0.240, 95% CI: 0.02, 0.452, P = 0.042; PPARγ: r = 0.256, 95% CI: 0.020, 0.464, P = 0.029). Gene expression remained statistically different between lean and obese groups following adjustment for maternal age, race, gestational weight gain, parity, and fetal sex, except for SCD1 (P = 0.056). In addition, placental PPARγ, SCD1, and DGAT1 protein concentrations were higher in obese women [Fig. 2(b) and 2(c)]. We did not detect any gender differences in any of these outcomes (Supplemental Table 3 (33.3KB, docx) ).
Figure 2.
Effect of maternal obesity on placental FA esterification pathway: (a) mRNA expression of placental genes involved in FA esterification from 40 lean and 40 obese placentas. Data (means ± standard error of the mean) are expressed as the ratio of gene of interest:reference gene (L19). (b) Representative Western blot of PPARγ, SCD1, and DGAT1 in the placentas of seven lean and seven obese women. (c) Densitometry analysis. After normalization to β-actin, the mean density of lean protein samples was assigned an arbitrary value of 1. Subsequently, obese density values were expressed relative to this mean. *P < 0.05 compared with lean by Student t test.
Effect of maternal obesity on placental FA oxidation gene and protein expression
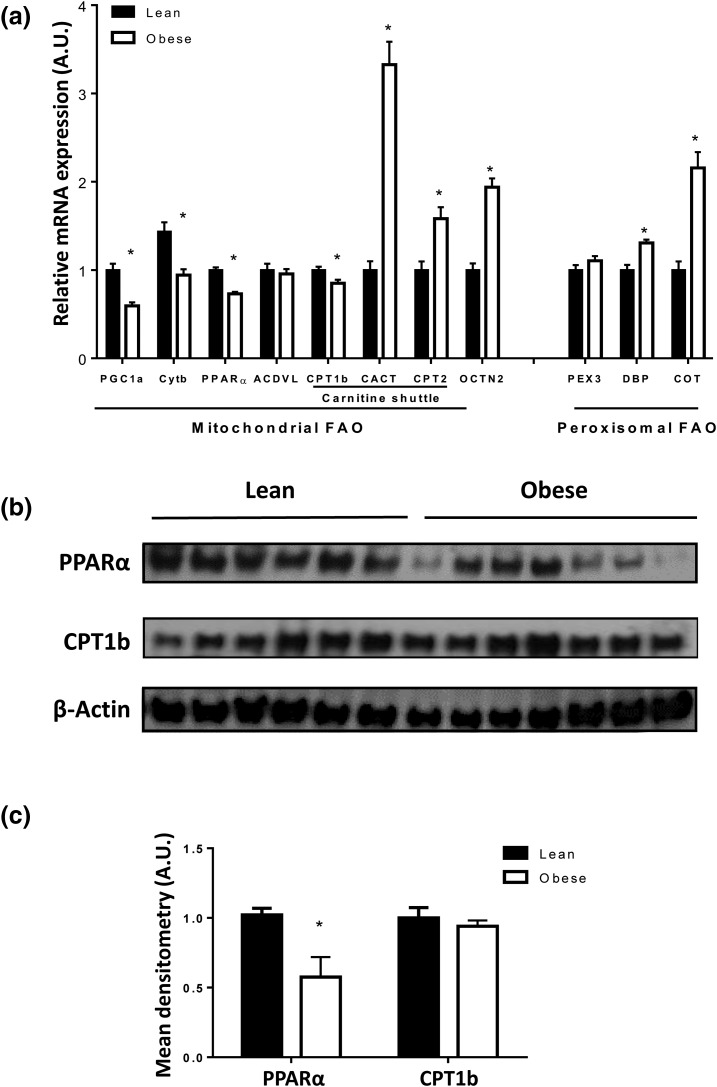
Because 95% of FAO occurs in the mitochondria (14), we estimated placental mitochondrial biogenesis and number by measuring PGC1-α [a master regulator of mitochondrial biogenesis (28)] mRNA expression, and mtDNA relative to nuclear DNA (CytB/β-actin) (11), respectively. Based on these markers, maternal obesity was associated with reduced placental mitochondrial biogenesis and number [P < 0.05, Fig. 3(a)]. In addition, placental mRNA expression of PPARα, and its target gene CPT1b, was significantly lower in obese compared with lean women [Fig. 3(a)]. Placental PPARα protein levels were also reduced in obese mothers [Fig. 3(b) and 3(c)]. CPT1b protein levels were not significantly affected by maternal obesity in whole tissue [Fig. 3(b)] or isolated mitochondria (data not shown). Placental mRNA expression of PPARα was negatively correlated with placental lipid content (r = −0.256, 95% CI: −0.464, −0.020, P = 0.029).
Figure 3.
Effect of maternal obesity on placental FA oxidation pathway: (a) mRNA expression of placental genes involved in FA oxidation from 40 lean and 40 obese placentas. Data (means ± standard error of the mean) are expressed as the ratio of gene of interest:reference gene (L19). Mitochondrial number was indicated by the ratio of CytB/β-actin DNA. (b) Representative Western blot of PPARα and CPT1b in the placentas of six lean and seven obese women. (c) Densitometry analysis. After normalization to β-actin, the mean density of lean protein samples was assigned an arbitrary value of 1. Subsequently, obese density values were expressed relative to this mean. *P < 0.05 compared with lean by Student t test.
Placental and umbilical venous plasma AC are lower in obese women
Total carnitine content [AC + free carnitine (FC)] and short- and medium-chain AC were decreased in placentas of obese women (P < 0.05) (Table 3). The placental AC/FC ratio was significantly higher in obese women (1.43 ± 0.22 vs 1.14 ± 0.21, P = 0.009), suggesting a decreased mitochondrial capacity for energy production (15). Consistent with its role in AC formation, placental CPT1 expression was positively correlated with total placental AC content (r = 0.52, 95% CI: 0.060, 0.801, P = 0.03). Interestingly, neonatal body fat percentage was negatively correlated with placental linoleoylcarnitine (r = −0.52, 95% CI: −0.799, −0.054, P = 0.027), the AC of the long-chain essential FA, linoleate (18:2, n-6), suggesting that fetal fat accrual may be inhibited by placental FAO (for individual placental AC data, see Supplemental Table 4 (33.3KB, docx) ).
Table 3.
Carnitine Profile in Mother–Baby–Placenta Triads
|
Maternal Plasma (μmol/L) |
Cord Plasma (μmol/L) |
Placenta (nmol/mg tissue) |
||||
|---|---|---|---|---|---|---|
| Lean | Obese | Lean | Obese | Lean | Obese | |
| Total carnitine | 14.8 ± 2.1 | 13.5 ± 2.0 | 21.7 ± 6.0 | 16.6 ± 3.5a | 242 ± 47 | 183 ± 45a |
| Free carnitine | 13.7 ± 2.1 | 12.5 ± 2.3 | 19.1 ± 5.8 | 15.3 ± 3.4 | 114 ± 28 | 76 ± 19a |
| Acylcarnitines: | ||||||
| Total short chain (<C8) | 0.92 ± 0.90 | 0.73 ± 0.80 | 1.97 ± 1.65 | 0.81 ± 0.28 | 129 ± 19 | 106 ± 28a |
| Total medium chain (C10–C14) | 0.24 ± 0.12 | 0.27 ± 0.24 | 0.14 ± 0.05 | 0.10 ± 0.03a | 4.2 ± 0.9 | 3.1 ± 0.7a |
| Total long chain (>C16) | 0.13 ± 0.06 | 0.15 ± 0.09 | 0.17 ± 0.04 | 0.13 ± 0.03 | 19.3 ± 8.8 | 15.3 ± 4.3 |
| Total acylcarnitine | 1.41 ± 0.98 | 1.30 ± 0.86 | 2.46 ± 1.74 | 1.18 ± 0.28a | 156 ± 25 | 128 ± 30a |
| Acylcarnitine/Free carnitine | 0.08 ± 0.07 | 0.09 ± 0.10 | 0.16 ± 0.12 | 0.09 ± 0.03 | 1.14 ± 0.21 | 1.44 ± 0.21a |
Data are expressed as mean ± standard deviation. N = 9 lean, N = 9 obese women.
P < 0.05 by Mann-Whitney U test.
Umbilical cord plasma total carnitine and medium-chain AC were significantly reduced in offspring of obese women (P < 0.05). Umbilical cord carnitine content may reflect spillover from the placenta, as rates of fetal FAO are very low (29, 30). We were unable to detect differences in maternal plasma FC or AC content between lean and obese women.
The placental mRNA expression of CACT and CPT2 (members of the carnitine shuttle involved in transport of AC and FC across the inner mitochondrial membrane), and organic cation/carnitine transporter 2 (the plasma membrane carnitine transporter), was significantly higher in placentas from obese vs lean women [Fig. 3(a)].
Effect of maternal obesity on placental lipid esterification and FAO in vitro
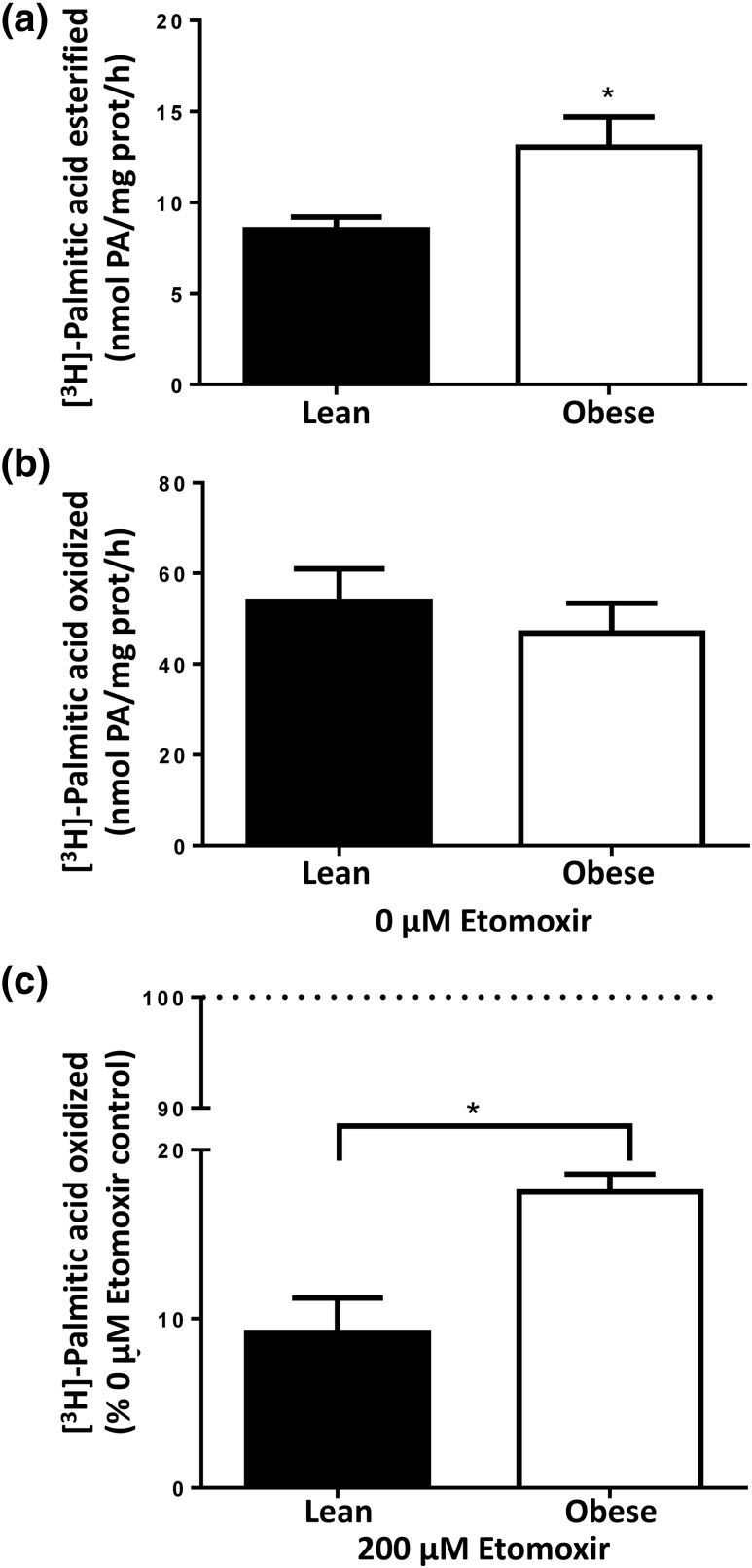
To determine the effect of the aforementioned molecular changes on cellular metabolism, we measured [3H]-palmitate oxidation and esterification rates in freshly isolated trophoblast cells (the most metabolically active cells in the placenta) from a separate cohort of healthy lean (n = 14) and obese (n = 15) women. Maternal and neonatal metabolic characteristics (Table 4) were similar to women included in the larger cohort. As shown in Fig. 4(a), esterification of [3H]-palmitate into total lipids was 53% higher in trophoblast cells isolated from obese women compared with lean women (13.0 ± 6.4 vs 8.5 ± 2.6 nmol/mg protein/h, P = 0.04). This association was not affected by adjustment for maternal age, parity, race, gestational weight gain, or fetal sex. Rate of [3H]-palmitate esterification in trophoblast cells was negatively correlated with the neonatal ponderal index (r = −0.500, 95% CI: −0.749, −0.128, P = 0.009), but no other neonatal anthropometric measures. This correlation was no longer significant (P = 0.18) after adjustment for the above confounders. Oxidation of [3H]-palmitate in isolated trophoblast cells was not significantly affected by maternal obesity [Fig. 4(b)].
Table 4.
Maternal and Neonatal Characteristics of Cohort Recruited for InVitro Metabolism Study
| Lean (n = 14) | Obese (n = 15) | Maternal Adiposity Effect (P Value)a | Adjusted Maternal Adiposity Effect (P Value)b | |
|---|---|---|---|---|
| Maternal | ||||
| Maternal age (y) | 27 ± 6 | 27 ± 4 | 0.804 | |
| Race (AA/Cauc/Hisp) | 4/9/1 | 10/5/0 | 0.096c | |
| Parity (previous live births) | 1.4 ± 1.2 | 1.7 ± 0.7 | 0.097 | |
| Prepregnancy BMI (kg/m2) | 21.6 ± 2 | 37.6 ± 7 | <0.0001 | |
| Gestational weight gain (kg) | 16.6 ± 5.6 | 11.7 ± 6.4 | 0.026 | |
| Placenta weight (g) | 687 ± 111 | 647 ± 137 | 0.426 | 0.716 |
| Neonatal | ||||
| Gestational age at delivery (wk) | 38.9 ± 0.7 | 38.9 ± 0.3 | 0.721 | 0.599 |
| Sex (M/F) | 6/8 | 4/11 | 0.359c | |
| Birth weight (kg) | 3.31 ± 0.40 | 3.32 ± 0.35 | 0.837 | 0.018 |
| Length (cm) | 49.5 ± 2.9 | 50.6 ± 1.8 | 0.326 | 0.058 |
| Fat mass (kg) | 0.45 ± 0.11 | 0.40 ± 0.15 | 0.327 | 0.129 |
| Fat-free mass (kg) | 2.94 ± 0.26 | 2.92 ± 0.22 | 0.962 | 0.025 |
| % Body fat | 13.0 ± 2.6 | 11.8 ± 3.4 | 0.287 | 0.522 |
Data are mean ± standard deviation.
Abbreviations: AA/Cauc/Hisp, African American, Caucasian, Hispanic.
P value calculated by nonparametric Mann-Whitney U test, unless otherwise noted.
P value calculated after adjustment for maternal age, parity, race, gestational weight gain, and fetal sex.
P value calculated via χ2 test.
Figure 4.
FA esterification and oxidation assay in trophoplasts isolated from lean (n = 14) and obese (n = 15) women. (a) [3H]-palmitate esterification was significantly higher in isolated trophoblast cells from obese women. (b) [3H]-palmitate oxidation was not significantly affected by maternal obesity. Data are calculated as nmol palmitate/mg protein/h and expressed as means ± standard error of the mean. (c) Etomoxir-mediated inhibition of mitochondrial FA oxidation. Data were calculated as a percentage relative to untreated placental trophoblast cells (0 µM etomoxir) for each independent experiment in triplicate and are expressed as means ± standard error of the mean. *P < 0.05 compared with untreated control by Mann-Whitney U test.
Long-chain FA, such as palmitate, require conversion to their AC via CPT1 to enter mitochondria for FAO. Alternatively, they may be partially oxidized in nearby peroxisomes, the newly shortened FA, then able to bypass CPT1 and enter the mitochondria for complete oxidation (31). We used etomoxir (a specific and irreversible inhibitor of CPT1) (32) to measure the rate of [3H]-palmitate oxidation in the absence of the typical mitochondrial pathway—representing the peroxisomal contribution to FAO (33). CPT1 inhibition had a weaker effect on cellular FAO rates in obese compared with lean women (82.9 ± 3.3% vs 92.2 ± 1.2% inhibition, P < 0.05), suggesting that extramitochondrial (peroxisomal) FAO capacity is greater in trophoblasts of obese women [Fig. 4(c)]. Consistent with enhanced peroxisomal activity, placental mRNA expression of DBP and COT (key enzymes regulating peroxisomal β-oxidation of FA) was significantly increased in obese compared with lean women [P < 0.05, Fig. 3(a)].
Discussion
Placental lipid metabolism is critical for placental function and positive pregnancy outcomes and affects fetal growth (4, 34). This study uses multiple molecular approaches (i.e., labeled lipid tracers, chromatography, quantitative PCR, immunohistochemistry, Western blotting) to characterize lipid metabolism in placentas of healthy lean and obese women. Our key finding is that maternal obesity is associated with an increase in lipid esterification and storage, and a decrease in mitochondrial FAO, which is compensated for by an upregulation of peroxisomal FAO. Altogether, these changes may serve to limit the amount of maternal lipid transferred to the fetus.
Consistent with previous reports (6, 7), lipid content was higher in placentas from obese as compared with lean women. Our data suggest this is secondary to an upregulation in expression of key proteins in the lipid esterification pathway (e.g., PPARγ, DGAT1, SCD1), driving FA taken up by the trophoblast toward esterification and storage pathways. Supporting this, [3H]-palmitate esterification occurred at a higher rate in trophoblasts isolated from obese women, in a second independent cohort. Similarly, [3H]-palmitate esterification was increased in triglyceride-rich placental explants of nonobese women with diabetes (35), suggesting that both diabetes and obesity stimulate placental lipid storage pathways. Our findings indicate that the excess lipids esterified increased both the neutral lipid fraction (triglycerides, cholesterol esters) and PL concentrations (PE, PC) in placentas of obese women, which may affect membrane fluidity and nutrient transport (5, 36).
The causal mechanism driving the upregulation of the esterification pathway in placentas of obese women is not known. PPARγ, a lipid-activated transcription factor, regulates the expression of lipid esterification and storage genes in several tissues (37–39) and was upregulated in placentas of obese women. Placental FA concentrations may be elevated in obese women due to either increased maternal supply, secondary to maternal insulin resistance and increased lipolysis (40), or lower placental FA oxidation. We speculate that elevated FA concentrations within the placenta activate PPARγ. In turn, PPARγ stimulates the transcription of DGAT1, SCD1, and ACC, key genes involved in lipid esterification and storage (37, 41, 42). Further studies are necessary to confirm this hypothesis in placental tissue, although others have described such a mechanism in adipose and hepatic tissues in mice and nonpregnant subjects (38, 39). Interestingly, in a mouse model, rosiglitazone-induced placental PPARγ activation resulted in higher placental FA uptake, but lower transfer to the fetus, due to increased lipid storage in the placenta (38). Indeed, based on our findings that higher placental lipid content correlated with lower neonatal fat mass and percent body fat, we speculate that higher placenta lipid storage partially protects the fetus from excess maternal lipid supply in obese pregnancies. In our model, 8% of variation in neonatal adiposity could be explained by placental lipid content. These results support the idea that the mother buffers the effects of the ex utero environment on the developing fetus (43, 44). Impairments in this feto-protective mechanism (e.g., inhibition of esterification pathways) may expose the fetus to excess maternal lipids, potentially explaining our recent finding that fish oil supplementation in obese women, which lowered placental lipid content, was associated with higher, not lower, neonatal fat mass (10).
In addition to influencing tissue FA content and their availability for esterification and storage, FA oxidation within the placenta provides ATP for critical placental functions such as hormone synthesis, which controls maternal adaptations to pregnancy and parturition (11, 45–47). We found that expression of CPT1, which catalyzes the rate-limiting step in mitochondrial FAO (35, 48), and PPARα, a positive regulator of CPT1 transcription (49), was lower in placentas of obese women, in addition to markers of mitochondrial biogenesis (PGC1-α) and number (CytB). These molecular indicators of placental FAO capacity were further validated by the quantity of the FAO intermediates, AC. The strong positive correlation between CPT1 mRNA expression and total AC concentration in the placenta demonstrates the importance of CPT1 for AC synthesis (50). Although we did not detect a decrease in protein expression, the reduction in placental AC content in obese women suggests that CPT1 activity was impaired. Upregulation of genes involved in the mitochondrial carnitine shuttle (e.g., CACT, CPT2) may indicate a compensatory response to the low AC concentration, attempting to boost mitochondrial AC uptake to maintain FAO.
To assess the outcome of these molecular changes on FAO activity, we measured the oxidation of [3H]-palmitate in trophoblasts isolated from an independent cohort of lean and obese women. We were unable to detect significant changes in the rate of palmitate oxidation between groups. Although mitochondria are responsible for 95% of cellular FAO, peroxisomes can also oxidize lipids (51). Peroxisomal participation in FAO may be enhanced in the setting of a high-fat diet or obesity in some tissues (20, 52). We used etomoxir, a specific and irreversible inhibitor of CPT1, to block mitochondrial long-chain FAO in isolated trophoblasts, and assessed the rate of palmitate oxidation. Etomoxir inhibited palmitate oxidation 92% in trophoblasts from lean women, but only 83% in trophoblasts from obese women. This indicates that the extramitochondrial contribution (peroxisomal) to FAO in placentas of obese women is ∼10% greater and may compensate for the reduced mitochondrial number or function, thereby maintaining overall placental lipid oxidation capacity (depicted in Fig. 5). Consistent with this, peroxisomal FAO enzymes [e.g., COT, DBP (19, 53)] were more highly expressed in placentas of obese women. Although an increase in peroxisomal function can maintain crucial FAO capacity, the byproduct of peroxisomal FAO is hydrogen peroxide (H2O2), an oxidant that may damage the mitochondria, particularly if cellular antioxidant production is inadequate, as has been reported in placentas of obese women (6, 12), thus leading to further cellular damage.
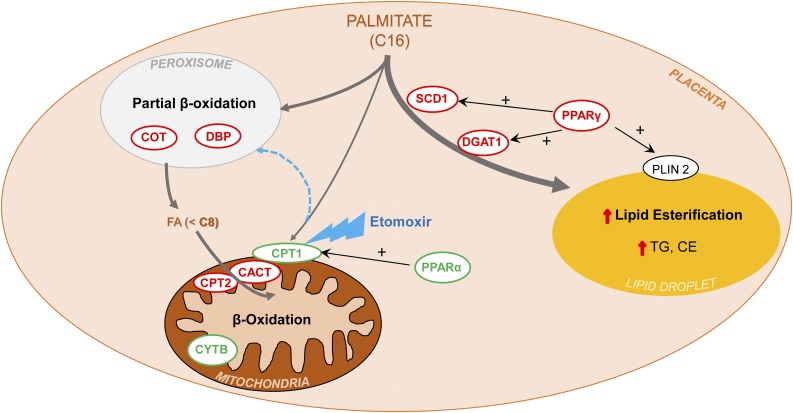
Figure 5.
Schematic summarizing findings and proposed mechanism of placental lipid metabolism in obese women. Long-chain FA (e.g., palmitate, C16:0), once taken up by the trophoblast, preferentially enter the esterification pathway, due to the upregulation (red) of several key components under the transcriptional control of PPARγ: SCD1 catalyzes the desaturation of palmitate to its monounsaturated counterpart, palmitoleate (C16:1), one of the most abundant FA in PL, triglycerides, and cholesterol esters; DGAT1 is the final committed step in triglycerides synthesis. As a result, rates of palmitate esterification are higher in placentas of obese women, as are content of PL, triglycerides, and cholesterol esters. Low expression (green) of PPARα, a transcriptional activator of CPT1 (a key enzyme necessary for palmitate entry into mitochondria), and PGC1-α (involved in mitochondrial biosynthesis), results in fewer mitochondria (evidenced by low CytB). Lower mitochondrial content drives palmitate to peroxisomes, whose β-oxidation machinery (DBP, COT) is upregulated, shortening the excess palmitate, thus allowing the smaller FA (<8 carbons) to bypass CPT1 and enter the mitochondria via diffusion, or the upregulated components of the carnitine shuttle (CACT, CPT2), thus maintaining overall β-oxidation capacity.
Our study has several strengths, such as the use of mRNA, protein, and in vitro activity assays to assess metabolic pathways, in two independent cohorts. However, our conclusions are limited by the fact that we are only studying the placenta at term. Further prospective studies are necessary to determine the ontogeny of alterations in placental lipid metabolism in pregnancies complicated by obesity and diabetes.
Figure 5, a depiction of how FA metabolism pathways are altered in placentas of obese women, summarizes our findings. We found that extramitochondrial (peroxisomal) FAO was enhanced in placentas of obese women, compensating for impaired mitochondrial function. Additionally, placenta lipid esterification and storage were greater. These adaptations of placental lipid metabolism pathways in obese women may limit excess fetal fat delivery, although further work on placenta FA flux throughout gestation among these women is necessary.
Acknowledgments
We thank Dr. Prem Shekhawat for helpful critiques of the manuscript. We are grateful for the support of the participating mothers, the clinical staff of the Department of Obstetrics and Gynecology, and staff of the Clinical Research Unit at MetroHealth Medical Center.
Acknowledgments
This work was supported by Grant R00HD062841 from the National Institute of Child Health and Development and MetroHealth Clinical Research Unit/Clinical & Translational Science Collaborative (UL1 RR024989).
Author contributions: V.C.-N. conducted the molecular analyses and in vitro studies, analyzed the data, and drafted the manuscript. M.H. performed the placental TLC assays. J.M. recruited volunteers and isolated trophoblast cells. P.G. conducted the immunohistochemistry assays. G.C.R. performed PCR analysis. C.H. conducted the carnitine profile assay. S.H.d.-M. and P.C. initiated the original database, provided samples, and helped design the study. P.O.-G. conceived and designed the study aims and drafted the manuscript. All authors read and approved the final manuscript.
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibodies Used
| Peptide/Protein Target | Name of Antibody | Species Raised in; Monoclonal or Polyclonal | Manufacturer and (Catalog No.) | Research Resource Identifier | Dilution Used |
|---|---|---|---|---|---|
| Plin 2 | Anti-Plin2 | Rabbit; polyclonal | LSBio (LS-B4850) | AB_10801440 | 1:200 |
| Cytokeratin 7 | Anti-cytokeratin 7 | Mouse; monoclonal | Dako (M7018) | AB_2134589 | 1:50 |
| PPARγ | Anti-PPARγ | Rabbit; polyclonal | Santa Cruz (sc-7196) | AB_654710 | 1:2000 |
| PPARα | Anti-PPARα | Rabbit; polyclonal | Santa Cruz (sc-9000) | AB_2165737 | 1:2000 |
| SCD1 | Anti-SCD1 | Rabbit; polyclonal | Cell Signaling (2438) | AB_823634 | 1:1000 |
| DGAT1 | Anti-DGAT1 | Rabbit; polyclonal | Abcam (54037) | AB_869453 | 1:500 |
| CPT1b | Anti-CPT1b | Rabbit; polyclonal | Abcam (134988) | AB_2650476 | 1:500 |
| β-actin | Anti–β-actin | Mouse; monoclonal | Cell Signaling (3700) | AB_ 2242334 | 1:2000 |
Footnotes
- AC
- acylcarnitine
- ACC
- acetyl-CoA carboxylase
- BMI
- body mass index
- BSA
- bovine serum albumin
- CACT
- carnitine/acylcarnitine translocase
- CI
- confidence interval
- COT
- peroxisomal carnitine O-octanoyltransferase
- CPT
- carnitine palmitoyltransferase
- CytB
- cytochrome B
- DBP
- D-bifunctional protein
- DGAT1
- diacylglycerol O-acyltransferase-1
- FA
- fatty acid
- FAO
- FA β-oxidation
- FC
- free carnitine
- HOMA-IR
- homeostasis model assessment-estimated insulin resistance
- mRNA
- messenger RNA
- mtDNA
- mitochondrial DNA
- PBS
- phosphate-buffered saline
- PC
- phosphatidylcholine
- PCR
- polymerase chain reaction
- PE
- phosphatidylethanolamine
- PGC1-α
- peroxisome proliferator-activated receptor γ coactivator 1α
- PL
- phospholipid
- PPAR
- peroxisome proliferator-activated receptor
- RRID
- Research Resource Identifier
- SCD
- steroyl-CoA desaturase
- TLC
- thin-layer chromatography
- v/v
- volume-to-volume ratio.
References
- 1.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003-2009. Prev Med. 2013;56(6):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C. Maternal size in pregnancy and body composition in children. J Clin Endocrinol Metab. 2007;92(10):3904–3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forsén T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother’s weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997;315(7112):837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myatt L, Maloyan A. Obesity and placental function. Semin Reprod Med. 2016;34(1):42–49. [DOI] [PubMed] [Google Scholar]
- 5.Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H, Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschmugl B, Desoye G, Catalano P, Klymiuk I, Scharnagl H, Payr S, Kitzinger E, Schliefsteiner C, Lang U, Wadsack C, Hauguel-de Mouzon S. Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int J Obes. 2017;41(2):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brass E, Hanson E, O’Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34(6):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dube E, Gravel A, Martin C, Desparois G, Moussa I, Ethier-Chiasson M, Forest JC, Giguere Y, Masse A, Lafond J. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod. 2012;87(1):14,1–11. [DOI] [PubMed] [Google Scholar]
- 10.Calabuig-Navarro V, Puchowicz M, Glazebrook P, Haghiac M, Minium J, Catalano P, Hauguel deMouzon S, O’Tierney-Ginn P. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr. 2016;103(4):1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassance L, Haghiac M, Minium J, Catalano P, Hauguel-de Mouzon S. Obesity-induced down-regulation of the mitochondrial translocator protein (TSPO) impairs placental steroid production. J Clin Endocrinol Metab. 2015;100(1):E11–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307(5):E419–E425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hastie R, Lappas M. The effect of pre-existing maternal obesity and diabetes on placental mitochondrial content and electron transport chain activity. Placenta. 2014;35(9):673–683. [DOI] [PubMed] [Google Scholar]
- 14.Mendez-Figueroa H, Chien EK, Ji H, Nesbitt NL, Bharathi SS, Goetzman E. Effects of labor on placental fatty acid β oxidation. J Matern Fetal Neonatal Med 2013;26(2):150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma S, Black SM. Carnitine homeostasis, mitochondrial function and cardiovascular disease. Drug Discov Today Dis Mech. 2009;6(1-4):e31–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. Role of carnitine in disease. Nutr Metab. 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryckman KK, Donovan BM, Fleener DK, Bedell B, Borowski KS. Pregnancy-related changes of amino acid and acylcarnitine concentrations: the impact of obesity. AJP Rep. 2016;6(3):e329–e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmuth C, Lindsay KL, Uhl O, Buss C, Wadhwa PD, Koletzko B, Entringer S. Association of maternal prepregnancy BMI with metabolomic profile across gestation. Int J Obes. 2017;41(1):159–169. [DOI] [PubMed] [Google Scholar]
- 19.Wanders RJA, Waterham HR, Ferdinandusse S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front Cell Dev Biol. 2016;3(83):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith JJ, Aitchison JD. Peroxisomes take shape. Nat Rev Mol Cell Biol. 2013;14(12):803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173(4):1176–1181. [DOI] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 23.Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Validated method for the quantification of free and total carnitine, butyrobetaine, and acylcarnitines in biological samples. Anal Chem. 2015;87(17):8994–9001. [DOI] [PubMed] [Google Scholar]
- 24.Minkler PE, Stoll MS, Ingalls ST, Kerner J, Hoppel CL. Quantitative acylcarnitine determination by UHPLC-MS/MS: going beyond tandem MS acylcarnitine “profiles.” Mol Genet Metab. 2015;116(4):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varastehpour A, Radaelli T, Minium J, Ortega H, Herrera E, Catalano P, Hauguel-de Mouzon S. Activation of phospholipase A2 is associated with generation of placental lipid signals and fetal obesity. J Clin Endocrinol Metab. 2006;91(1):248–255. [DOI] [PubMed] [Google Scholar]
- 26.Hughes SD, Quaade C, Johnson JH, Ferber S, Newgard CB. Transfection of AtT-20ins cells with GLUT-2 but not GLUT-1 confers glucose-stimulated insulin secretion: relationship to glucose metabolism. J Biol Chem. 1993;268(20):15205–15212. [PubMed] [Google Scholar]
- 27.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48(12):2547–2559. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93(4):884S–890S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt-Sommerfeld E, Penn D, Wolf H. The influence of maternal fat metabolism on fetal carnitine levels. Early Hum Dev. 1981;5(3):233–242. [DOI] [PubMed] [Google Scholar]
- 30.Bargen-Lockner C, Hahn P, Wittmann B. Plasma carnitine in pregnancy. Am J Obstet Gynecol. 1981;140(4):412–414. [DOI] [PubMed] [Google Scholar]
- 31.Violante S, Ijlst L, Te Brinke H, Koster J, Tavares de Almeida I, Wanders RJ, Ventura FV, Houten SM. Peroxisomes contribute to the acylcarnitine production when the carnitine shuttle is deficient. Biochim Biophys Acta. 2013;1831(9):1467–1474. [DOI] [PubMed] [Google Scholar]
- 32.Schlaepfer IR, Rider L, Rodrigues LU, Gijón MA, Pac CT, Romero L, Cimic A, Sirintrapun SJ, Glodé LM, Eckel RH, Cramer SD. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol Cancer Ther. 2014;13(10):2361–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaikaus RM, Sui Z, Lysenko N, Wu NY, Ortiz de Montellano PR, Ockner RK, Bass NM. Regulation of pathways of extramitochondrial fatty acid oxidation and liver fatty acid-binding protein by long-chain monocarboxylic fatty acids in hepatocytes: effect of inhibition of carnitine palmitoyltransferase I. J Biol Chem. 1993;268(36):26866–26871. [PubMed] [Google Scholar]
- 34.Desoye G, Shafrir E. Placental metabolism and its regulation in health and diabetes. Mol Aspects Med. 1994;15(6):505–682. [DOI] [PubMed] [Google Scholar]
- 35.Visiedo F, Bugatto F, Sánchez V, Cózar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305(2):E205–E212. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Jain A, Baumann M, Körner M, Surbek D, Bütikofer P, Albrecht C. Increased placental phospholipid levels in pre-eclamptic pregnancies. Int J Mol Sci. 2013;14(2):3487–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab. 2005;90(7):4267–4275. [DOI] [PubMed] [Google Scholar]
- 38.Schaiff WT, Knapp FF Jr, Barak Y, Biron-Shental T, Nelson DM, Sadovsky Y. Ligand-activated peroxisome proliferator activated receptor gamma alters placental morphology and placental fatty acid uptake in mice. Endocrinology. 2007;148(8):3625–3634. [DOI] [PubMed] [Google Scholar]
- 39.Ferré P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53(Suppl 1):S43–S50. [DOI] [PubMed] [Google Scholar]
- 40.Lindsay KL, Hellmuth C, Uhl O, Buss C, Wadhwa PD, Koletzko B, Entringer S. Longitudinal metabolomic profiling of amino acids and lipids across healthy pregnancy. PLoS One. 2015;10(12):e0145794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49(11):2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56(4):952–964. [DOI] [PubMed] [Google Scholar]
- 43.Wells JC. An evolutionary perspective on the trans-generational basis of obesity. Ann Hum Biol. 2011;38(4):400–409. [DOI] [PubMed] [Google Scholar]
- 44.Wells JC. A critical appraisal of the predictive adaptive response hypothesis. Int J Epidemiol. 2012;41(1):229–235. [DOI] [PubMed] [Google Scholar]
- 45.Mouzon SH, Lassance L. Endocrine and metabolic adaptations to pregnancy; impact of obesity. Horm Mol Biol Clin Investig. 2015;24(1):65–72. [DOI] [PubMed] [Google Scholar]
- 46.Oey NA, den Boer ME, Ruiter JP, Wanders RJ, Duran M, Waterham HR, Boer K, van der Post JA, Wijburg FA. High activity of fatty acid oxidation enzymes in human placenta: implications for fetal-maternal disease. J Inherit Metab Dis. 2003;26(4):385–392. [DOI] [PubMed] [Google Scholar]
- 47.Shekhawat P, Bennett MJ, Sadovsky Y, Nelson DM, Rakheja D, Strauss AW. Human placenta metabolizes fatty acids: implications for fetal fatty acid oxidation disorders and maternal liver diseases. Am J Physiol Endocrinol Metab. 2003;284(6):E1098–E1105. [DOI] [PubMed] [Google Scholar]
- 48.Sebastián D, Guitart M, García-Martínez C, Mauvezin C, Orellana-Gavaldà JM, Serra D, Gómez-Foix AM, Hegardt FG, Asins G. Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells. J Lipid Res. 2009;50(9):1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feingold KR, Wang Y, Moser A, Shigenaga JK, Grunfeld C. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J Lipid Res. 2008;49(10):2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schooneman MG, Vaz FM, Houten SM, Soeters MR. Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes. 2013;62(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le Borgne F, Demarquoy J Interaction between peroxisomes and mitochondria in fatty acid metabolism. Open J Mol Integr Physiol. 2012;2:27–33. [Google Scholar]
- 52.Huang T-Y, Zheng D, Muller-Borer B, Collins M, Noland R, Funai K, Hickner R, Cortright R. Peroxisomal biogenesis occurs in response to obesity and to a high lipid environment in human skeletal muscle (abstract 1159.5). FASEB J. 2014; 28(1)(Suppl). [Google Scholar]
- 53.Wanders RJ, Vreken P, Ferdinandusse S, Jansen GA, Waterham HR, van Roermund CW, Van Grunsven EG. Peroxisomal fatty acid alpha- and beta-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem Soc Trans. 2001;29(Pt 2):250–267. [DOI] [PubMed] [Google Scholar]