Abstract
Growth hormone (GH) and insulinlike growth factor 1 (IGF-1) are anabolic hormones that facilitate somatic and skeletal growth and regulate metabolism via endocrine and autocrine/paracrine mechanisms. We hypothesized that excess tissue production of GH would protect skeletal growth and integrity in states of reduction in serum IGF-1 levels. To test our hypothesis, we used bovine GH (bGH) transgenic mice as a model of GH hypersecretion and ablated the liver-derived acid-labile subunit, which stabilizes IGF-1 complexes with IGF–binding protein-3 and -5 in circulation. We used a genetic approach to create bGH/als gene knockout (ALSKO) mice and small interfering RNA (siRNA) gene-silencing approach to reduce als or igf-1 gene expression. We found that in both models, decreased IGF-1 levels in serum were associated with decreased body and skeletal size of the bGH mice. Excess GH produced more robust bones but compromised mechanical properties in male mice. Excess GH production in tissues did not protect from trabecular bone loss in response to reductions in serum IGF-1 (in bGH/ALSKO or bGH mice treated with siRNAs). Reduced serum IGF-1 levels in the bGH mice did not alleviate the hyperinsulinemia and did not resolve liver or kidney pathologies that resulted from GH hypersecretion. We concluded that reduced serum IGF-1 levels decrease somatic and skeletal growth even in states of excess GH.
Reduced serum IGF-1 levels decreased somatic and skeletal growth in both sexes even in states of excess GH.
Growth hormone (GH) and insulinlike growth factor 1 (IGF-1) are anabolic hormones that control somatic and skeletal growth (1). Clinical studies (2, 3) and numerous animal models of the GH/IGF-1 axis (1) have established that excesses in GH/IGF-1 enhance somatic growth, whereas lack of GH/IGF-1 signals retards growth. GH controls liver IGF-1 production and, by inference, serum IGF-1 levels. In serum, IGF-1 forms binary complexes with IGF-binding proteins (IGFBPs) that protect it from proteolytic degradation and regulate its binding to the IGF-1 receptor in tissues (4). Most IGF-1 in serum is found in a high-molecular-weight (∼150 kDa) ternary complex with IGFBP-3 and an acid-labile subunit (ALS), both of which are predominantly produced by the liver under GH regulation (5–7). ALS binds to IGFBP-3 (or IGFBP-5) and plays an important role in stabilizing the ternary complex, thus regulating levels of serum IGF-1. In humans, mutations in ALS lead to marked reductions in serum IGF-1 level, growth retardation, and metabolic abnormalities (8). Ablation of the als gene in the mouse [als gene knockout (ALSKO)] recapitulates the human phenotype (9). ALSKO mice show reduced serum IGF-1 levels, reduced body weight, and skeletal and metabolic abnormalities (10–14).
Previous studies from our laboratory have shown that reductions in serum IGF-1 levels due to ablation of als (ALSKO) (10) or liver-specific IGF-1 gene deletion (LID) (15) resulted in mild reductions in body weight (∼6% to 10%) and body and bone lengths despite 65% and 75% reduced serum IGF-1 levels, respectively. However, skeletal integrity was substantially compromised. Long bones of ALSKO and LID mice were more slender and mechanically inferior than those of controls (10, 15). In contrast, studies of mouse models overexpressing bovine GH (bGH) or human GH reported increases in serum and tissue IGF-1 levels, as well as increases in body weight and skeletal robustness, a structural measure of longitudinal vs. radial bone growth [e.g., total cross-sectional area/bone length (Le)](1).
We hypothesized that excess tissue production of GH/IGF-1 would protect the skeleton from impairments caused by reductions in serum IGF-1 level. We used bGH transgenic mice that express bGH ubiquitously under the metallothionein promoter/enhancer (16) as a model of excess GH. To test our hypothesis, we used a genetic approach in which we crossed bGH mice with ALSKO mice to generate bGH/ALSKO mice and a small interfering RNA (siRNA)–gene-silencing approach whereby hepatic transcription of IGF-1 or ALS was inhibited in the bGH mice. Using both approaches, we created mice with excesses of bGH and IGF-1 in tissues that showed significant reductions in serum IGF-1 levels.
Methods
Ethics statement
All procedures involving mice were reviewed and approved by the Institutional Animal Care and Use Committee of the New York University School of Medicine; the Public Health Service animal welfare assurance identification number is A3435-01. The program is licensed under the United States Department of Agriculture as research facility no. 465.
Mice
Generation of bGH (17) and ALSKO mice (5) was previously described. All mice were in the C57BL/6J (B6) genetic background. Weaned mice were randomly allocated into cages separated according to their sex. Mice were housed two to five animals per cage in a facility with 12-hour light/12-hour dark cycles and free access to food and water. Different analyses were performed in male and female mice at the indicated ages.
Serum parameters
Serum and plasma were collected via orbital bleeding immediately after euthanasia between 8 and 10 am. Serum hormone levels were measured by enzyme-linked immunosorbent assay: IGF-1 (catalog no. 22-IG1MS-E01; ALPCO, Salem, NH), insulin (NC9440604; Mercodia, Winston-Salem, NC), IGFBP-3 levels (catalog no. EMIGFBP3; Thermo Fisher, Waltham, MA), osteocalcin (catalog no. 60-1305; Immutopics, Inc., Athens, OH), and carboxy-terminal collagen crosslink [(CTX) catalog no. MBS703094; MyBioSource, San Diego, CA]. Serum ALS levels were determined by Western immunoblotting; 2.0 μL of serum was separated on 4% to 20% gradient SDS-PAGE (catalog no. NP0335; Life Technologies, New York, NY) and transferred to nitrocellulose membranes (catalog no. 170-4158; Bio-Rad, Hercules, CA). They were then detected using the anti-ALS primary antibody (catalog no. AF1436; R&D, Minneapolis, MN) and secondary antibody (catalog no. HAF109; R&D). Blood glucose levels were measured from the tail vein using an automated glucometer (Elite; Bayer, Mishawaka, IN) at the indicated time points.
Gene expression
Total RNA was extracted from tissues or femurs (cortical bone shells flushed of the bone marrow) using TRIzol (Invitrogen, Carlsbad, CA) or by RNeasy Plus kit (catalog no. 74134; Qiagen, Germantown, MD). RNA samples (1 μg) were reverse-transcribed using a complementary DNA synthesis system (catalog no.18080-051; Invitrogen), and quantitative real-time polymerase chain reaction (RT-PCR) was performed using SYBR Master Mix (catalog no. 4385612; Life Technologies/Applied Biosystems) on a BioRad CFX384TM real-time machine with a C1000 TouchTM Thermal Cycler detection system following the manufacturer’s instructions. Transcript levels were assayed three times in each sample, and the ratio of the fold-change between experimental and control samples was calculated relative to GAPDH or 18S, as indicated. The following primers were used: IGF-1 forward 5′-GGACCAGAGACCCTTTGCGGGG-3′; reverse 5′-GGCTGCTTTTGTAGGCTTCAGTGG-3′, ALS forward 5′-CCTGCAGAATCTCTACCATCT-3′; reverse 5′-CAAACTGAGTGAAGCCAGAC-3′; IGFBP-3 forward 5′-ATTCCAAGTTCCATCCACTC-3′; reverse 5′-AGGAGAAGTTCTGGGTGTCT-3′; GHR forward 5′-CCTCGATTCACCAAGTGT-3′; reverse 5′-ATCCTGGGGTCTTTAAATC-3′; insulin receptor forward 5′-TCTATCTGGATGGTCAGTGTG-3′; reverse 5′- GAGAGTTCCTGCATTTGAAGT -3′; CTSK forward 5′-CAGCAGAGGTGTGTACTATG-3′; reverse 5′-GCGTTGTTCTTATTCCGAGC-3′; GAPDH forward 5′-TTGTGCAGTGCCAGCCTCGTC-3′; reverse 5′-GCGCCCAATACGGCCAAATCC-3′; 18S forward 5′-CGGCTACCACATCCAAGGAA-3′; and reverse 5′-GCTGGAATTACCGCGGCT-3′.
siRNA silencing
siRNAs that target IGF-1 or ALS messenger RNA were administered subcutaneously once a week from 4 to 11 weeks of age. The siRNAs were conjugated to an N-acetylgalactosamine (GalNAc) for optimized delivery as previously described (18). The siRNA duplexes targeting mouse IGF-1 and ALS were designed to maximize predicted efficacy and minimize off-target effects using proprietary algorithms. Potent GalNAc-siRNAs targeting IGF-1 and ALS were selected using in vitro cell-based activity screens with confirmation of activity in wild-type (WT) mice. Reactive siRNA-ALS or siRNA−IGF-1 duplexes that inhibited als or igf-1 gene transcription, respectively, by more than 80% in WT mice were chosen for experiments with the bGH mice. A dose-response study revealed that 3 mg/kg given once a week was sufficient to inhibit gene transcription and show sustained reductions in serum level of ALS or IGF-1 for at least 7 days. Thirty-two WT (C57Bl/6) male mice at 3 weeks of age were randomly assigned to four groups and were injected intraperitoneally with siRNA-ALS (AD-66807.1, 3 mg/kg), SiRNA−IGF-1 (AD-68112.2, 3 mg/kg), siRNA-ALS/IGF-1 (3 mg/kg each), or vehicle [phosphate-buffered saline (PBS)] once a week for 7 to 8 weeks.
Histology
Tissues were fixed in 10% zinc formalin and then processed for paraffin sectioning (5-μm sections) and stained with hematoxylin and eosin. Bones were decalcified prior to paraffin embedding. Sections were stained with cathepsin K antibody (catalog no. ab19027; dilution 1:200). Cathepsin K−positive cells on trabecular bone surface were counted.
Microcomputed tomography
Microcomputed tomography (microCT) was performed according to published guidelines (19). The left femora were scanned using a high-resolution SkyScan microCT system (SkyScan 1172; Kontich, Belgium). Images were acquired using a 10-MP digital detector, 10W of energy (70 kV and 142 μA), and a 0.5-mm aluminum filter with a 9.7-μm image voxel size. A fixed global threshold method was used according to manufacturer’s recommendations and preliminary studies, which showed that mineral variation between groups was not high enough to warrant adaptive thresholds. The cortical region of interest was selected as the 2-mm mid-diaphyseal region directly below the third trochanter, which includes the mid-diaphysis and more proximal cortical regions. The following cortical bone measurements were made: total cross-sectional area (Tt.Ar), cortical bone area (Ct.Ar), marrow area, cortical bone thickness (Cs.Th), polar moment of inertia (MMI), and tissue mineral density (TMD). The trabecular measurements assessed as the 2-mm region at the distal femur metaphysis included the bone volume relative to the total volume, bone mineral density (BMD), trabecular number (Tb.N), trabecular spacing, and trabecular thickness (Tb.Th). Additional whole bone measurements were made, including femoral Le, robustness (Tt.Ar/Le), and relative cortical area (RCA; Ct.Ar/Tt.Ar).
Three-point bending assay
Harvested femurs were stored frozen at −20°C and wrapped in PBS-soaked gauze. At testing time, samples were brought to room temperature in a saline bath. Three-point bending tests to failure were carried out using a BOSE materials testing machine (ElectroForce 3220; MN) according to published guidelines (20). The upper loading span width was 3 mm, and the lower support span width was 6 mm. Femora were positioned in a saline bath posterior side down on the supports to generate bending moments about the medial-lateral axis. Samples were carefully centered on the supports to ensure maximum load at the midpoint of the diaphysis. A preload of 1 N was briefly applied to secure the sample, and then a ramp waveform at a constant displacement rate of 0.10 mm/s was applied until failure. Load-displacement curves were recorded and subsequently analyzed.
Primary osteoclast cultures
Bone marrow was flushed from long bones with 3 mL of minimum essential medium α (αMEM) and washed three times using αMEM. Bone marrow cells in αMEM were supplemented with 10% fetal bovine serum and plated in a 10-mL dish overnight. Nonadherent cells were collected and loaded on Ficoll, and the interphase, including myeloid cells, was collected. Cells were then washed three times with αMEM and seeded in triplicates on a 96-well plate (0.15 × 106 cells/mL) in αMEM supplemented with 10% fetal bovine serum 40 ng/mL macrophage-colony stimulating factor and 60 ng/mL receptor activator of nuclear factor κB ligand. Cultures were followed for 6 days and subsequently stained for tartrate-resistant acid phosphatase using a kit (catalog #387A-1K; Sigma). Multinucleated (at least three nuclei) tartrate-resistant acid phosphatase−positive cells were counted.
Statistical analyses
Data are presented as mean ± standard error of the mean. Differences between groups were tested using two-way analysis of variance/Tukey post hoc test. Significance was accepted at P < 0.05. Scientists were blinded to sample identifications in all experimental procedures.
Results
Body and organ growth of bGH mice in the absence of ALS (a genetic approach)
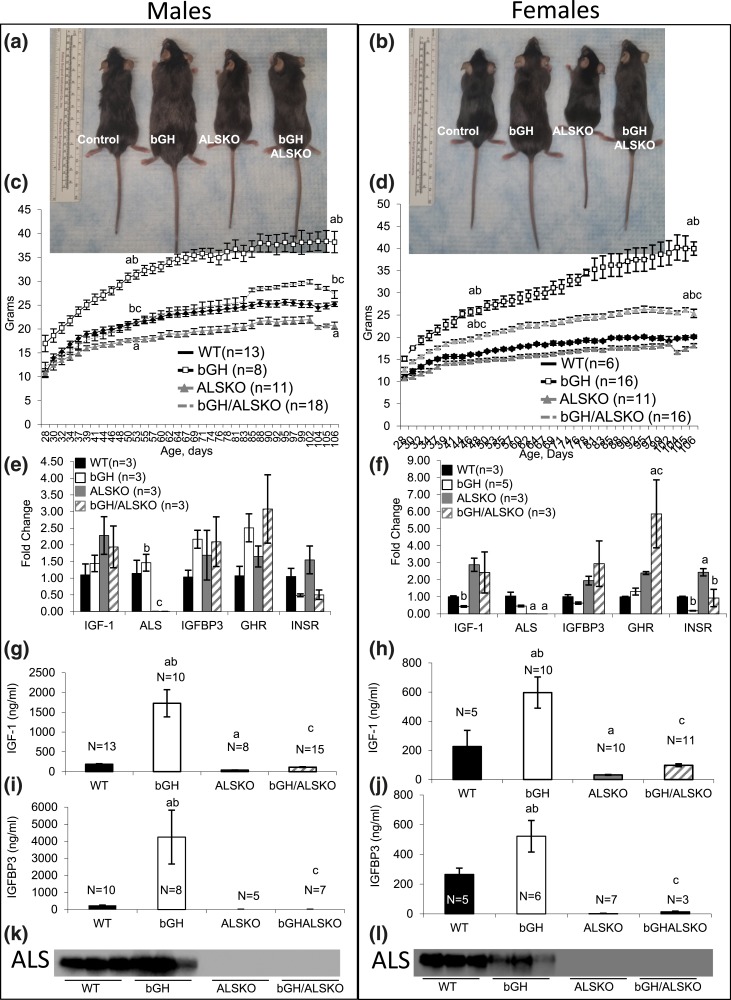
To ablate the ALS in the bGH transgenic mice, we crossed them with the ALS null (ALSKO) mice that were characterized previously (10). We followed four groups of mice: control, bGH, ALSKO, and bGH/ALSKO [Fig. 1(a) and 1(b)] of both sexes. Body weight was recorded three times a week starting at 21 days to ∼112 days of age. As established previously (17), the bGH male and female mice exhibited significant increases in body weight starting at 3 to 4 weeks of age [Fig. 1(c) and 1(d)]. Likewise, the previously described ALSKO mice (10) exhibited reduced body weight in both sexes compared with controls [Fig. 1(c) and 1(d)].
Figure 1.
Genetic ablation of ALS in bGH mice. Pictures of (a) male mice and (b) female mice used in the study. Body weights of (c) male and (d) female mice followed up from 4 to 16 weeks of age. Liver expression of the Igf-1, als, Igfbp3, Ghr, and Insr genes in (e) male and (f) female mice at 16 weeks of age. Serum IGF-1 levels in (g) male and (h) female mice at 16 weeks of age. Serum levels of IGFBP-3 in 16-week-old (i) male and (j) female mice. Western immunoblotting of serum ALS in (k) male and (l) female mice at 16 weeks of age. Data are presented as mean ± standard error of the mean. Significance is accepted at P < 0.05 [(a) = vs. controls; (b) = vs. ALSKO mice; (c) = vs. bGH mice].
Liver expression of als was undetectable in ALSKO or bGH/ALSKO mice, whereas igf-1 gene expression was upregulated in these groups in both sexes [Fig. 1(e) and 1(f)]. Ghr gene expression was not affected by deletion of als in the male bGH/ALSKO mice compared with the bGH mice [Fig. 1(e)], but it was increased in female mice [Fig. 1(f)] compared with bGH mice. In accordance with previous reports (21, 22), we found that insulin receptor gene expression in the liver was reduced in bGH and bGH/ALSKO mice in both sexes [Fig. 1(e) and 1(f)], most likely because of insulin resistance in these mice.
A fivefold increase in serum IGF-1 level was exhibited in male bGH mice [Fig. 1(g)] as well as an approximately twofold increase in female mice [Fig. 1(h)]. Ablation of the als gene normalized serum IGF-1 levels in male and female bGH/ALSKO mice. Likewise, serum IGFBP-3 increased by approximately sixfold in males [Fig. 1(i)] and by ∼2.5-fold in female mice [Fig. 1(j)]. Ablation of als in the bGH/ALSKO mice resulted in almost undetectable levels of serum IGFBP-3. Finally, ALS protein levels in serum were undetectable in the ALSKO and bGH/ALSKO groups in both sexes [Fig. 1(k) and 1(l), representative blots].
The metabolic abnormalities of the bGH mice were extensively studied (23). bGH mice are insulin resistant and show increased serum insulin levels but do not always show hyperglycemia (24). We found hyperinsulinemia in bGH male mice (2.599 ± 0.435 ng/mL vs. 0.831 ± 0.226 ng/mL in controls) and bGH female mice (2.673 ± 0.080 ng/mL vs. 0.474 ± 0.080 ng/mL in controls) that persisted even with ablation of ALS in bGH/ALSKO male mice (4.073 ± 0.703 ng/mL) and female mice (2.452 ± 0.330 ng/mL) (Supplemental Table 1 (155.5KB, doc) ). Fasting glucose levels were slightly elevated in bGH male and female mice, but fed glucose levels did not differ between the groups (Supplemental Table 1 (155.5KB, doc) ).
Organ weights were recorded at 16 weeks of age (Table 1). Despite reductions in body weight of bGH/ALSKO mice compared with bGH mice, relative liver weights were similar in these groups in both sexes. We found that relative weights of the kidney, spleen, and heart increased in bGH/ALSKO mice compared with weights in bGH mice in both sexes. In contrast, relative muscle (quadriceps) weights in bGH/ALSKO mice decreased compared with weights in bGH mice, but only in males.
Table 1.
Body and Organ Weights of bGH Mice With Genetic Ablation of ALS
|
Male Mice Euthanized at 16 Weeks of Age |
Female Mice Euthanized at 16 Weeks of Age |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control (n = 13) | bGH (n = 6) | ALSKO (n = 11) | bGH/ALSKO (n = 18) | Control (n = 6) | bGH (n = 10) | ALSKO (n = 11) | bGH/ALSKO (n = 16) | |
| Body weight | 25.279 ± 0.497 | 40.835 ± 1.499a,b | 20.403 ± 0.546a | 28.728 ± 0.435a,b,c | 19.745 ± 0.253 | 34.492 ± 0.823a,b | 17.706 ± 0.281 | 26.724 ± 0.550a,b,c |
| Body length | 9.723 ± 0.060 | 12.050 ± 0.257a,b | 9.209 ± 0.116a | 10.883 ± 0.077a,b,c | 9.167 ± 0.161 | 11.760 ± 0.121a,b | 9.122 ± 0.036 | 10.738 ± 0.072a,b,c |
| Liver | 1.240 ± 0.037 | 2.938 ± 0.167a,b | 1.103 ± 0.037 | 2.231 ± 0.036a,b,c | 0.943 ± 0.033 | 2.562 ± 0.060a,b | 0.922 ± 0.017 | 1.983 ± 0.083a,b,c |
| Liver, % of BW | 4.898 ± 0.083 | 7.169 ± 0.154a,b | 5.423 ± 0.176a | 7.769 ± 0.071a,b | 4.782 ± 0.184 | 7.032 ± 0.135a,b | 5.207 ± 0.100 | 7.408 ± 0.246a,b |
| Muscle | 0.375 ± 0.011 | 0.437 ± 0.016a,b | 0.285 ± 0.009a | 0.284 ± 0.006a,c | 0.280 ± 0.013 | 0.439 ± 0.015a,b | 0.246 ± 0.005a | 0.275 ± 0.009c |
| Muscle, % of BW | 1.484 ± 0.026 | 1.076 ± 0.053a,b | 1.401 ± 0.032 | 0.990 ± 0.013a,b | 1.416 ± 0.055 | 1.201 ± 0.024a,b | 1.384 ± 0.022 | 1.031 ± 0.028a,b,c |
| Gonadal fat | 0.258 ± 0.025 | 0.637 ± 0.094a,b | 0.245 ± 0.016 | 0.396 ± 0.022a,b,c | 0.145 ± 0.017 | 0.371 ± 0.031a,b | 0.226 ± 0.013 | 0.352 ± 0.028a,b |
| gFat, % of BW | 1.022 ± 0.096 | 1.529 ± 0.188a | 1.197 ± 0.071 | 1.371 ± 0.070 | 0.733 ± 0.081 | 1.009 ± 0.066 | 1.277 ± 0.077a | 1.301 ± 0.088a |
| Sc fat | 0.225 ± 0.017 | 0.422 ± 0.056a,b | 0.155 ± 0.013 | 0.338 ± 0.014a,b,c | 0.195 ± 0.022 | 0.430 ± 0.019a,b | 0.196 ± 0.017 | 0.312 ± 0.018a,b,c |
| Sc fat, % of BW | 0.886 ± 0.060 | 1.017 ± 0.108 | 0.757 ± 0.058 | 1.180 ± 0.050a,b | 0.985 ± 0.106 | 1.178 ± 0.041 | 1.098 ± 0.086 | 1.159 ± 0.055 |
| Brown fat | 0.065 ± 0.004 | 0.117 ± 0.006a,b | 0.052 ± 0.005 | 0.103 ± 0.006a,b | 0.065 ± 0.003 | 0.114 ± 0.008a,b | 0.047 ± 0.004 | 0.111 ± 0.009a,b |
| bFat, % of BW | 0.256 ± 0.017 | 0.288 ± 0.020 | 0.255 ± 0.026 | 0.357 ± 0.016a,b | 0.330 ± 0.019 | 0.313 ± 0.022 | 0.264 ± 0.021 | 0.411 ± 0.031b |
| Kidney | 0.319 ± 0.010 | 0.522 ± 0.018a,b | 0.358 ± 0.020 | 0.437 ± 0.008a,b,c | 0.255 ± 0.006 | 0.456 ± 0.008a,b | 0.256 ± 0.010 | 0.387 ± 0.007a,b,c |
| Kidney, % of BW | 1.263 ± 0.031 | 1.281 ± 0.038b | 1.761 ± 0.093a | 1.523 ± 0.023a,b,c | 1.292 ± 0.030 | 1.254 ± 0.030b | 1.441 ± 0.051a | 1.452 ± 0.026a,c |
| Spleen | 0.071 ± 0.004 | 0.210 ± 0.008a,b | 0.081 ± 0.008 | 0.198 ± 0.006a,b | 0.073 ± 0.007 | 0.185 ± 0.009a,b | 0.102 ± 0.006 | 0.183 ± 0.010a,b |
| Spleen, % of BW | 0.279 ± 0.013 | 0.517 ± 0.022a,b | 0.391 ± 0.030a | 0.692 ± 0.018a,b,c | 0.371 ± 0.036 | 0.509 ± 0.024a | 0.575 ± 0.030a | 0.682 ± 0.033a,c |
| Heart | 0.132 ± 0.006 | 0.218 ± 0.012a,b | 0.128 ± 0.006 | 0.182 ± 0.004a,b,c | 0.112 ± 0.002 | 0.197 ± 0.006a,b | 0.106 ± 0.004 | 0.173 ± 0.006a,b,c |
| Heart, % of BW | 0.521 ± 0.022 | 0.533 ± 0.014b | 0.630 ± 0.025a | 0.632 ± 0.008a,c | 0.566 ± 0.010 | 0.540 ± 0.015 | 0.594 ± 0.019 | 0.646 ± 0.017a,c |
| Lung | 0.132 ± 0.003 | 0.217 ± 0.010a,b | 0.120 ± 0.004 | 0.163 ± 0.003a,b,c | 0.135 ± 0.004 | 0.188 ± 0.008a,b | 0.118 ± 0.004 | 0.171 ± 0.006a,b |
| Lung, % of BW | 0.524 ± 0.012 | 0.535 ± 0.035 | 0.592 ± 0.024 | 0.567 ± 0.008 | 0.684 ± 0.019 | 0.515 ± 0.018a,b | 0.664 ± 0.021 | 0.641 ± 0.024c |
| Pancreas | 0.258 ± 0.010 | 0.577 ± 0.033a,b | 0.205 ± 0.015 | 0.392 ± 0.012a,b,c | 0.177 ± 0.011 | 0.505 ± 0.026a,b | 0.233 ± 0.007 | 0.349 ± 0.015a,b,c |
| Pancreas, % of BW | 1.019 ± 0.034 | 1.409 ± 0.050a,b | 1.005 ± 0.072 | 1.363 ± 0.035a,b | 0.893 ± 0.048 | 1.381 ± 0.055a | 1.315 ± 0.029a | 1.307 ± 0.049a |
Data are presented as mean ± standard error of the mean. Significance was tested with two-way analysis of variance/Tukey post hoc test and accepted at P < 0.05. Bolded numbers indicate significant interaction (P < 0.05) between bGH and ALSKO.
Abbreviations: bFat, brown fat; BW, body weight; gFat, gonadal fat; Sc, subcutaneous fat.
vs. control.
vs. ALSKO.
vs. bGH.
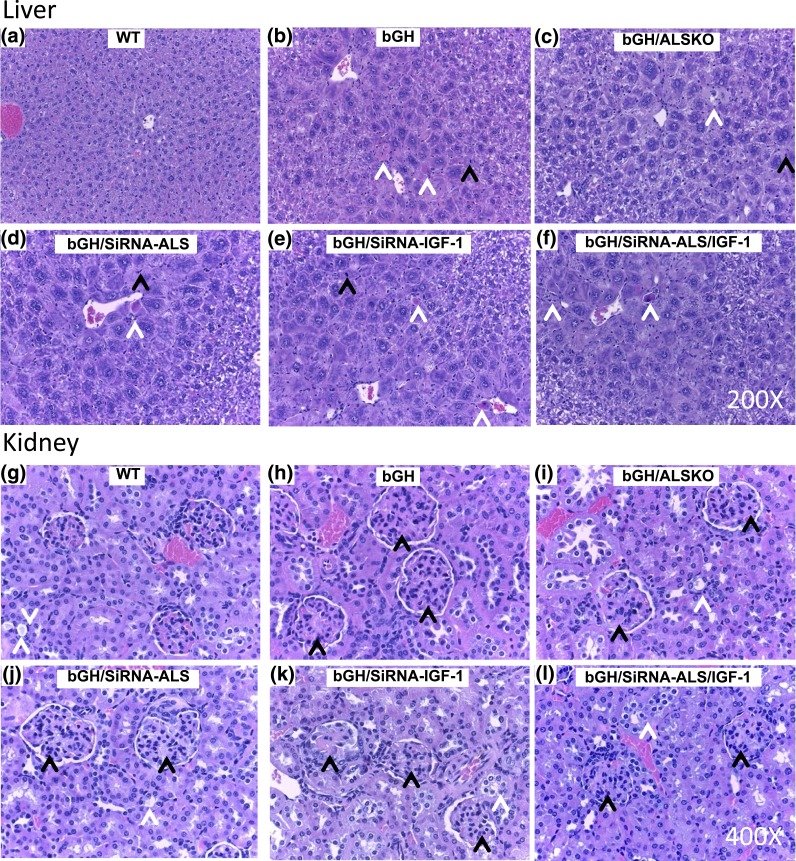
Livers from bGH mice exhibited hepatocellular hypertrophy with karyomegaly, single-cell necrosis, and cellular infiltrates as expected for this model [Fig. 2(a–c); Supplemental Table 2 (155.5KB, doc) ]. Livers from bGH/ALSKO mice were similar to those from bGH mice; thus, genetic ablation of als had no effect on the incidence or severity of histopathological findings observed in the bGH mouse [Fig. 2(a–c); Supplemental Table 2 (155.5KB, doc) ]. There were no sex differences in liver histology (Supplemental Table 2 (155.5KB, doc) ). Histological findings in livers from control and ALSKO mice were within normal limits and showed variable levels of glycogen consistent with variability in feeding cycle. bGH mice have exhibited severe glomerulosclerosis (25–27). Kidneys from bGH and bGH/ALSKO mice showed similar incidence and severity of membranoproliferative glomerulopathy [Fig. 2(g–i); Supplemental Table 2 (155.5KB, doc) ]. All groups, including controls, showed similar incidence and severity of kidney tubular lesions of uncertain pathophysiology.
Figure 2.
Microscopic effects of serum IGF-1 reductions on kidney and liver of bGH mice. (a) Livers from WT mice had expected background levels of hepatocellular glycogen. Livers from (b) bGH and (c) bGH/ALSKO mice, as well as from mice administered siRNA targeting (d) ALS, (e) IGF-1, or (f) ALS/IGF-1, had hepatocellular hypertrophy with no differences between groups. (b–f) Black arrowheads indicate single-cell necrosis, and white arrowheads indicate mitotic figures. (g) Kidneys from WT mice had thin capillary loops containing erythrocytes in glomerular capillaries. Kidneys from (h) bGH and (i) bGH/ALSKO mice, as well as (j) mice administered siRNA duplexes ALS, had membranoproliferative glomerulonephropathy characterized by increased glomerular mesangium and hypercellularity. Mice administered siRNA targeting (k) IGF-1 or (l) ALS/IGF-1 had slightly increased severity of glomerulonephropathy compared with that of bGH mice. (h–l) Black arrowheads indicate glomerular lesions, and white arrowheads indicate tubules with basophilic, often vacuolated, cytoplasm and occasional casts.
Skeletal growth of bGH mice in the absence of ALS (genetic approach)
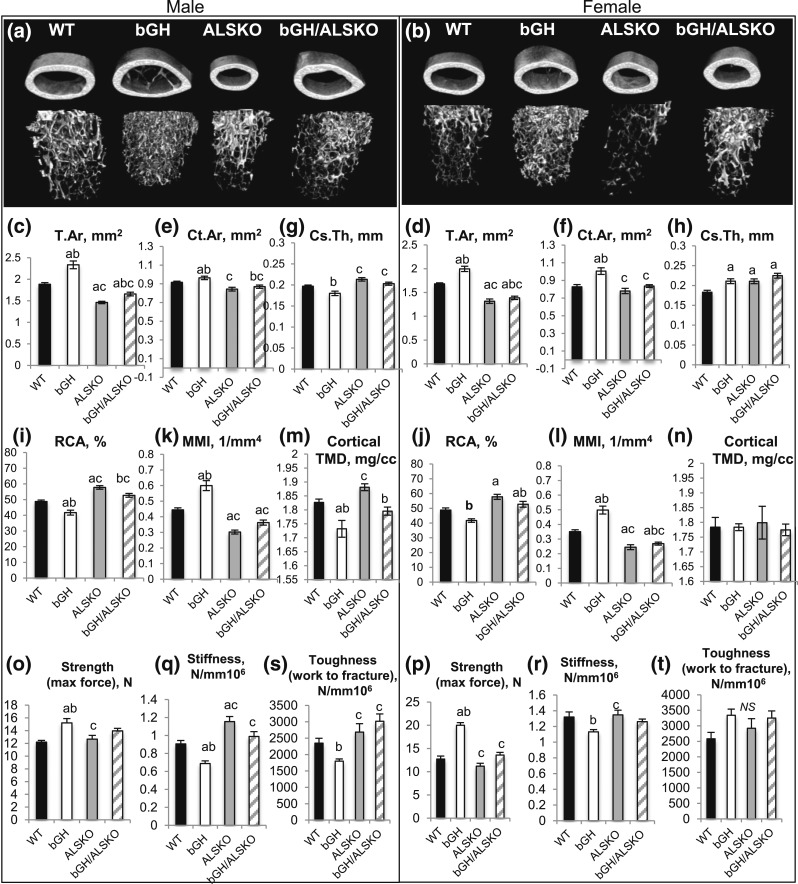
Increases in GH levels in bGH mice result in skeletal overgrowth. To study how reductions in serum IGF-1 levels affect the skeletal properties of the bGH/ALSKO mice, we used microCT [detailed in Fig. 3(a) and 3(b); Supplemental Table 3 (155.5KB, doc) ] and three-point bending assays (Fig. 3). Cortical bone morphology assessed at the femur mid-diaphysis showed increased Tt.Ar [Fig. 3(c) and 3(d)] in both sexes of bGH mice that significantly decreased in male and female bGH/ALSKO mice. Ct.Ar [Fig. 3(e) and 3(f)] increased in bGH mice in both sexes and decreased with ablation of ALS (bGH/ALSKO) in both sexes. In male bGH mice, Cs.Th was reduced compared with Cs.Th in controls but did not reach significance [Fig. 3(g)]; this was manifested in reduced relative cortical bone area (RCA = Ct.Ar/Tt.Ar × 100) [Fig. 3(i)] compared with that of control and bGH/ALSKO mice, suggesting increased endosteal bone resorption in bGH male mice. In females, however, Cs.Th significantly increased in bGH and bGH/ALSKO groups compared with controls [Fig. 3(h)]. RCA in bGH/ALSKO females significantly increased compared with Cs.Th in bGH mice [Fig. 3(j)], suggesting decreased endosteal bone resorption in that group.
Figure 3.
Effects of genetic ablation of ALS in bGH mice on skeletal morphology and mechanical properties. Femurs dissected from 16-week-old mice were analyzed by microCT (WT males, n = 13; ALSKO males, n = 10; bGH males, n = 9; bGH/ALSKO males, n = 14; WT females, n = 11; ALSKO females, n = 6; bGH females, n = 13; bGH/ALSKO females, n = 13). (a, b) Three-dimensional images of cortical and trabecular volumes at the mid-diaphysis and distal metaphysis, respectively, from male and female mice. (c, d) Total cross-sectional area (T.Ar), (e, f) bone area, (g, h) cortical bone thickness, (i, j) relative cortical bone area (RCA; Ct.Ar/T.Ar × 100), and (k, l) polar MMI of cortical bone taken at the femur mid-diaphysis in male and female mice. (m, n) Cortical bone mineral density. Mechanical properties of the femur were determined using a three-point bending assay (WT males, n = 7; ALSKO males, n = 6; bGH males, n = 5; bGH/ALSKO males, n = 10; WT females, n = 6; ALSKO females, n = 6; bGH females, n = 8; bGH/ALSKO females, n = 10). (o, p) Bone strength (maximal force), (q, r) bone stiffness (work to fracture), and (s, t) bone toughness (yield stress). Data are presented as mean ± standard error of the mean. Significance is accepted at P < 0.05 [(a) = vs. control; (b) = vs. ALSKO; (c) = vs. bGH]. NS, not significant.
Trabecular bone morphology at the distal metaphysis of the femur varied largely. Male and female bGH mice did not show significant differences in trabecular bone traits compared with controls (Supplemental Table 3 (155.5KB, doc) ); however, in male bGH mice, Tb.Th decreased. Ablation of ALS in bGH male mice did not affect the trabecular bone compartment, such that no significant differences were found between bGH and bGH/ALSKO males (Supplemental Table 3 (155.5KB, doc) ). However, in female mice, the effects of ALS ablation in bGH was more profound, such that bGH/ALSKO females showed decreased Tb.N, decreased trabecular BMD, and increased trabecular spacing compared with values in bGH females. This could indicate a development-related decrease in trabecular bone volume in bGH/ALSKO mice or increased trabecular bone resorption in that group.
Bone mechanical properties were estimated from the polar MMI [Fig. 3(k) and 3(l)], a trait derived from the microCT analyses and three-point bending assays [Fig. 3(q–t)]. In contrast to our estimations from the microCT morphological parameters, which suggest that bGH bones are more robust (Tt.Ar/Le) with significant increases in polar MMI [Fig. 3(k) and 3(l)], we found that male bGH mice showed reduced bone toughness and stiffness and that their strength does not relate proportionally to their morphological traits. These data are in line with decreased cortical bone TMD in bGH mice compared with controls [Fig. 3(m)] (controls: 1.82 ± 0.012 mg/mL; bGH mice: 1.73 ± 0.03 mg/mL; P = 0.004), suggesting that bone quality is compromised in bGH male mice. Ablation of ALS in bGH males restored femur toughness and stiffness in the bGH/ALSKO mice and was associated with increased cortical TMD (bGH/ALSKO mice: 1.80 ± 0.01 mg/mL; P = 0.046 compared with bGH mice alone). In female bGH mice, we found increases in polar MMI and increased maximum strength by three-point bending assay, but their toughness and stiffness did not show the expected mechanical strength from the microCT data [Fig. 3(p), 3(r), and 3(t)], again suggesting compromised bone tissue quality. In females, bone stiffness increased in bGH/ALSKO mice compared with bGH mice alone. Cortical TMD did not differ between the female groups [Fig. 3(n)].
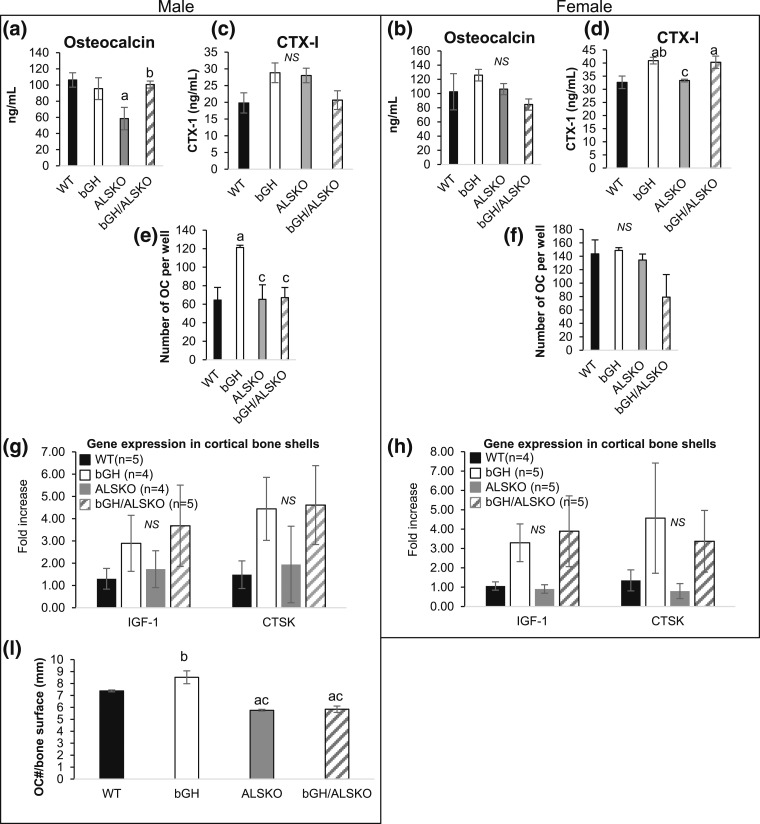
Subsequently, we measured serum levels of osteocalcin [Fig. 4(a) and 4(b)], a bone formation marker, and CTX, a bone resorption marker [Fig. 4(c) and 4(d)]. Osteocalcin levels did not differ between bGH and bGH/ALSKO groups in either sex. CTX levels were significantly higher in bGH and bGH/ALSKO females, whereas no significant differences were detected in the male groups.
Figure 4.
Effects of genetic ablation of ALS in bGH mice on serum osteocalcin, CTX, in vivo osteoclastogenesis, and gene expression in bone. (a, b) Serum osteocalcin levels (n > 8 per group). (c, d) Serum CTX levels (n > 6 per group). (e, f) Primary osteoclast (OC) cultures (n > 3 samples per group). (g, h) Gene expression in cortical bone shells. (i) Cathepsin K−positive cells/bone surface (n = 3 per group). Data are presented as mean ± standard error of the mean. Significance is accepted at P < 0.05 [(a) = vs. control; (b) = vs. ALSKO; (c) = vs. bGH]. CTSK, cathepsin K; NS, not significant.
We also determined the number of osteoclast (OC) progenitors in bone marrow extracted from long bones [Fig. 4(e) and 4(f)] in all groups. We found that marrow from male bGH mice had significantly more OC progenitors than the marrow of controls and that the OC number was reduced in bGH/ALSKO males. Females did not show significant differences in the number of OC progenitors in cultures; however, the total number of OCs increased in female mice. Expression levels of the Igf-1 gene in the femur cortical shells increased (but did not reach significance) in male and female bGH and bGH/ALSKO mice, and the expression of cathepsin K, an OC marker, showed a similar pattern (but did not reach significance), suggesting more resorption in these groups [Fig. 4(g) and 4(h)]. Lastly, bone sections from bGH male mice showed increased anticathepsin K−positive cells on trabecular bone surface [Fig. 4(i)], suggesting an increased bone resorption. Our data are in accordance with those from a previous study showing that overexpression of IGF-1 in osteoblasts increased bone resorption (28).
Validation of siRNA approach to silence liver production of ALS or IGF-1 in WT mice
Our second approach was aimed at understanding whether exogenous intervention to reduce serum IGF-1 levels postnatally (by siRNA) would affect body and skeletal growth in a manner similar to the genetic approach with congenital ablation of ALS (bGH/ALSKO). To do that, we used GalNAc-conjugated siRNA. GalNAc is a carbohydrate moiety that allows the siRNA to be efficiently internalized by the hepatocyte-specific cell surface asialoglycoprotein receptor. Using the siRNA-conjugates does not elicit an inflammatory response in the liver (29, 30).
To validate the efficacy of the siRNA duplexes, we used WT mice. We found only minor reductions in body weight in the different groups of WT mice injected with siRNA, which did not reach significance [Supplemental Fig. 1A] (425.5KB, ppt) . These findings were in accordance with our previous reports of LID (15) and ALSKO mice (10), which showed only mild reductions in body weight despite significant reductions in serum IGF-1 levels. Organ weights of WT mice injected with als and Igf-1 siRNA duplexes revealed that the relative liver weight to body weight increased (Supplemental Table 4 (155.5KB, doc) ). Injection of siRNA−IGF-1 increased the relative weight of the gonadal fat pad and significantly decreased kidney and pancreas weights compared with those of PBS-injected WT mice (Supplemental Table 4 (155.5KB, doc) ).
Liver expression of the als or igf-1 gene was evaluated by RT-PCR. As expected, we found >90% inhibition of als or igf-1 gene expression in the siRNA-injected WT mice (Supplemental Fig. 1B) (425.5KB, ppt) . Accordingly, serum samples collected at euthanasia showed 75% reductions in IGF-1 levels in WT mice injected with siRNA-ALS, 85% reductions in WT mice injected with siRNA−IGF-1, and 95% reductions in WT mice injected with SiRNA-ALS/IGF-1 (Supplemental Fig. 1C) (425.5KB, ppt) . IGFBP-3, the main carrier of IGF-1 in circulation, is stabilized via binding to ALS (31). Genetic ablation of ALS has shown significant reductions in serum IGFBP-3 levels, most likely because of increased degradation (32). Accordingly, we found 60% reductions in serum IGFBP-3 levels in WT mice injected with siRNA-ALS or siRNA-ALS/IGF-1 Supplemental Fig. 1D) (425.5KB, ppt) . Likewise, serum ALS levels were reduced by 60% in WT mice injected with siRNA−IGF-1 and was virtually undetectable in WT mice injected with siRNA-ALS or siRNA-ALS/IGF-1 (Supplemental Fig. 1E) (425.5KB, ppt) .
The aforementioned studies with the WT mice established the efficacy of the siRNA duplexes in reducing liver production of IGF-1 and ALS.
Body and organ growth in bGH mice in response to als and igf-1 gene silencing (using siRNA-ALS or siRNA−IGF-1)
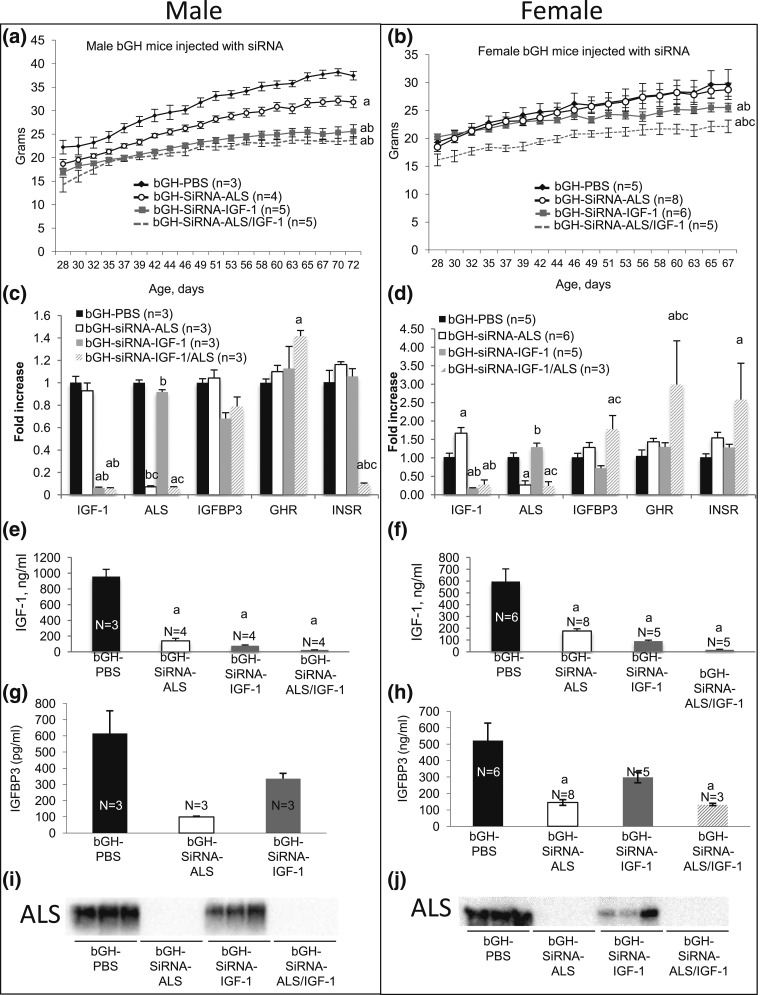
Male and female bGH mice were allocated randomly to four groups. Starting at 21 days, siRNA-ALS (3 mg/kg), SiRNA−IGF-1 (3 mg/kg), siRNA-ALS/IGF-1 (3 mg/kg each), or vehicle (PBS) was injected once a week for 7 to 8 weeks. Male bGH mice injected with siRNA-ALS, siRNA−IGF-1, or both siRNA-ALS/IGF-1 showed decreased body weight [Fig. 5(a)]. However, in female bGH mice injected with siRNA-ALS, body weight did not decrease [Fig. 5(b)]. bGH females injected with siRNA−IGF-1 showed significant reductions in body weight starting 2 weeks after the first injection, whereas those injected with both siRNA-ALS/IGF-1 exhibited further reductions in body weight that were evident as early as a few days after the first injection [Fig. 5(b)].
Figure 5.
siRNA approach to silence Igf-1 and als gene expression in bGH mice. Body weights of (a) male and (b) female mice followed up from 4 to 11 weeks of age. Liver expression of the Igf-1, als, Igfbp3, Ghr, and Insr genes in (c) male and (d) female mice at 11 weeks of age. Serum IGF-1 levels in (e) male and (f) female mice at 11 weeks of age. Serum IGFBP-3 levels in (g) male and (h) female mice at 11 weeks of age. Western immunoblotting of serum ALS in (i) male and (j) female mice at 11 weeks of age. Data are presented as mean ± standard error of the mean. Significance is accepted at P < 0.05 [(a) = vs. bGH-PBS; (b) = vs. bGH-siRNA-ALS; (c) = vs. bGH-siRNA-IGF-1].
Liver expression of the als or igf-1 genes was evaluated by RT-PCR. As expected, we found >90% inhibition of als andr igf-1 gene expression in bGH mice injected with siRNA-ALS and siRNA−IGF-1, respectively, in both males and females [Fig. 5(c) and 5(d)]. Expression of IGFBP-3 in livers of bGH mice injected with siRNA−IGF-1 decreased in both sexes [Fig. 5(c) and 5(d)]. GH receptor gene expression was elevated in both sexes of bGH mice injected with siRNA-ALS/IGF-1. Insulin receptor expression in the livers of male bGH mice injected with both siRNA-ALS/IGF-1 decreased significantly compared with expression in mice injected with PBS.
bGH mice injected with siRNA-ALS showed ∼60% to 80% reductions in serum IGF-1 levels [Fig. 5(e) and 5(f)], ∼40% to 70% reductions in IGFBP-3 levels [Fig. 5(g) and 5(h)], and significant reductions in serum ALS levels determined by Western blot analyses [Fig. 5(i) and 5(j); representative blots]. bGH mice injected with siRNA−IGF-1 showed similar reductions in serum IGF-1 and IGFBP-3 levels in both sexes [Fig. 5(e–j)].
siRNA injections in the bGH mice did not affect fed blood glucose levels in either sex (Supplemental Table 1 (155.5KB, doc) ). bGH mice showed increased serum insulin levels (all >2.5 ng/mL) in both sexes (Supplemental Table 1 (155.5KB, doc) ), which were not changed with siRNA-duplex injection.
Organ weights of bGH mice treated with siRNA complexes for 7 weeks were recorded at euthanasia (Table 2). Relative organ weights of bGH mice injected with siRNA-ALS did not differ from those of vehicle-injected mice. In male bGH mice injected with siRNA−IGF-1 or in bGH mice injected with siRNA-ALS/IGF-1, we recorded reductions in relative weights of muscle, kidney, heart, and pancreas compared with weights in the bGH-PBS group (Table 2). In female mice, we found that injection of siRNA-ALS/IGF-1 decreased kidney, spleen, and pancreas sizes, whereas the subcutaneous fat pad increased (Table 2).
Table 2.
Body and Organ Weights of bGH Mice Treated With siRNA Duplexes From 4 Weeks of Age for 7 Weeks
|
Male bGH Mice Injected From 4 to 11 Weeks of Age |
Female bGH Mice Injected From 4 to 11 Weeks of Age |
|||||||
|---|---|---|---|---|---|---|---|---|
| PBS (n = 3) | siRNA-ALS (n = 4) | siRNA-IGF-1 (n = 5) | siRNA-ALS/IGF-1 (n = 5) | PBS (n = 5) | siRNA-ALS (n = 8) | siRNA-IGF-1 (n = 6) | siRNA-ALS/IGF-1 (n = 5) | |
| Body weight | 37.787 ± 1.070 | 31.925 ± 0.862a | 26.064 ± 1.242a,b | 23.864 ± 0.503a,b | 30.520 ± 2.247 | 29.390 ± 1.204 | 26.272 ± 0.699 | 22.684 ± 0.882a,b |
| Body length | 11.467 ± 0.033 | 10.900 ± 0.212 | 10.400 ± 0.176a | 10.080 ± 0.080a,b | 10.640 ± 0.294 | 10.763 ± 0.171 | 10.283 ± 0.140 | 9.700 ± 0.265a,b |
| Liver | 2.780 ± 0.125 | 2.185 ± 0.125a | 1.978 ± 0.123a | 1.808 ± 0.028a | 2.150 ± 0.170 | 2.100 ± 0.118 | 1.963 ± 0.065 | 1.790 ± 0.059a,b |
| Liver, % of BW | 7.350 ± 0.128 | 6.828 ± 0.222 | 7.572 ± 0.138b | 7.582 ± 0.102b | 7.049 ± 0.210 | 7.109 ± 0.160 | 7.471 ± 0.115 | 7.901 ± 0.123 |
| Muscle | 0.373 ± 0.003 | 0.310 ± 0.016a | 0.254 ± 0.012a,b | 0.232 ± 0.012a,b | 0.322 ± 0.022 | 0.301 ± 0.012 | 0.247 ± 0.006a | 0.208 ± 0.017a,b |
| Muscle, % of BW | 0.989 ± 0.026 | 0.971 ± 0.041 | 0.977 ± 0.035a | 0.970 ± 0.033a,b | 1.059 ± 0.038 | 1.028 ± 0.024 | 0.941 ± 0.027 | 0.917 ± 0.073 |
| Gonadal fat | 0.667 ± 0.047 | 0.603 ± 0.119 | 0.344 ± 0.032a | 0.330 ± 0.036a,b | 0.342 ± 0.037 | 0.284 ± 0.014 | 0.280 ± 0.014 | 0.234 ± 0.044 |
| gFat, % of BW | 1.761 ± 0.084 | 1.903 ± 0.404 | 1.328 ± 0.134 | 1.375 ± 0.126 | 1.153 ± 0.172 | 0.985 ± 0.082 | 1.070 ± 0.061 | 1.012 ± 0.145 |
| Sc fat | 0.447 ± 0.018 | 0.455 ± 0.080 | 0.358 ± 0.049 | 0.320 ± 0.031 | 0.382 ± 0.043 | 0.409 ± 0.033 | 0.387 ± 0.030 | 0.372 ± 0.042 |
| Sc fat, % of BW | 1.182 ± 0.026 | 1.440 ± 0.284 | 1.358 ± 0.126 | 1.335 ± 0.118 | 1.248 ± 0.084 | 1.390 ± 0.089 | 1.470 ± 0.107 | 1.627 ± 0.133 |
| Brown fat | 0.123 ± 0.003 | 0.100 ± 0.004 | 0.084 ± 0.007a | 0.086 ± 0.005a | 0.106 ± 0.008 | 0.098 ± 0.008 | 0.092 ± 0.008 | 0.078 ± 0.011 |
| bFat, % of BW | 0.327 ± 0.008 | 0.315 ± 0.020 | 0.320 ± 0.015 | 0.361 ± 0.023 | 0.348 ± 0.009 | 0.330 ± 0.020 | 0.348 ± 0.025 | 0.340 ± 0.036 |
| Kidney | 0.527 ± 0.028 | 0.423 ± 0.044a | 0.282 ± 0.011a,b | 0.260 ± 0.010a,b | 0.368 ± 0.032 | 0.375 ± 0.022 | 0.307 ± 0.015 | 0.242 ± 0.014a,b |
| Kidney, % of BW | 1.392 ± 0.037 | 1.316 ± 0.105 | 1.085 ± 0.024a | 1.090 ± 0.039a | 1.202 ± 0.019 | 1.271 ± 0.040 | 1.167 ± 0.047 | 1.070 ± 0.062b |
| Spleen | 0.163 ± 0.019 | 0.143 ± 0.017 | 0.082 ± 0.007a,b | 0.086 ± 0.007a,b | 0.154 ± 0.015 | 0.149 ± 0.005 | 0.108 ± 0.006a,b | 0.088 ± 0.005a,b |
| Spleen, % of BW | 0.431 ± 0.041 | 0.445 ± 0.049 | 0.314 ± 0.018 | 0.361 ± 0.032 | 0.502 ± 0.016 | 0.516 ± 0.039 | 0.413 ± 0.022 | 0.389 ± 0.019b |
| Heart | 0.197 ± 0.009 | 0.163 ± 0.010 | 0.154 ± 0.010a | 0.142 ± 0.002a | 0.178 ± 0.017 | 0.183 ± 0.011 | 0.173 ± 0.011 | 0.146 ± 0.011 |
| Heart, % of BW | 0.520 ± 0.010 | 0.508 ± 0.019 | 0.590 ± 0.014a,b | 0.596 ± 0.013a,b | 0.580 ± 0.021 | 0.617 ± 0.018 | 0.660 ± 0.038 | 0.645 ± 0.050 |
| Lung | 0.180 ± 0.010 | 0.163 ± 0.014 | 0.150 ± 0.008 | 0.140 ± 0.003 | 0.158 ± 0.012 | 0.154 ± 0.007 | 0.153 ± 0.003 | 0.144 ± 0.005 |
| Lung, % of BW | 0.476 ± 0.014 | 0.507 ± 0.034 | 0.579 ± 0.036 | 0.587 ± 0.011 | 0.518 ± 0.017 | 0.523 ± 0.011 | 0.586 ± 0.019 | 0.638 ± 0.030a,b |
| Pancreas | 0.470 ± 0.021 | 0.380 ± 0.046 | 0.262 ± 0.011 | 0.242 ± 0.021 | 0.366 ± 0.017 | 0.321 ± 0.013 | 0.237 ± 0.015a,b | 0.190 ± 0.020a,b |
| Pancreas, % of BW | 1.243 ± 0.022 | 1.184 ± 0.124 | 1.013 ± 0.061a,b | 1.015 ± 0.087a,b | 1.215 ± 0.072 | 1.099 ± 0.042 | 0.906 ± 0.066a | 0.831 ± 0.066a,b |
Data are presented as mean ± standard error of the mean. Significance was tested with two-way analysis of variance/Tukey post hoc test and accepted at P < 0.05. Bolded numbers indicate significant interaction (P < 0.05) between SiRNA-ALS and SiRNA-IGF-1.
Abbreviations: bFat, brown fat; BW, body weight; gFat, gonadal fat; Sc, subcutaneous fat.avs. bGH-PBS.
vs. bGH-SiRNA-ALS.
vs. bGH-SiRNA-IGF-1.
Histological evaluation of livers from bGH mice injected with vehicle or siRNA duplexes revealed hepatocellular hypertrophy with karyomegaly [Fig. 2(d–f); Supplemental Table 2 (155.5KB, doc) ]. No differences were found between groups in the incidence or severity of histological findings with siRNA treatment individually or in combination. Kidneys from vehicle-injected bGH mice showed minimal to mild membranoproliferative glomerulonephropathy (with only rare tubular lesions) that was exacerbated in females but not in males by the administration of siRNA−IGF-1 with or without concurrent administration of siRNA-ALS [Fig. 2(j–l); Supplemental Table 2 (155.5KB, doc) ].
Skeletal characterization of bGH mice in response to reductions in serum IGF-1 level using siRNA and genetic approaches
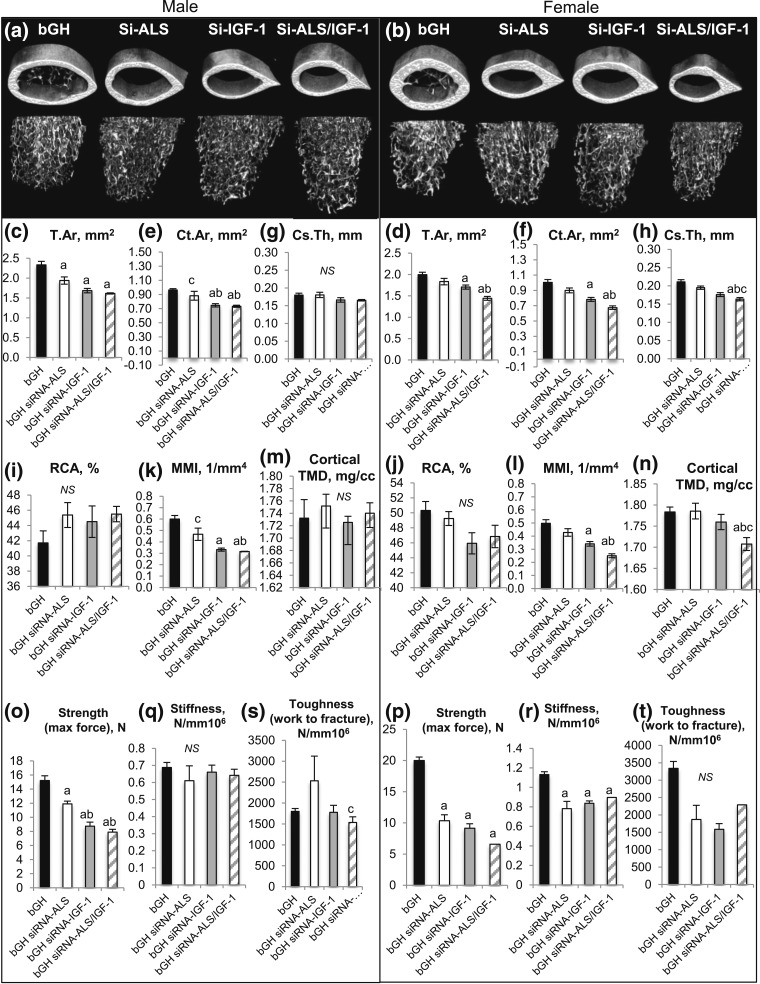
In both male and female bGH mice, the cortical bone compartment (detailed in Supplemental Table 5 (155.5KB, doc) ) showed response to siRNA injections [Fig. 6(c–n)]. A more profound response was seen following injection of siRNA−IGF-1 as opposed to injection of siRNA-ALS. Male bGH mice injected with siRNA-ALS showed decreased Tt.Ar, whereas injection of siRNA−IGF-1 decreased Tt.Ar, Ct.Ar, and polar MMI. Injection of siRNA-ALS/IGF-1 in bGH males had similar effects to those seen with injection of siRNA−IGF-1. In female bGH mice, siRNA-ALS did not affect cortical bone morphology, whereas siRNA−IGF-1 or siRNA-ALS/IGF-1 injection caused significant reductions in Tt.Ar, Ct.Ar, Ct.Th, and MMI. As in the genetic model, trabecular bone morphology at the femur distal metaphysis dissected from siRNA-injected bGH groups varied widely (detailed in Supplemental Table 5 (155.5KB, doc) ). In females, we found significant reductions in Tb.Th in all injected groups, which may indicate increased resorption (Supplemental Table 5 (155.5KB, doc) ).
Figure 6.
Effects of siRNA silencing of Igf-1 and als gene expression on skeletal morphology and mechanical properties. Femurs dissected from 11-week-old mice were analyzed by microCT (bGH-PBS males, n = 9; bGH-siRNA-ALS males, n = 5; bGH-siRNA-IGF-1 males, n = 5; bGH-siRNA-ALS/IGF-1 males, n = 5; bGH-PBS females, n = 13; bGH-siRNA-ALS females, n = 8; bGH-siRNA-IGF-1 females, n = 5; bGH-siRNA-ALS/IGF-1 females, n = 5). (a, b) Three-dimensional images from male and female mice of cortical and trabecular volumes at the mid-diaphysis and distal metaphysis, respectively. (c, d) Total cross-sectional area; (e, f) bone area; (g, h) cortical bone thickness; (i, j) relative cortical bone area (RCA; Ct.Ar/T.Ar × 100); and (k, l) polar MMI of cortical bone taken at the femur mid-diaphysis in male and female mice. (m, n) Cortical BMD. Mechanical properties of the femur were determined using a three-point bending assay (bGH-PBS males, n = 5; bGH-siRNA-ALS males, n = 5; bGH-siRNA-IGF-1 males, n = 5; bGH-siRNA-ALS/IGF-1 males, n = 5; bGH-PBS females, n = 8; bGH-siRNA-ALS females, n = 8; bGH-siRNA-IGF-1 females, n = 5; bGH-siRNA-ALS/IGF-1 females, n = 5). (o, p) Bone strength (maximal force), (q, r) bone toughness (work to fracture), and (s, t) bone stiffness (yield stress). Data are presented as mean ± standard error of the mean. Significance is accepted at P < 0.05 [(a) = vs. bGH; (b) = vs. bGH-SiRNA-ALS; (c) = vs. bGH-SiRNA-IGF-1]. NS, not significant.
Polar MMI [Fig. 6(k) and 6(l)] decreased in both sexes of bGH mice injected with siRNA duplexes, with the largest effect seen in the siRNA-ALS/IGF-1−injected groups. In female but not male mice, cortical TMD was significantly reduced with siRNA-ALS/IGF-1 injection (bGH-PBS: 1.78 ± 0.01 mg/mL; bGH-siRNA-ALS: 1.78 ± 0.01 mg/mL; bGH-siRNA−IGF-1: 1.76 ± 0.02 mg/mL; bGH-siRNA-ALS/IGF-1: 1.70 ± 0.01 mg/mL) [Fig. 6(m) and 6(n)]. In this model, female mice in all groups injected with siRNA duplexes showed significant reductions in bone strength (max force) [Fig. 6(p)], bone stiffness [Fig. 6(r)], and toughness [Fig. 6(t)]. Male bGH mice showed reduced max force when injected with siRNA duplexes; however, bone stiffness and toughness did not change with injection [Fig. 6(o), 6(q), and 6(s)].
Discussion
Our study shows that congenital ablation of ALS or the siRNA-duplex approach to silence liver expression of the igf-1 and als genes effectively reduced serum IGF-1 and IGFBP-3 levels in male and female bGH mice. Subsequently, reductions in serum IGF-1 levels (using the two approaches) decreased body weight and reduced skeletal size in both sexes. The changes in body and skeletal size of bGH mice following ALS inactivation were not accompanied by changes in glucose or insulin levels.
Excess GH in bGH mice was associated with increased hepatocellular mitosis and hypertrophy, increased single-cell necrosis, and increased mononuclear infiltrates, irrespective of serum IGF-1 levels in both sexes of the two models (genetic and siRNA duplex). These findings are consistent with the hepatic histology of mice expressing the bGH transgene under the phosphoenolpyruvate-carboxykinase promoter (33). In this report, the authors showed that hepatic pathology remained even after crossing the mice with the IGF-1 null mice. GH-induced enlargement of the liver was also reported in IGF-1 null mice with elevated endogenous GH secretion (34, 35). In contrast, IGF-1 transgenic mice did not show increased liver size (36) even in the absence of endogenous GH (37), and hepatocyte-specific IGF-1 transgenic mice did not show alterations in liver size (38, 39). These data suggest that the effects of GH on hepatocellular morphology are IGF-1 independent.
Excesses in GH/IGF-1 are associated with changes in renal size, morphology, and glomerular/tubular function. We found that all groups of bGH mice (in both models and both sexes) showed membranoproliferative glomerulonephropathy characterized by increased glomerular mesangium and glomerular hypercellularity. Previous reports indicated that both IGF-1 transgenic and bGH mice show glomerular hypertrophy; however, even though IGF-1 transgenic mice have higher serum IGF-1 levels than bGH mice, they show less severe phenotype and do not develop glomerulosclerosis (25, 27). This suggests that activation of the GH receptor is most likely the cause of glomerular hypertrophy and not GH-mediated local production of IGF-1 or high serum IGF-1 levels. Similarly, in the current study, despite significant reductions in serum IGF-1 levels (in bGH/ALSKO and bGH mice injected with siRNA duplexes), we did not observe improvement in kidney pathology. Therefore, reductions in serum IGF-1 levels are insufficient to alleviate kidney abnormalities in cases of extremely elevated GH levels (as seen in the bGH transgenic mice).
GH affects skeletal size and integrity via its direct actions on skeletal cells (osteoblasts, osteocytes, and OCs) and via its effects on vitamin D production in the kidney and secondary effects on parathyroid hormone production (40). In man, excess GH/IGF-1 is associated with high bone turnover (40–42), which may lead to bone loss and increased fracture risk. In mice, osteoblast-specific expression of IGF-1 associated with decreased bone volume and increased bone turnover favored more resorption (28). In our study, serum levels of the bone turnover marker osteocalcin did not differ between bGH and control mice, whereas serum levels of the bone resorption marker CTX were elevated in female bGH and bGH/ALSKO mice but did not reach significance in male mice. Note that serum levels of osteocalcin and CTX are only crude measurements of bone turnover because we do not know which bone compartment is affected (cortical vs. trabecular bone) or whether their source is the axial or appendicular skeleton. bGH male (genetic model) mice showed increased numbers of OC progenitors in bone marrow compared with numbers in controls and increased cathepsin K−positive cells on trabecular bone surface, which suggests more bone resorption. However, bGH females did not show such differences. Further studies are needed to address sex-specific bone remodeling in the bGH mice at different ages during skeletal acquisition.
MicroCT data suggested compromised bone modeling, which was consistent with findings from a previous study (43) showing that excess GH in male mice was associated with impaired skeletal morphological and mechanical properties. Reductions in serum IGF-1 levels in bGH/ALSKO and bGH mice injected with siRNA duplexes reduced skeletal growth compared with levels in naive bGH mice. This was evident by decreases in the radial cortical bone properties T.Ar, Ct.Ar, and Cs.Th. Overall, the data show that reductions in serum IGF-1 levels in mice with excess GH is associated with reduced skeletal acquisition. We showed that despite the robust morphology of bGH femurs (in both sexes) with increased Tt.Ar, bone Le, and MMI, male bone mechanical properties were impaired, indicating reduced bone quality. This was evident by reduced bone stiffness and toughness in bGH male bones, tested with the three-point bending assay, and in agreement with reduced cortical bone TMD in bGH male mice. In contrast, in female bGH mice, we did not find significant differences in bone toughness and stiffness compared with values in WT controls, and no differences in cortical bone TMD were detected. Because changes in igf-1 expression in bone tissue were not evident in either sex of bGH and bGH/ALSKO mice, we cannot attribute the differences in stiffness and toughness between male and female mice to the autocrine/paracrine mode of IGF-1 action. Thus, other GH-dependent local or systemic factors must play a role in skeletal sexual dimorphism.
With regard to the trabecular bone compartment, most of the differences were detected using the genetic model. bGH/ALSKO males showed decreased bone volume relative to total volume, BMD, and Tb.Th compared with controls, and female bGH/ALSKO mice showed reduced BMD, Tb.Th, and Tb.N compared with controls. Together, these data suggest either a defect in trabecular bone gain during growth or increases in trabecular bone resorption postnatally.
Several possible mechanisms may explain the skeletal sexual dimorphism in the bGH mice; among them are interactions between the GH/IGF-1 and the sex steroid axes (44). Unlike in the liver (45), in the skeleton molecular mechanisms implicated in GH-mediated sexual dimorphism have not been discovered. It is conceivable that GH together with sex steroids regulates bone modeling and osteoblast function (matrix deposition and mineralization) in a sex-specific manner via induction of a subset of genes that are expressed in different levels in each sex. These may be transcription regulators, such as runx2 (46, 47), or matrix proteins, such as collagen (48). Clearly, the somatotropic (GH/IGF-1) and gonadotropic (sex steroids) axes cross-react during puberty to regulate linear and radial bone growth. Studies in GH receptor null (49) and IGF-1 null mice (50), which also exhibit impaired gonadal development, revealed that skeletal sexual dimorphism was lost.
Our data with the genetic and siRNA models showed that the effects of bGH and ALS variables on body weight, organ weight, and skeletal properties are sex independent, such that the effects of bGH and ALS were seen in both sexes for different magnitudes (by three-way analysis of variance; data not shown). By studying both sexes, we found that congenital ablation of ALS in bGH mice reduced body weight by ∼29% and ∼25% in male and female bGH/ALSKO mice, respectively (compared with bGH alone). Injections of siRNA-ALS reduced body weight by ∼15% in male bGH mice but was ineffective in female bGH mice.
With regard to skeletal parameters, the Tt.Ar was reduced by ∼28% and ∼30% in bGH/ALSKO male and female mice, respectively, compared with areas in bGH alone mice. The bone area was reduced by ∼10% and ∼17% in male and female bGH/ALSKO mice, respectively, compared with the area in bGH alone mice. Injections of siRNA duplexes effectively reduced cortical bone traits, with the most effective inhibition found in siRNA-ALS/IGF-1−injected mice. Overall, we found that male and female bGH mice responded similarly to reductions in serum IGF-1 levels.
In summary, we showed that congenital ablation of als or silencing liver production of Igf-1/als significantly reduced serum IGF-1 levels, even in states of GH excess. Reductions in serum IGF-1 level were associated with overall decreased body size, with proportional reductions in organ sizes. Excess GH was associated with increased skeletal size, with more robust bones (in bGH mice). However, in male but not female mice, mechanical properties were compromised, most likely because of impaired tissue quality. Reductions in serum IGF-1 levels were also associated with decreased trabecular bone fraction and BMD. Ongoing studies will determine whether the reductions in serum IGF-1 levels affect longevity and the overall health of the bGH/ALSKO mice.
Acknowledgments
Acknowledgments
Financial support was received from Alnylam, National Institutes of Health Grant DK100246 (to S.Y.), Binational Science Foundation Grant 2013282 (to S.Y.), and Grant S10OD010751-01A1 (microCT instrument grant; to the New York University College of Dentistry).
Author contributions: Z.L. conducted the experiments. S.F., J.B., T.Z., and F.T. designed, generated, and verified the efficacy of siRNA complexes for ALS and IGF-1. C.H. (pathologist) examined the liver and kidney sections. N.A. (endocrinologist) discussed the data and participated in manuscript preparation. J.J.K. generated the bGH transgenic mice, discussed the data, and participated in manuscript preparation. M.B.S. discussed bone morphology and mechanics data and participated in manuscript preparation. S.Y. designed the experiments and wrote the article.
Disclosure Summary: S.F., J.B., T.Z., F.T., and C.H. are employed by Alnylam and have equity interest in Alnylam. The remaining authors have nothing to disclose.
Appendix:
Antibody Table
| Name of Antibody | Company, Catalog # | RRID |
|---|---|---|
| Cathepsin K antibody | Abcam, ab19027 | AB_2261274 |
| ALS primary antibody | R&D, AF1436 | AB_2122936 |
| Donkey anti-goat IgG HRP affinity purified PAb antibody | R&D, HAF109 | AB_357236 |
| Anti-rabbit IgG, HRP-linked antibody | Cell Signaling Technology, 7074 | AB_2099233 |
Abbreviations: HRP, horseradish peroxidase; IgG, immunoglobulin G; PAb, polyclonal antibody; RRID, research resource identifier.
Footnotes
- ALS
- acid-labile subunit
- ALSKO
- als gene knockout
- bGH
- bovine growth hormone
- BMD
- bone mineral density
- Cs.Th
- cortical bone thickness
- Ct.Ar.
- cortical bone area
- CTX
- carboxy-terminal collagen crosslinks
- GalNAc
- N-acetylgalactosamine
- GH
- growth hormone
- IGF-1
- insulinlike growth factor 1
- IGFBP
- insulinlike growth factor−binding protein
- Le
- length
- LID
- liver-specific IGF-1 gene deletion
- microCT
- microcomputed tomography
- MMI
- moment of inertia
- OC
- osteoclast
- PBS
- phosphate-buffered saline
- RCA
- relative cortical area (Ct.Ar/Tt.Ar)
- RT-PCR
- real-time polymerase chain reaction
- siRNA
- small interfering RNA
- Tb.N
- trabecular number
- Tb.Th
- trabecular thickness
- TMD
- tissue mineral density
- Tt.Ar
- total cross-sectional area
- WT
- wild-type
- αMEM
- minimum essential medium α.
References
- 1.Yakar S, Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: lessons from mouse models. Growth Horm IGF Res. 2016;28:26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigerlova E, Hwa V, Derr MA, Rosenfeld RG. Current issues on molecular diagnosis of GH signaling defects. Endocr Dev. 2013;24:118–127. [DOI] [PubMed] [Google Scholar]
- 3.Wit JM, Oostdijk W, Losekoot M. Spectrum of insulin-like growth factor deficiency. Endocr Dev. 2012;23:30–41. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175(1):19–31. [DOI] [PubMed] [Google Scholar]
- 5.Boisclair YR, Rhoads RP, Ueki I, Wang J, Ooi GT. The acid-labile subunit (ALS) of the 150 kDa IGF-binding protein complex: an important but forgotten component of the circulating IGF system. J Endocrinol. 2001;170(1):63–70. [DOI] [PubMed] [Google Scholar]
- 6.Baxter RC. Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278(6):E967–E976. [DOI] [PubMed] [Google Scholar]
- 7.Rhoads RP, Greenwood PL, Bell AW, Boisclair YR. Organization and regulation of the gene encoding the sheep acid-labile subunit of the 150-kilodalton insulin-like growth factor-binding protein complex. Endocrinology. 2000;141(4):1425–1433. [DOI] [PubMed] [Google Scholar]
- 8.Domené HM, Hwa V, Argente J, Wit JM, Camacho-Hübner C, Jasper HG, Pozo J, van Duyvenvoorde HA, Yakar S, Fofanova-Gambetti OV, Rosenfeld RG; International ALS Collaborative Group . Human acid-labile subunit deficiency: clinical, endocrine and metabolic consequences. Horm Res. 2009;72(3):129–141. [DOI] [PubMed] [Google Scholar]
- 9.Domené HM, Bengolea SV, Jasper HG, Boisclair YR. Acid-labile subunit deficiency: phenotypic similarities and differences between human and mouse. J Endocrinol Invest. 2005;28(5 Suppl):43–46. [PubMed] [Google Scholar]
- 10.Courtland HW, DeMambro V, Maynard J, Sun H, Elis S, Rosen C, Yakar S. Sex-specific regulation of body size and bone slenderness by the acid labile subunit. J Bone Miner Res. 2010;25(9):2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritton JC, Kawashima Y, Mejia W, Courtland HW, Elis S, Sun H, Wu Y, Rosen CJ, Clemmons D, Yakar S. The insulin-like growth factor-1 binding protein acid-labile subunit alters mesenchymal stromal cell fate. J Biol Chem. 2010;285(7):4709–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yakar S, Rosen CJ, Bouxsein ML, Sun H, Mejia W, Kawashima Y, Wu Y, Emerton K, Williams V, Jepsen K, Schaffler MB, Majeska RJ, Gavrilova O, Gutierrez M, Hwang D, Pennisi P, Frystyk J, Boisclair Y, Pintar J, Jasper H, Domene H, Cohen P, Clemmons D, LeRoith D. Serum complexes of insulin-like growth factor-1 modulate skeletal integrity and carbohydrate metabolism. FASEB J. 2009;23(3):709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, Cohen P, Hwang D, Boisclair Y, Leroith D, Rosen CJ. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol. 2006;189(2):289–299. [DOI] [PubMed] [Google Scholar]
- 14.Haluzik M, Yakar S, Gavrilova O, Setser J, Boisclair Y, LeRoith D. Insulin resistance in the liver-specific IGF-1 gene-deleted mouse is abrogated by deletion of the acid-labile subunit of the IGF-binding protein-3 complex: relative roles of growth hormone and IGF-1 in insulin resistance. Diabetes. 2003;52(10):2483–2489. [DOI] [PubMed] [Google Scholar]
- 15.Yakar S, Canalis E, Sun H, Mejia W, Kawashima Y, Nasser P, Courtland HW, Williams V, Bouxsein M, Rosen C, Jepsen KJ. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009;24(8):1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen NY, Chen WY, Striker LJ, Striker GE, Kopchick JJ. Co-expression of bovine growth hormone (GH) and human GH antagonist genes in transgenic mice. Endocrinology. 1997;138(2):851–854. [DOI] [PubMed] [Google Scholar]
- 17.Kaps M, Moura AS, Safranski TJ, Lamberson WR. Components of growth in mice hemizygous for a MT/bGH transgene. J Anim Sci. 1999;77(5):1148–1154. [DOI] [PubMed] [Google Scholar]
- 18.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel’in AV, Milstein S, Taneja N, O’Shea J, Shaikh S, Zhang L, van der Sluis RJ, Jung ME, Akinc A, Hutabarat R, Kuchimanchi S, Fitzgerald K, Zimmermann T, van Berkel TJ, Maier MA, Rajeev KG, Manoharan M. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–16961. [DOI] [PubMed] [Google Scholar]
- 19.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- 20.Jepsen KJ, Silva MJ, Vashishth D, Guo XE, van der Meulen MC. Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J Bone Miner Res. 2015;30(6):951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balbis A, Bartke A, Turyn D. Overexpression of bovine growth hormone in transgenic mice is associated with changes in hepatic insulin receptors and in their kinase activity. Life Sci. 1996;59(16):1363–1371. [DOI] [PubMed] [Google Scholar]
- 22.Balbis A, Dellacha JM, Calandra RS, Bartke A, Turyn D. Down regulation of masked and unmasked insulin receptors in the liver of transgenic mice expressing bovine growth hormone gene. Life Sci. 1992;51(10):771–778. [DOI] [PubMed] [Google Scholar]
- 23.Olsson B, Bohlooly-Y M, Fitzgerald SM, Frick F, Ljungberg A, Ahrén B, Törnell J, Bergström G, Oscarsson J. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146(2):920–930. [DOI] [PubMed] [Google Scholar]
- 24.Kopchick JJ, Bellush LL, Coschigano KT. Transgenic models of growth hormone action. Annu Rev Nutr. 1999;19:437–461. [DOI] [PubMed] [Google Scholar]
- 25.Doi T, Striker LJ, Gibson CC, Agodoa LY, Brinster RL, Striker GE. Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I. I. Relationship between increased glomerular size and mesangial sclerosis. Am J Pathol. 1990;137(3):541–552. [PMC free article] [PubMed] [Google Scholar]
- 26.Doi T, Striker LJ, Kimata K, Peten EP, Yamada Y, Striker GE. Glomerulosclerosis in mice transgenic for growth hormone: increased mesangial extracellular matrix is correlated with kidney mRNA levels. J Exp Med. 1991;173(5):1287–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doi T, Striker LJ, Quaife C, Conti FG, Palmiter R, Behringer R, Brinster R, Striker GE. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulinlike growth factor-1. Am J Pathol. 1988;131(3):398–403. [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang J, Lichtler AC, Gronowicz GA, Adams DJ, Clark SH, Rosen CJ, Kream BE. Transgenic mice with osteoblast-targeted insulin-like growth factor-I show increased bone remodeling. Bone. 2006;39(3):494–504. [DOI] [PubMed] [Google Scholar]
- 29.Winkler J. Oligonucleotide conjugates for therapeutic applications. Ther Deliv. 2013;4(7):791–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakefield DH, Klein JJ, Wolff JA, Rozema DB. Membrane activity and transfection ability of amphipathic polycations as a function of alkyl group size. Bioconjug Chem. 2005;16(5):1204–1208. [DOI] [PubMed] [Google Scholar]
- 31.Domené HM, Hwa V, Jasper HG, Rosenfeld RG. Acid-labile subunit (ALS) deficiency. Best Pract Res Clin Endocrinol Metab. 2011;25(1):101–113. [DOI] [PubMed] [Google Scholar]
- 32.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blutke A, Schneider MR, Renner-Müller I, Herbach N, Wanke R, Wolf E. Genetic dissection of IGF1-dependent and -independent effects of permanent GH excess on postnatal growth and organ pathology of mice. Mol Cell Endocrinol. 2014;394(1-2):88–98. [DOI] [PubMed] [Google Scholar]
- 34.Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141(12):4436–4441. [DOI] [PubMed] [Google Scholar]
- 35.Liu JL, LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140(11):5178–5184. [DOI] [PubMed] [Google Scholar]
- 36.Quaife CJ, Mathews LS, Pinkert CA, Hammer RE, Brinster RL, Palmiter RD. Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology. 1989;124(1):40–48. [DOI] [PubMed] [Google Scholar]
- 37.Behringer RR, Lewin TM, Quaife CJ, Palmiter RD, Brinster RL, D’Ercole AJ. Expression of insulin-like growth factor I stimulates normal somatic growth in growth hormone-deficient transgenic mice. Endocrinology. 1990;127(3):1033–1040. [DOI] [PubMed] [Google Scholar]
- 38.Liu Z, Cordoba-Chacon J, Kineman RD, Cronstein BN, Muzumdar R, Gong Z, Werner H, Yakar S. Growth hormone control of hepatic lipid metabolism. Diabetes. 2016;65(12):3598–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu Y, Sun H, Basta-Pljakic J, Cardoso L, Kennedy OD, Jasper H, Domené H, Karabatas L, Guida C, Schaffler MB, Rosen CJ, Yakar S. Serum IGF-1 is insufficient to restore skeletal size in the total absence of the growth hormone receptor. J Bone Miner Res. 2013;28(7):1575–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ezzat S, Melmed S, Endres D, Eyre DR, Singer FR. Biochemical assessment of bone formation and resorption in acromegaly. J Clin Endocrinol Metab. 1993;76(6):1452–1457. [DOI] [PubMed] [Google Scholar]
- 41.Bolanowski M, Daroszewski J, Medraś M, Zadrozna-Sliwka B. Bone mineral density and turnover in patients with acromegaly in relation to sex, disease activity, and gonadal function. J Bone Miner Metab. 2006;24(1):72–78. [DOI] [PubMed] [Google Scholar]
- 42.Kotzmann H, Bernecker P, Hübsch P, Pietschmann P, Woloszczuk W, Svoboda T, Geyer G, Luger A. Bone mineral density and parameters of bone metabolism in patients with acromegaly. J Bone Miner Res. 1993;8(4):459–465. [DOI] [PubMed] [Google Scholar]
- 43.Lim SV, Marenzana M, Hopkinson M, List EO, Kopchick JJ, Pereira M, Javaheri B, Roux JP, Chavassieux P, Korbonits M, Chenu C. Excessive growth hormone expression in male GH transgenic mice adversely alters bone architecture and mechanical strength. Endocrinology. 2015;156(4):1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Mohan S, Yakar S. Does the GH/IGF-1 axis contribute to skeletal sexual dimorphism? Evidence from mouse studies. Growth Horm IGF Res. 2016;27:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waxman DJ, O’Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20(11):2613–2629. [DOI] [PubMed] [Google Scholar]
- 46.Cardelli M, Aubin JE. ERRγ is not required for skeletal development but is a RUNX2-dependent negative regulator of postnatal bone formation in male mice. PLoS One. 2014;9(10):e109592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamper BD, Park SS, Beyer RP, Bammler TK, Cunningham ML. Unique sex-based approach identifies transcriptomic biomarkers associated with non-syndromic craniosynostosis. Gene Regul Syst Bio. 2012;6:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasatzky E, Schwartz Z, Boyan BD, Soskolne WA, Ornoy A. Sex-dependent effects of 17-beta-estradiol on chondrocyte differentiation in culture. J Cell Physiol. 1993;154(2):359–367. [DOI] [PubMed] [Google Scholar]
- 49.Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, Vanderschueren D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res. 2010;25(3):617–626. [DOI] [PubMed] [Google Scholar]
- 50.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16(12):2320–2329. [DOI] [PubMed] [Google Scholar]