Abstract
Aim:
The aim of this study is to examine the elevation of MYOC in long-term treatment of human trabecular meshwork (HTM) cells using dexamethasone (DEX) encapsulated pentablock (PB) copolymer-based nanoparticles (NPs) (DEX-PB-NPs).
Materials & methods:
PB copolymers and DEX-PB-NPs were synthesized and characterized using nuclear magnetic resonance, gel permeation chromatography, and X-ray diffraction analyses. MYOC levels secreted from HTM cells were measured by western blot (WB) analysis.
Results:
DEX-PB-NPs were formulated in the size range of 109 ± 3.77 nm (n = 3). A long term DEX release from the NPs was observed over three months. Cell viability and cytotoxicity were not affected up to 12 weeks of treatment with PB-copolymer or DEX-PB-NPs. WB data from five HTM cell strains showed that MYOC levels increased by 5.2 ± 1.3, 7.4 ± 4.3, and 2.8 ± 1.1-fold in the presence of DEX-PB-NPs compared with 9.2 ± 3.8, 2.2 ± 0.5, and 1.5 ± 0.3-fold at 4, 8 and 12 weeks in control-DEX treatment group, respectively (n = 5). Based on the decline in MYOC levels after withdrawal of DEX from control wells, DEX-PB-NPs released the DEX for at least 10 weeks.
Conclusion:
The treatment of HTM cells using DEX-PB-NPs were analyzed in this study. The in vitro cell-based system developed here is a valuable tool for determining the safety and effects of steroids released from polymeric NPs.
Keywords: : block copolymer, MYOC, nanoparticle, steroids, sustained release, trabecular meshwork cells, western blot
Nanotechnology has opened exciting therapeutic options in drug delivery approaches. These systems offer several benefits in ocular drug delivery due to their small size and use of biodegradable materials in their formulation [1,2]. Dexamethasone (DEX), one of the most effective corticosteroids, has been widely indicated in the clinical practice of ophthalmology as an anti-inflammatory and immunosuppressive agent. It can be administered via topical, periocular (i.e., subconjunctival) or intraocular (i.e., intravitreal) routes. Topical DEX has proven efficacious in the management of postoperative inflammation in the anterior segment after cataract surgery, treatment of anterior uveitis (iritis) and dry-eye disease symptoms [3–5]. Intravitreal administration of DEX has been effective in the treatment of macular edema following retinal vein occlusion, diabetic macular edema [6–8], and non-infectious uveitis [9], particularly when other therapeutic agents have failed to provide treatment benefits. However, DEX has a short half-life [10], and requires multiple applications. As a consequence, technologies that achieve slow, sustained, and controlled-release of DEX may prevent frequent administrations or multiple invasive treatments [11,12]. In this regard, US FDA approved biodegradable polymers such as polycaprolactone (PCL), polylactic acid (PLA), polyglycolic acid (PGA) polyethylene glycol (PEG) and polylactide-co-glycolide (PLGA) have been comprehensively studied for the sustained delivery of the corticosteroid [13,14]. These polymers have been widely tested preparing various diblock (DB) [15], and triblock (TB) copolymers [15] for drug delivery technologies. Investigators have applied various block copolymers combinations such as PLGA-PEG-PLGA [16], PEG-PLA-PEG [17,18], for the development of sustained release formulations. Several of these polymers are incorporated in microparticle [19], nanoparticle [14], and liposomal preparations [20] for long-term drug release. Recently, the FDA has approved a DEX intravitreal implant for the treatment of macular edema following retinal vein occlusion, diabetic macular edema, or non-infectious uveitis [9,21].
Several attempts have been made to overcome the initial burst of therapeutic molecules using DB or TB copolymers. However, to overcome the above problem, there is an unmet need of designing the optimized block copolymer based delivery system to provide continuous delivery of corticosteroids for longer duration with the minimal burst release. In this regard, pentablock (PB) copolymers were designed to overcome the limitation of the burst release associated with the NP and to provide long-term delivery of therapeutic molecules [22,23]. In this approach, the in vitro drug release profile was optimized by adjusting the block length, arrangement, and the ratio of the PCL/PLA/PGA with PEG. The arrangements can be further optimized by changing the molecular weight (MW) of each polymeric block. Considering these facts, a novel PB copolymer (PGA-PCL-PEG-PCL-PGA) was developed to encapsulate DEX in PB-NPs attempting to achieve a long-term delivery. The PB copolymer displays a unique block arrangement, ratio and MW, which can influence the drug release profile of hydrophobic molecules. The purpose of the present study is to examine the DEX release profile of PB copolymer in physiological solution and in vitro cell culture media in the presence of human trabecular meshwork (HTM) cell. In addition, the activity and safety over time in ocular cell culture were examined using primary cultures of HTM cells. The approach developed here will be applied to generate an animal model for corticosteroid induced ocular hypertension.
Materials & methods
Materials
Poly(ethylene glycol) (PEG 1 kDa), poly(vinyl alcohol) (PVA), stannous octoate, and dexamethasone (DEX) were obtained from Sigma-Aldrich (MO, USA). The ϵ-caprolactone, glycolide and L-lactide were procured from Acros Organics (NJ, USA). HPLC solvents and other reagents utilized in this study were of analytical grade.
Methods
Synthesis of copolymers
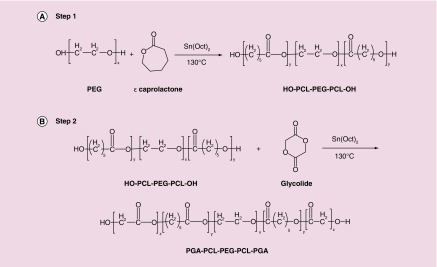
Novel PB copolymer, poly(glycolic acid)-poly (caprolactone)-poly (ethylene glycol)-poly (caprolactone)-poly (glycolic acid) (PGA-PCL-PEG-PCL-PGA) was synthesized in two steps by sequential ring-opening polymerization reaction [23]. PEG (1 kDa) was utilized as the macroinitiator and stannous octoate act as the catalyst. In the first step, TB copolymer PCL-PEG-PCL was synthesized by polymerization of ϵ-caprolactone on two open hydroxyl ends of PEG. ϵ-caprolactone and stannous octoate (0.5% w/w) were added to anhydrous PEG and temperature was raised to 130°C. After 24 h, the reaction mixture was dissolved in methylene chloride followed by precipitation in cold ether. Purified TB copolymer was then used for the preparation of PB copolymer. Stannous octoate (0.5% w/w) was added as a catalyst in the reaction mixture containing a predetermined quantity of TB copolymer. The synthesis of PB copolymer was carried out at 130°C for 24 h under inert atmosphere. After 24 h, the reaction mixture was dissolved in methylene chloride followed by precipitation in cold petroleum ether. The purified PB copolymer was vacuum-dried and stored at -20°C until further analysis. Reaction schemes for the synthesis of TB and PB copolymers were depicted in Figure 1A & B, respectively.
Figure 1. . Synthesis scheme for (A) triblock (TB: PCL-PEG-PCL) copolymer and (B) pentablock (PB: PGA-PCL-PEG-PGA-PCL) copolymer by ring opening bulk copolymerization method.
Characterization of copolymers
The synthesized TB and PB copolymers were characterized for their MW, polydispersity index (PDI), and purity by proton (1H) nuclear magnetic resonance (1H-NMR) spectroscopy, gel permeation chromatography (GPC) and powder x-ray diffraction (PXRD). The structures and MWs of copolymers (TB and PB) are described in Table 1.
Table 1. . Characterization of block copolymers.
| Copolymers | Structure | Total Mn† (theoretical) | Total Mn‡ (calculated) | Total Mn§ (calculated) | Mw§ (GPC) | PDI§ |
|---|---|---|---|---|---|---|
| TB |
PCL7000-PEG1000-PCL7000 |
15,000 |
14,278 |
12,289 |
17,562 |
1.83 |
| PB | PGA3000-PCL7000-PEG1000-PCL7000-PGA3000 | 21,000 | 20,264 | 17,952 | 23,158 | 1.36 |
†Theoretical value, calculated according to the feed ratio.
‡Calculated from 1H-NMR.
§Determined by GPC analysis.
GPC: Gel permeation chromatography; NMR: Nuclear magnetic resonance; PDI: Polydispersity index.
1H-NMR spectroscopy
1H-NMR spectra of TB and PB copolymers were acquired on a 400 MHz NMR instrument (Varian Inc., CA, USA). The chemical shift (δ) values were reported in parts per million (ppm). NMR samples were prepared by dissolving TB and PB copolymers in deuterated chloroform in a 5 mm outer diameter NMR tubes (Wilmad-LabGlass, NJ, USA).
GPC analysis
The purity, MW and PDI of TB and PB copolymers were further analyzed by GPC analysis. Polymer samples were analyzed with Waters 410 refractive index (RI) detector (Waters, MA, USA). Briefly, samples were prepared by dissolving 1 mg of copolymers in tetrahydrofuran (THF) (THF was utilized as eluting agent at the flow rate of 1 ml/min). Separation was carried out on a Styragel HR-3 column (Waters, MA, USA). The internal and external temperatures of the SEC column were maintained at 35°C using a Waters column heater module controlled by 410 RI detector. The data were acquired and processed with Waters Millenium32 software (version 3.2). A calibration curve was prepared by using Dextran SEC standards (Polymer Standards Service-USA, MA, USA) in the MW range of 5.2–410 kDa. A volume of 200 μl was injected into the SEC system in each analysis.
PXRD analysis
To analyze the crystallinity of copolymers, PXRD analysis was performed using Rigaku MiniFlex automated x-ray diffractometer (Rigaku, TX, USA) equipped with Ni-filtered Cu-Kα radiation (30 kV and 15 mA). The analysis was performed at room temperature at the scanning rate of 5°/min.
Formulation of DEX-encapsulated PB nanoparticles (DEX-PB-NPs)
DEX-loaded PB copolymer NPs were prepared by oil-in-water (O/W) single emulsion solvent evaporation method. Briefly, DEX (5 mg) and PB copolymer (25 mg) were dissolved in methylene chloride copolymers to make the organic phase. The aqueous phase was comprised of 5 ml of 2% PVA. The O/W emulsion was formed using probe sonication. The organic phase was then evaporated by stirring the emulsion overnight. NPs were separated by ultra-centrifugation at 20,000 rpm for 45 min at 4°C. NPs were washed twice with distilled deionized water (DDW), and centrifuged to remove the traces of PVA and unentrapped DEX. The purified NPs were freeze-dried with mannitol (50 mg) as a cryoprotectant and stored at -20°C until further use.
Physicochemical characterization of NPs
Size distribution measurements
DEX-PB-NPs were analyzed for their particle mean diameter: nm, and size distribution by nanoparticle tracking analysis (NTA) using a Nanosight LM10 instrument (Nanosight, Salisbury, UK). Freeze dried DEX-PB-NPs (1 mg/ml) suspended in DDW were subjected to particle size analysis at room temperature and 90° scattering angle. All the samples were analyzed in triplicate (n = 3).
Entrapment efficiency & drug loading
The entrapment efficiency (EE) (%) and drug loading (DL) (%) were estimated by the ultrafast liquid chromatography (UFLC) assay for the amount of DEX in the supernatants obtained from the NP preparation. Equations 1 and 2 were used for the calculation of EE (%) and DL (%), respectively.
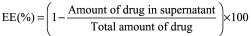 |
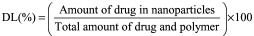 |
In vitro drug release profile of DEX-PB-NPs
To analyze the in vitro drug release profile, 1 mg of DEX equivalent freeze-dried NPs were suspended in a dialysis tube. DEX-loaded NPs were suspended in 25 ml of phosphate-buffered saline (PBS) pH -7.4 at 37°C. The tube containing dialysis bag was placed in a water bath at 37°C (GFL 3032 Shaker, LABOTECT, Rosdorf, Germany). At predetermined time intervals, 1 ml of clear supernatant was collected and replaced with the same volume of fresh PBS (preincubated at 37°C). Drug concentrations were measured by UFLC analysis. All experiments were conducted in triplicate (n = 3). In vitro release data were expressed as cumulative drug released (%) with time.
UFLC assay
Reversed phase UFLC assay was employed to analyze EE, DL and in vitro release of the DEX-PB-NPs. A Shimadzu UFLC system (Shimadzu Scientific Instruments, MD, USA) coupled with pumps (LC-20AT), degasser (DGU-20A3R), DAD detector (SPD-20AV) and autosampler (SIL-20AHT) was employed. A Phenomenex column (Phenomenex C18 kinetex column (100 × 4.6 mm, 5 mm) was used at a total flow rate of 0.5 ml/min. An isocratic elution method was employed for the separation with mobile Phase A (water with 0.1% formic acid) at 60% and mobile phase B (acetonitrile [ACN] with 0.1% formic acid) at 40% were running for 8 min. Concentration of DEX standards ranged from 250 to 0.488 µg/ml prepared in the mobile phase. Injection volume of 30 µl was used for each analysis and the DAD detector was set at 254 nm for DEX quantification.
In vitro tolerability studies of PB copolymer & PB-NPs
To analyze the cytotoxic effects of PB copolymers on corneal, conjunctival and retinal cell lines, cell cytotoxicity (lactate dehydrogenase [LDH]) and cell viability (MTT) assays were performed according to the supplier's instructions (Promega Corp., WI, USA). SV-40 (human corneal epithelial transfected with a recombinant SV-40-adenovirus vector cell), CCL20.2 (human conjunctival epithelial cell/Chang's conjunctival cell line) and D407 (human retinal pigment epithelium cell) cells are immortalized and can be subcultured many times, while maintaining their physiological properties. SV-40 cells were cultured in DMEM/F-12 medium supplemented with 15% (v/v) heat-inactivated fetal bovine serum (FBS), 22 mM NaHCO3, 15 mM HEPES and 5 mg/l insulin, 10 μg/l human epidermal growth factor, 100 mg penicillin and 100 mg streptomycin each. Both cell lines were incubated at 37°C, 5% CO2 and 98% humidity. CCL20.2 cells were maintained in a cell culture flask containing Minimum Essential Medium (MEM), Earle's Balanced Salt Solution medium supplemented with 10% FBS, 100 U/L of penicillin, 100 mg/l of streptomycin, NaHCO3 (2.2 mg/ml) and 2 mM l-glutamine. D407 cells were grown at 37°C, humidified 5% CO2/95% air atmosphere in a DMEM culture medium supplemented with 10% (v/v) FBS (heat inactivated), 29 mM NaHCO3, 20 mM HEPES, 100 mg of penicillin and streptomycin each, and 1% nonessential amino acids at pH 7.4. The cells were harvested at 80–90% confluency with TrypLE™ Express (a superior replacement for trypsin) [15,24].
Human trabecular meshwork (HTM) cell culture
Five strains of trabecular meshwork cells (HTM120, 136, 126, 134 and 141) were isolated from eyes of human donors of ages 11 and 3 months old (HTM120 and 136), 88, 51 and 38 years old (HTM126, 134 and 141), respectively, with no documented history of eye disease. The genders for the HTM cells were HTM126, 136: females; HTM134, 141: males; HTM120: unknown, no records available from the Eye Bank. Cells were isolated and characterized as previously described [25,26]. Human eye tissues were sourced ethically from Miracles in Sight (NC, USA), accredited by the Eye Bank Association of America. The research uses of eye tissues were in accordance with the terms of the informed consents of the donors and/or donor family. HTM cells (passages 3–6) were seeded into 24- or 96-well culture plates in DMEM containing 10% FBS (Atlanta Biologicals, GA, USA) until cells reached confluency. As a differentiation step, the cells were then switched to DMEM medium containing 1% FBS for at least 7 days prior to experimentation.
Dexamethasone nanoparticle (DEX-PB-NPs) treatment
HTM cells were treated with PB-NPs (1 mg/ml) containing a total of 23 μg DEX (DEX-PB-NPs) or Con-NPs (without DEX), DEX (39.25 ng/ml) or control (Con: 0.1% ethanol) in fresh 1 ml DMEM containing 1% FBS (1% DMEM). Two days after initial treatment, cell culture supernatant was removed and replaced with fresh 1% DMEM medium containing either 0.1% ethanol vehicle or DEX (39.25 ng/ml in 0.1% ethanol). Similarly, medium in Con-NPs or DEX-PB-NPs treated wells were replaced with fresh 1% DMEM. Then, the cell culture supernatant was collected and replaced with fresh 1% DMEM once/week for a total of 12 weeks for DEX-NP or Con-NP treatment wells. Other wells were replaced with fresh 1% DMEM medium containing either Con or DEX once/week. After 4 weeks, all wells were replaced with fresh 1% FBS medium once in a week for an additional 8 weeks and stored at -80°C until further analysis.
Western blot analysis
Secreted MYOC levels in cell culture supernatants were detected and normalized following our previously published methods [27]. Briefly, cell culture supernatants were collected from wells of culture plates after 2 days of treatment, and then once/week for 12 weeks. At the end of 12 weeks, cells were harvested and rinsed twice with cold PBS. Cells were scraped into 80 μl of lysis buffer (25% glycerol, 0.0625M Tris.HCl, 2% SDS) containing 5% beta mercaptoethanol. Cell culture media at each time point was mixed with 4 × loading buffer (50% glycerol, 0.125M Tris.HCl, 4% SDS) containing 5% beta mercaptoethanol and boiled for 10 min before storing at -20°C. For WB analysis, 24 μl of solubilized proteins in the cell culture supernatant containing 4 × loading buffer were loaded into 8% polyacrylamide gel slabs; and 10 μl of cell lysates were loaded to 10% polyacrylamide gel slabs. The proteins were separated via SDS-PAGE. Fractionated proteins were then transferred electrophoretically to nitrocellulose membranes. Nonspecific binding of antibodies to membranes containing transferred proteins was reduced by incubating with Tris-buffered saline with 0.1% Tween 20 (TBS-T) containing 5% nonfat dry milk (blocking buffer).
Rabbit polyclonal antibodies against MYOC or a mouse monoclonal antibody against beta-actin (Sigma-Aldrich, MO, USA) in blocking buffer were incubated overnight with membranes at 4°C. The next day, the membranes were first washed in TBS-T (three times for 10 min) and then were incubated in blocking buffer containing horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., PA, USA) for 1 h. After incubation, membranes were washed with TBS-T as before. Protein-antibody complexes were visualized using a chemiluminescent horseradish peroxidase (HRP) antibody detection reagent spray (HyGLO; Denville Scientific, Inc., NJ, USA) and exposure to x-ray film (Phonix Research Company, NC, USA). The protein abundance in each band was quantified by densitometry using ImageJ image analysis software (GeneSnap/GeneTools, Syngene, MD, USA).
Cell cytotoxicity assay
The LDH assay was performed using previously published protocol with minor modifications [22]. Briefly, 5, 25 and 50 mg/ml of PB copolymer were dissolved in ACN and 100 μl was aliquoted in each well of the 96-well plate. Plates were exposed overnight under UV light (laminar flow) for polymer sterilization as well as evaporation of ACN. Similarly, 5, 25 and 50 mg/ml of PB-NP was dispersed in culture media and filtered through 0.22 μM filter. One hundred microliter of this solution was added to the 96-well cell culture plate. D407, SV-40 and CCL.20.2 cells at the density of 1.0 × 104 were seeded in each well and incubated at 37°C, 5% CO2 in a humidified atmosphere for 48 h. After completion of the incubation period, cell supernatants were analyzed for the quantification of LDH release using the LDH assay kit (Takara Bio, Inc., Otsu, Japan). Absorbance of each well was measured at 450 nm using a DTX 800 Multimode microplate reader (Beckman Coulter, CA, USA). The LDH release (%) was calculated according to Equation 3 and more than 10% of LDH release was considered as cytotoxic.
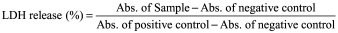 |
To test the cytotoxicity in HTM cells, three strains of confluent HTM cells (HTM141, HTM126 and HTM136) kept in 1% DMEM media for 1 week were treated with Con vehicle (0.1% ethanol), DEX (39.25 ng/ml in 0.1% ethanol), Con-NPs (1 mg/ml) or DEX-PB-NPs (1 mg/ml) in 1% DMEM media. The treatment protocol was the same as for collecting media for MYOC analysis. At the end of 12 weeks of incubation, cytotoxicity was determined by measuring LDH release from the cells using an LDH assay kit (Roche, IN, USA). Briefly, the supernatant was carefully removed, centrifuged and transferred to a 96-well plate. The cells were lysed and then transferred to a 96-well plate. A reaction mixture consisting of catalyst/dye combination was prepared, and 100 μl was added directly to each of 100 μl of the cell supernatant, cell lysates, plain media and lysate solution. After incubation at 25°C for 30 min, absorbance was measured using a spectrophotometer at 490 nm with a reference wavelength at 690 nm. Released LDH in the cell supernatant was calculated by subtraction of media background and then normalized by total LDH in cell lysates from each treatment group.
Cell viability assay
The safety and biocompatibility of PB copolymers was further established by performing an in vitro cell viability assay (MTS; (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) tetrazolium reduction). MTS assay (Promega Corp., WI, USA) was performed according to a previously reported protocol with minor modifications [22]. PB copolymer solutions at the concentration of 5, 25 and 50 mg/ml in ACN were prepared, aliquoted and sterilized. Following sterilization, D407, SV-40 and CCL20.2 cells were seeded in each well of 96-well plate at a cell density of 1.0 × 104, and incubated at 37°C and 5% CO2 in a humidified atmosphere for 48 h. Similarly, 5, 25 and 50 mg/ml of PB-NP solution was prepared in culture media and filtered through 0.22 μM filter. From this, 100 μl was added to the 96-well plate. At the end of the incubation period, cell culture medium was aspirated and the cells were incubated for 4 h (37°C and 5% CO2) in the presence of 100 μl of serum-free medium containing 20 μl of the MTS solution. The absorbance was measured at 450 nm using the above microplate reader. The percent (%) cell viability was calculated using Equation 4. The PB copolymers exhibiting more than 90% of cell viability were considered suitable for ocular applications.
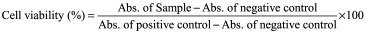 |
To test the cell viability of HTM cells with long-term DEX, Con-NPs or DEX-PB-NPs, a colorimetric assay was performed based on the cleavage of the tetrazolium salt WST-1 (4-[3-(4-lodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1.3-benzene disulfonate) by mitochondrial dehydrogenases in viable cells (Roche, Mannheim, DE, Germany). Twelve weeks after initial treatment, the WST-1 solution (10 μl/well) was added to each well containing 100 μl of cell culture supernatant. Cells were further incubated for 30 min at 37°C. The plate was read on a spectrophotometer at 440 nm with a reference wavelength of 690 nm.
Extraction method of DEX from cell culture media
Cell culture medium samples were analyzed using a UFLC method as described earlier. Sample preparation was carried out using liquid–liquid extraction technique. Hydrocortisone (HC), a corticosteroid, similar in structure to DEX was used as an internal standard for analysis. Briefly, samples were thawed at room temperature and 50 μl of internal standard was added to the samples (300 μl). Solutions were vortexed for 30 s, to which 100 μl of ACN was added, and again vortexed for 30 s to deactivate the serum proteins and enzymes. Each sample was mixed with 300 μl of organic solvent and vortexed again for another 2.5 min to allow equilibration between the phases. For efficient separation of the aqueous and organic layers, samples were extracted twice and centrifuged at 10,000 rpm for 7 min. Aliquots (500 μl) were collected and dried under reduced pressure for 45 min. The residue was reconstituted with 300 μl of mobile phase (ACN [40%] & water [60%]), vortexed for 30 s and transferred into a pre-labeled UFLC autosampler vial with silanized inserts. A 30 μl of the resulting solution was injected into the UFLC system and analyzed for DEX quantification.
Statistics
Mann–Whitney U test was used to analyze the statistically significant differences between groups. A p-value of ≤ 0.05 was considered statistically significant.
Results & discussion
Synthesis of PB copolymer & formulation of DEX-PB-NPs
Characterization of PB copolymer
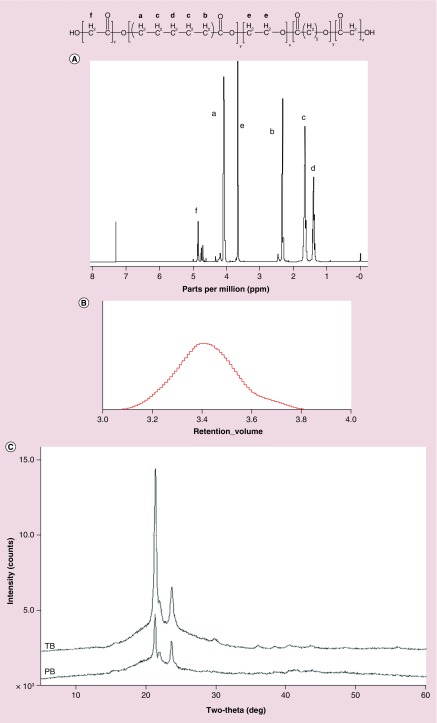
PB copolymer designed for the preparation of NPs was successfully synthesized by ring-opening bulk copolymerization. Figure 2A depicts the typical 1H-NMR peaks comprised of glycolic acid which displayed a series of singlets between 4.6 and 4.9 ppm confirming methylene protons of PGA block. A sharp peak at 3.65 ppm was attributed to methylene protons (-CH2CH2O-) of PEG. The 1H-NMR characteristic peaks of PCL unit were observed at 1.40, 1.65, 2.30 and 4.06 ppm represented the methylene (–CH2–) protons of -(CH2)5-, -OCO-CH2-, and -CH2OOC-, respectively. Molecular weights (number average MW: Mn) of PB copolymers were calculated from the integration values of 1H-NMR peaks of individual blocks. Moreover, the absence of any additional peaks in the 1H-NMR spectrum confirmed the purity of PB copolymers. MWs (Mn) calculated from 1H-NMR are reported in Table 1.
Figure 2. . Charcaterizations of pentablock copolymers.
(A) Proton nuclear magnetic resonance (1H NMR) spectra depicts peaks comprised of glycolic acid which displayed a series of singlets between 4.6 and 4.9 ppm confirming methylene protons of PGA block; (B) GPC depicts a single peak indicating mono distribution of molecular weight; (C) PXRD patterns of TB and PB copolymers exhibit crystalline peaks of PCL at 2θ = 21.5° and 23.9°.
GPC: Gel permeation chromatography; PB: Pentablock; PCL: Polycaprolactone; TB: Triblock; PXRD: Power x-ray diffraction.
Purity, MWs (Mn and weight average MW: Mw) and PDI were further evaluated by GPC are represented in Figure 2B. The PDI of PB copolymers was below 1.5, suggesting a narrow distribution of MWs. Moreover, PB copolymers depicted a single peak in GPC chromatogram indicating mono distribution of MW. Calculated MWs appeared to be very similar to the theoretical MWs obtained from feed ratio. Therefore, theoretical MWs were considered instead of calculating MWs subsequently. PXRD study revealed the crystallinity of block copolymers, which are represented in Figure 2C. Interestingly, TB and PB copolymers exhibited crystalline peaks of PCL at 2θ = 21.5° and 23.9°. PXRD patterns of TB and PB indicated that PCL blocks have retained semicrystalline structure, even after covalent conjugation with PGA blocks. Conjugation of PGA blocks at the terminals of TB copolymers slightly affected the intensity of crystalline peak. Previous published reports suggested that the decrease in crystallinity significantly enhances the degradation of block copolymer [22]. Hence, it is anticipated that PB copolymer can display a slower rate of degradation due to its semicrystalline nature.
Characterization of DEX-PB-NPs
Particle size
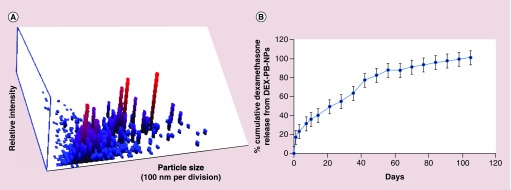
The particle size of DEX-PB-NPs was approximately 109 ± 3.77 nm (n = 3) observed by NTA reported in Figure 3A.
Figure 3. . Nanoparticle size distribution and drug release analysis of dexamethasone-encapsulated-pentablock-copolymer-nanoparticle.
(A) Particle size distribution of DEX-PB-NPs by NTA. The particle size was in the range of 109 ± 3.77 nm (n = 3). (B) In vitro release of dexamethasone (DEX) from DEX-PB-NPs. Representative graph shows the long-term release of DEX from DEX-PB-NPs.
DEX-PB-NP: Dexamethasone-encapsulated-pentablock-copolymer nanoparticle; NTA: Nanoparticle tracking analysis.
Entrapment efficiency (EE%) and drug loading (DL%)
DEX-PB-NPs were successfully prepared by single emulsion solvent evaporation method. The percent EE and DL was calculated as 63.23 (± 2.31) and 10.53 (± 0.38), respectively (n = 3) (Table 2).
Table 2. . Characterization of dexamethasone-encapsulated-pentablock-copolymer-nanoparticles.
| Polymer | Particle size (nm) (n = 3) | Entrapment efficiency (% w/w) (n = 3) | Loading (% w/w) (n = 3) |
|---|---|---|---|
| PB | 109 ± 3.77 | 63.23 ± 2.31 | 10.53 ± 0.38 |
PB: Pentablock.
In vitro DEX release study from DEX-PB-NPs
The release study was performed by suspending 1 mg of DEX equivalent PB-NPs in PBS at 37°C and sampling from the dialysis chamber. Burst release (20%) in 2 days has been observed due to surface bound drug of NPs. Cumulative %DEX released versus time profile is illustrated in Figure 3B. DEX release from the PB-NPs was continuously over 3 months. About 50% of DEX was released within 6 weeks, which appears to be responsible for interactions with PGA chains resulting in a relatively faster release pattern. A biphasic release pattern of DEX release was evident from NPs with an initial burst release, followed by a sustained release phase. DEX demonstrated slow release rate from NP because of the hydrophobicity and low crystallinity of PB copolymer. Hence, PB copolymer-based NPs may be considered more effective relative to existing PLGA and other polymers-based system. An advantage associated with this sustained release formulation (DEX loaded NPs) offers higher drug residence at the site of absorption.
In vitro tolerability of PB copolymer & PB-NP on ocular cell lines
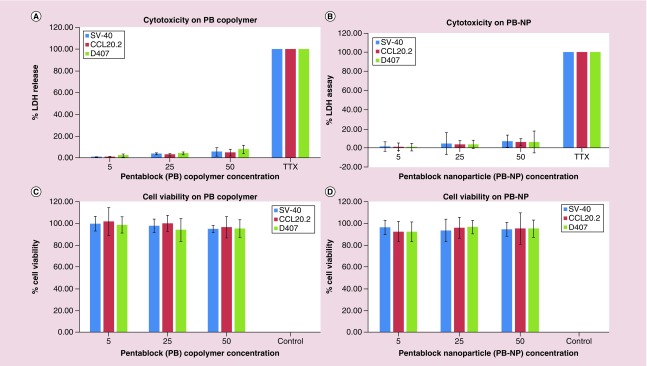
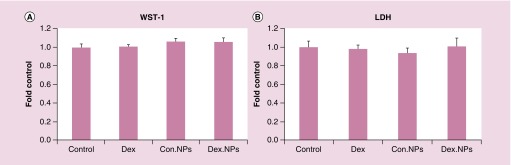
In order to investigate the toxicity of PB copolymer and PB-NPs with the biological system, transformed ocular cell lines (SV-40, CCL20.2 and D407 cells) were treated with 5, 25 and 50 mg/ml of PB copolymer and PB-NPs for 48 h. Primary cultures of HTM cells were treated with 1 mg/ml of PB copolymer and DEX-PB-NPs for 12 weeks. LDH is a cytoplasmic enzyme, secreted in cell culture medium following cell-membrane damage. Estimation of LDH concentration in the culture supernatant was used to provide PB copolymer toxicity information. Less than 10% of LDH release was observed after 48 h and 12 weeks exposure, indicating negligible toxicity (Figures 4 & 7A). Noticeably, results were comparable with negative controls.
Figure 4. . In vitro cytotoxicity and cell viability assays.
In vitro cytotoxicity assay (LDH) of (A) pentablock (PB) copolymers; (B) blank PB-NPs at different concentrations on D407, SV-40 and CCL20.2 cells. Less than 10% of LDH release was observed after 48 h and 12 weeks exposure, indicating negligible toxicity. In vitro cell viability assay (MTS) of: (C) PB copolymers; (D) blank PB-NPs at different concentrations on D407, SV-40 and CCL20.2 cells. The result demonstrated more than 90% cell viability after 48 hours exposure to PB copolymer.
LDH: Lactate dehydrogenase; MTS: (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2Htetrazolium, inner salt; PB-NP: Pentablock-copolymer-nanoparticle.
Figure 7. . Effect of dexamethasone-encapsulated-pentablock-copolymer-nanoparticle on human trabecular meshwork cytotoxicity over time.
Confluent HTM cells were treated with a single application of Con-NPs or DEX-PB-NPs (1 mg/ml). DEX (39.25 ng/ml) treatment was repeated once/week for four weeks. Cell culture media was replaced with fresh 1% FBS media once/week for 12 weeks. At the end of 12 weeks, cell viability was determined by WST-1 and cytotoxicity was examined by LDH release. The relative cell viability and cytotoxicity from DEX, Con-NPs and DEX-PB-NPs treatments were compared with vehicle controls. The data were summarized from three individual cell strains with four replicates in each strain.
DEX-PB-NP: Dexamethasone-encapsulated-pentablock-copolymer-nanoparticle; FBS: Fetal bovine serum; HTM: Human trabecular meshwork.
To further test the cytotoxicity of copolymers, MTS or WST-1 cell viability assay was performed. The WST-1 assay is a rapid and sensitive colorimetric assay based on the cleavage of the tetrazolium salt WST-1 (4-[3-(4-lodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1.3-benzene disulfonate) by mitochondrial dehydrogenases in viable cells (Roche, Mannheim, Germany). In MTS assay, only metabolically active cells convert tetrazolium compound to formazan. Hence, concentrations of formazan products provide a direct estimation of the cell viability. Results in Figure 4 demonstrated that more than 90% cell viability was observed (for all the cell lines) after 48 h and 12 weeks exposure to PB copolymer and PB-NP (Figure 7B). This suggested an excellent safety profile of PB copolymer and nanoformulation for ocular applications.
MYOC secretion from cultured HTM cells
The expression and secretion of MYOC from primary cultures of HTM cells were robustly enhanced following DEX treatment [27–32]. Our previous work [27] suggeste that MYOC was continually upregulated following prolonged 4 weeks DEX treatment and its expression declined over time in the absence of DEX. Thus, the secretion of MYOC can be used as a surrogate read out of biological activity of DEX released from DEX-PB-NPs. A single application of DEX-PB-NPs (1 mg/ml) or control NPs were given to HTM cells. Cell culture supernatant was collected and replaced with fresh 1% DMEM once/week for 12 weeks. DEX or Control vehicle served as controls to compare MYOC secretion levels by WB, being applied once/week for 4 weeks, and then switched to fresh 1% DMEM media for the final 8 weeks. Four HTM cell strains showed similar MYOC secretion patterns, having robust responses for the entire monitoring period (Figure 5). In contrast, one cell strain only responded over a few weeks (Supplementary Figure 1).
Figure 5. . Dexamethasone-encapsulated-pentablock-copolymer-nanoparticle induced prolonged MYOC secretion from cultured human trabecular meshwork cells.
Representative western blot (WB) images show MYOC secretion over time in response to DEX (39.25 ng/ml), Con-NPs and DEX-PB-NPs treatment. Cells were exposed to NP preparations for entire 12-week period, while only exposed to DEX for first 4 weeks. Rows 1–12 show MYOC protein levels from week 1 to 12. Last row shows beta-actin from the same cells collected at end of 12-week period. n = 5. Four HTM cell strains (HTM120, 126, 134 and 136) showed similar MYOC secretion pattern as representative profile from HTM136 shown.
DEX-PB-NP: Dexamethasone-encapsulated-pentablock-copolymer-nanoparticle; HTM: Human trabecular meshwork; WB: Western blot.
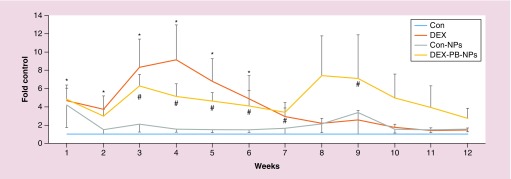
Quantitation of WB data from five HTM cell strains (Figure 6) showed that MYOC increased 5.2 ± 1.3, 7.4 ± 4.3 and 2.8 ± 1.1-fold in 4, 8 and 12 weeks in the presence of DEX-PB-NPs compared with 9.2 ± 3.8, 2.2 ± 0.5 and 1.5 ± 0.3-fold in 4, 8 and 12 weeks in the control DEX treatment group (n = 5). There were significant differences at early time points when DEX group is compared with the control group (*). The Dex-PB-NP group was compared with ghost-NP group (#) (Figure 6) using Mann–Whitney U Test. They did not reach statistical significant difference at later time points in DEX-PB-NPs compared with Con-NPs, primarily due to the unresponsiveness of one cell strain HTM141(out of five cell strains) at later time points. These control data are consistent with our previous results [27] where MYOC from cells treated with DEX were significantly upregulated (>4-fold) within the first 6 weeks and then gradually returned to near baseline levels by the end of 6 weeks. Based on the decline in MYOC levels after withdrawal of DEX from control wells, our data suggested that DEX-PB-NPs releases the biologically active DEX for at least 10 weeks. Interestingly, the first measurement of MYOC levels in Con-NPs-treated groups at week 1 showed a fourfold increase, then dramatically dropped back to near control levels by 2 weeks, where it remained. By comparison, MYOC levels in vehicle treated control wells remained unchanged.
Figure 6. . Quantification of MYOC secretion levels in response to dexamethasone-encapsulated-pentablock-copolymer-nanoparticle over time from human trabecular meshwork cells.
MYOC western blot images from all five HTM cell strains treated with DEX-NPs, Con NPs or DEX (39.25 ng/ml) for 12 time-points for each strain were digitized and quantified using ImageJ software whereby the band intensities were normalized to β-actin level observed for each individual cell strain. Cells were exposed to NP preparations for entire 12 weeks, while cells were only exposed to DEX for first 4 weeks. The relative MYOC secretion levels from DEX, Con-NPs and DEX-PB-NPs were compared with their individual controls at each time point. The combined data represent mean ± SE, n = 5. Symbols (* and #) indicate the significant differences compared with the Control group and control-NP group, respectively, using Mann–Whitney U Test.
DEX-PB-NP: Dexamethasone-encapsulated-pentablock-copolymer-nanoparticle; DEX: Dexamethasone; HTM: Human trabecular meshwork; NP: Nanoparticle.
Modification of HTM cell morphology by the PB copolymer
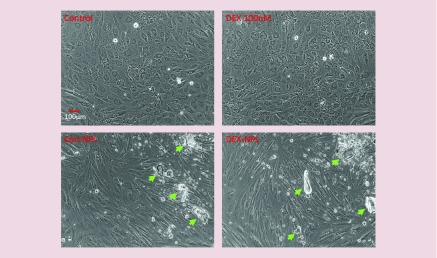
Although not showing any signs of cytotoxicity, the changes in HTM cell morphology were observed in the presence of either NP alone or DEX-PB-NP, becoming more elongated (Figure 8), possibly due to phagocytosis of NPs. A change in morphology was observed as early as 6 weeks and continued up to the last observation at 12 weeks. Interestingly, morphology alterations did not appear to change their MYOC secretion response to DEX released from PB-NPs. In contrast, DEX cells looked similar to vehicle control cells during the 4 weeks of treatment, compared with 12 weeks of Con-NPs. Regardless, changes in morphology were observed in cells treated with Con-NPs alone, suggesting that DEX was not responsible for morphological alterations.
Figure 8. . Modification of human trabecular meshwork cell morphology by nanoparticle polymers but not by dexamethasone.
Confluent HTM cells were treated with single application of Con-NPs or DEX-PB-NPs (1 mg/ml) for entire 12-week observation period. In contrast, DEX (39.25 ng/ml) treatment was repeated once/week for 4 weeks. Cell culture media was replaced with fresh 1% FBS media once/week for 12 weeks. At end of 12 weeks, cell morphology from each treatment was recorded under phase/contrast light microscope with 10× magnifications. n = 5. Arrows indicate aggregated polymers.
DEX-PB-NPs: Dexamethasone-encapsulated-pentablock-copolymer-nanoparticle; FBS: Fetal bovine serum; HTM: Human trabecular meshwork.
Extraction of DEX from cell culture media
The culture media thawed from different cell strains of HTM cells was collected from cells exposed to DEX-PB-NPs once a week. The DEX was then extracted from the collected media to determine the amount of DEX released from the NPs. The extraction efficiency was found to be more than 90% for both DEX and HC. Interestingly, the amount of DEX released from all three strains found approximately equal and detectable for 12 weeks.
Conclusion
In the current study, novel PB copolymer based DEX encapsulated NPs were synthesized and characterized. It was observed that the location of the PCL block in the middle of the PGA and PEG resulted in high EE and DL. However, positioning of the hydrophilic block (PGA) at the terminal resulted in an initial burst release. Regardless, the burst drug release was less than other block polymers or PLGA formulations studied earlier. In addition, the DEX release from the NPs followed diffusion through the polymer matrices or erosion of the polymeric materials due to the hydrolytic degradation of ester linkages. A possible explanation of enhanced drug release is due to conjugation of PGA to PCL which alters the crystallinity of the PB copolymer. PB copolymer is a semi-crystalline material which provided a longer DEX release owing to its slow degradation. Additionally, the longer DEX release from the PB-NP may be due to high MW of the PB copolymer which required a longer time for degradation.
The activity of a novel PB-NP loaded with DEX was examined by the in vitro cell culture system. The MYOC secretion levels in long-term treatment of HTM cells using DEX-PB-NPs were analyzed by WB. With a single application of the DEX-PB-NPs to cultured HTM cells, the robust upregulation of MYOC secretion from HTM cells was detected and maintained at least over 12 weeks. Consistent with our previous study, HTM cells from different donors can vary in response to DEX treatment [27]. Out of five HTM cell strains studied, four of them showed a consistent MYOC increase over the first 6 weeks (4 weeks of treatment). The level of MYOC in the media from these four cell strains decreased afterwards, but was still significantly higher than control levels 7 weeks later. Only one cell strain (HTM141) showed an increase in MYOC secretion in response to DEX-PB-NPs for only 3 weeks before equal to or below control levels for the remainder of the study (Supplementary Figure 1). The varied response to polymer treatments in different cell strains indicated that the polymers should be examined on various strains from multiple donors before the in vivo studies.
The individual in response to DEX treatment in the clinic varies; about 40% of non-glaucomatous individuals are steroid responders. The exact mechanism for the steroids-induced IOP elevation is uncertain, but due to its time course likely involves at least two cellular processes in the resistance-generation region of the conventional outflow pathway: increased barrier function of the inner wall of Schlemm's canal and/or alterations in cell contractility and/or extracellular matrix turnover in the TM [33]. Resident TM cells are responsible for maintenance of its unique architecture and their extracellular matrix constituents. The differential response of individual HTM cell strains to DEX and DEX-PB-NPs treatment in our study may partially explained the important role of TM cells in steroids-induced ocular hypertension. For these reasons, it is important that multiple cell strains are tested. Although the PB copolymer did not display any sign of cytotoxicity to HTM cells in this long-term study, it did modify the HTM cell morphology. HTM cell elongation was present in all strains following Con-NPs and DEX-PB-NPs treatment. Morphological modification of HTM cells by the polymers may accompany functional changes that could not be measured in the present study, but should be further investigated. In addition, MYOC elevation was also observed in three HTM cell strains with Con-NPs treatment during the first week of the treatment. Therefore, the safety of the polymer must be further ascertained.
This study provided evidence that the developed in vitro system is a valuable tool for analyzing the safety of polymers as well as biological effects on steroid release from the polymers. Although, the in vitro release condition was somewhat different compared with DEX release study with HTM cell strains, an approximately equal amounts of DEX released into the culture media was observed. Under both in vitro conditions, DEX was released for a longer period of time. Although, a slow and long-term (>1 month) DEX release was detected in the current study, an early burst release phenomenon still occurred with PB copolymer. It may be acceptable in some of the in vivo studies, but limit its applications especially in clinic. Our next goal is to develop the PB copolymers with different MWs, ratios, and arrangements so that the therapeutic agents could be released in more controlled release fashion without initial burst release. At the same time the PB copolymers must be biocompatible, biodegradable and should not modify the cellular functions and morphology.
Summary points.
Pentablock copolymer synthesis & characterization
Novel pentablock (PB) copolymer, poly (glycolic acid)-poly (caprolactone)-poly (ethylene glycol)-poly (caprolactone)-poly (glycolic acid) (PGA-PCL-PEG-PCL-PGA) was synthesized in two steps by sequential ring-opening bulk copolymerization reaction.
1H-NMR peaks comprised of glycolic acid displayed a series of singlets between 4.6 and 4.9 ppm confirming methylene protons of PGA block.
PB copolymers depicted a single peak in gel permeation chromatography chromatogram indicating mono distribution of molecular weight with polydispersity index was below 1.5.
X-ray diffraction patterns of copolymer indicated that PCL blocks have retained semicrystalline structure, even after covalent conjugation with PGA blocks.
Dexamethasone nanoparticle formulation development, characterization & in vitro release
Dexamethasone (DEX)-loaded PB copolymer based nanoparticles (NPs) were prepared with oil in water single emulsion solvent evaporation method. The percent entrapment efficiency and drug loading were determined as 63.23 ± 2.31 and 10.53 ± 0.38%w/w, respectively. The particle size of NPs was approximately 109 ± 3.77 nm, analyzed by nanoparticle tracking analysis.
Long-term DEX release from the PB-NPs was found continuous over 3 months. A biphasic release pattern of DEX release was evident from NPs with an initial burst release, followed by a sustained release phase.
In vitro cytotoxicity & cell viability of dexamethasone-encapsulated-pentablock-copolymer-nanoparticles
The cytotoxic effects of PB copolymers on corneal (SV-40; Human Corneal Epithelial Cell), conjunctival (CCL20.2; Human Conjunctival Epithelial Cell/Chang's Conjunctival Cell Line), retinal (D407; Human Retinal Pigment Epithelium Cell) and trabecular meshwork (HTM) cell lines, indicating negligible toxicity. In addition, more than 90% cell viability (for all the cell lines) after 48 h and 12-week exposure to PB copolymer and PB-NPs suggested excellent safety profiles.
MYOC elevation: quantitative analysis by western blot
Five strains of human trabecular meshwork cells (HTM120, 136, 126, 134 and 141) were isolated from eyes of human donors of ages11- and 3-month old (HTM120 and 136), 88-, 51- and 38-year old (HTM126, 134 and 141), respectively, and treated with dexamethasone-encapsulated-pentablock-copolymer-nanoparticles (DEX-PB-NPs). Expression and secretion of MYOC from primary cultures of trabecular meshwork cells are robustly enhanced following DEX treatment and quantitatively analyzed by western blot.
Based on the decline in MYOC levels after withdrawal of DEX from control wells, our data suggested that DEX-PB-NPs release biologically active DEX for at least 10 weeks. Interestingly, the first measurement of MYOC levels in Con-NPs-treated groups at week 1 showed fourfold increase, then dramatically dropped back to near control levels by 2 weeks, where it remained. By comparison, MYOC levels in vehicle treated control wells remain unchanged.
Conclusion & future prospective
Activity and safety over time in ocular cell culture were examined using primary cultures of human trabecular meshwork cells.
The developed system is a valuable and novel tool for determining the safety and effects of steroids released from polymeric NPs.
Our next goal is to develop the PB copolymers with different molecular weights, ratios and arrangements to provide the controlled zero-rate release without initial burst release.
PB copolymer did not show any sign of cytotoxicity to HTM cells in this long-term study, it did modify the HTM cell morphology. HTM cell elongation was present in all cell strains after both Con-NPs and DEX-PB-NPs treatment. Morphological modification of HTM cells by the polymers may accompany functional changes that were not measured in the present study, but should be further investigated.
This approach will be followed to generate an animal model for corticosteroid induced ocular hypertension. The developed system is a valuable and novel tool for determining the safety and effects of steroids released from polymeric NPs.
Supplementary Material
Acknowledgements
The authors are thankful to J Murowchick (Department of GeoSciences, UMKC) for helping in PXRD analysis, W-T Hung and LK Christenson (Department of Molecular and Integrative Physiology, University of Kansas Medical Center). D407 cells were generous gifts from R Hunt (University of South Carolina, SC, USA).
Footnotes
Financial & competing interests disclosure
This study is supported by the BrightFocus Foundation (G2015100) and NIH R01 EY09171–14. The authors are thankful to the School of Graduate Studies (SGS), UMKC Research Grant and Graduate Assistant Fund (GAF), UMKC Women's Council for providing the financial support. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Agrahari V, Agrahari V, Mandal A, Pal D, Mitra AK. How are we improving the delivery to back of the eye? Advances and challenges of novel therapeutic approaches. Expert Opin. Drug Deliv. 2016 doi: 10.1080/17425247.2017.1272569. Epub ahead or print. [DOI] [PubMed] [Google Scholar]; •• Advances in the development of several nanotechnology-based approaches to enhance drug bioavailability and overcome existing barriers for effective ocular delivery to posterior segment eye diseases are discussed.
- 2.Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine. 2017;12(6):683–702. doi: 10.2217/nnm-2016-0379. [DOI] [PubMed] [Google Scholar]
- 3.Bian F, Pelegrino FS, Henriksson JT, et al. Differential effects of dexamethasone and doxycycline on inflammation and MMP production in murine alkali-burned corneas associated with dry eye. Ocul. Surf. 2016;14(2):242–254. doi: 10.1016/j.jtos.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quentin CD, Behrens-Baumann W. [Double-blind study of the effectiveness of the prostaglandin synthesis inhibitor diclofenac and dexamethasone phosphate in the treatment of iritis after local administration] Fortschr. Ophthalmol. 1987;84(4):353–355. [PubMed] [Google Scholar]
- 5.Coassin M, Iovieno A, Soldani A, et al. Bromfenac ophthalmic solution 0.09% as an adjunctive therapy to topical steroids after cataract surgery in pseudoexfoliation syndrome. J. Cataract Refract. Surg. 2016;42(8):1119–1125. doi: 10.1016/j.jcrs.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Narayanan R, Kuppermann BD. Corticosteroids and anti-complement therapy in retinal diseases. Handb. Exp. Pharmacol. 2017;242:309–320. doi: 10.1007/164_2016_22. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz SG, Scott IU, Stewart MW, Flynn HW., Jr Update on corticosteroids for diabetic macular edema. Clin. Ophthalmol. 2016;10:1723–1730. doi: 10.2147/OPTH.S115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haller JA, Bandello F, Belfort R, Jr, et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118(12):2453–2460. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Tsang AC, Virgili G, Abtahi M, Gottlieb CC. Intravitreal dexamethasone implant for the treatment of macular edema in chronic non-infectious uveitis. Ocul. Immunol. Inflamm. 2016 doi: 10.3109/09273948.2016.1160130. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Brady ME, Sartiano GP, Rosenblum SL, Zaglama NE, Bauguess CT. The pharmacokinetics of single high doses of dexamethasone in cancer patients. Eur. J. Clin. Pharmacol. 1987;32(6):593–596. doi: 10.1007/BF02455994. [DOI] [PubMed] [Google Scholar]
- 11.Balzus B, Feleke Sahle F, Honzke S, et al. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epithelium. Eur. J. Pharm. Biopharm. 2017;115:122–130. doi: 10.1016/j.ejpb.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Gerecke C, Edlich A, Giulbudagian M, et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug delivery systems and their intracellular localization in keratinocytes. Nanotoxicology. 2017;11(2):267–277. doi: 10.1080/17435390.2017.1292371. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Trinh HM, Agrahari V, Sheng Y, Pal D, Mitra AK. Nanoparticle-based topical ophthalmic gel formulation for sustained release of hydrocortisone butyrate. AAPS PharmSciTech. 2016;17(2):294–306. doi: 10.1208/s12249-015-0354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boddu SH, Jwala J, Vaishya R, et al. Novel nanoparticulate gel formulations of steroids for the treatment of macular edema. J. Ocul. Pharmacol. Ther. 2010;26(1):37–48. doi: 10.1089/jop.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaishya RD, Gokulgandhi M, Patel S, Minocha M, Mitra AK. Novel dexamethasone-loaded nanomicelles for the intermediate and posterior segment uveitis. AAPS PharmSciTech. 2014;15(5):1238–1251. doi: 10.1208/s12249-014-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Chen J, Li B, et al. PLGA-PEG-PLGA triblock copolymeric micelles as oral drug delivery system: In vitro drug release and in vivo pharmacokinetics assessment. J. Colloid Interface Sci. 2017;490:542–552. doi: 10.1016/j.jcis.2016.11.089. [DOI] [PubMed] [Google Scholar]
- 17.Calucci L, Forte C, Buwalda SJ, Dijkstra PJ, Feijen J. Self-aggregation of gel forming PEG-PLA star block copolymers in water. Langmuir. 2010;26(15):12890–12896. doi: 10.1021/la101613b. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Li S, Coumes F, Darcos V, Lai Kee Him J, Bron P. Modeling and self-assembly behavior of PEG-PLA-PEG triblock copolymers in aqueous solution. Nanoscale. 2013;5(19):9010–9017. doi: 10.1039/c3nr02899b. [DOI] [PubMed] [Google Scholar]
- 19.Loftsson T, Hreinsdottir D, Stefansson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007;59(5):629–635. doi: 10.1211/jpp.59.5.0002. [DOI] [PubMed] [Google Scholar]
- 20.Ebrahim S, Peyman GA, Lee PJ. Applications of liposomes in ophthalmology. Surv. Ophthalmol. 2005;50(2):167–182. doi: 10.1016/j.survophthal.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Sudhalkar A, Chhablani J, Vasavada A, et al. Intravitreal dexamethasone implant for recurrent cystoid macular edema due to Irvine-Gass syndrome: a prospective case series. Eye. 2016;30(12):1549–1557. doi: 10.1038/eye.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agrahari V, Agrahari V, Hung WT, Christenson LK, Mitra AK. Composite nanoformulation therapeutics for long-term ocular delivery of macromolecules. Mol. Pharm. 2016;13(9):2912–2922. doi: 10.1021/acs.molpharmaceut.5b00828. [DOI] [PubMed] [Google Scholar]; •• The development of composite nanoformulations and their application in long-term delivery of macromolecule drugs are described.
- 23.Patel SP, Vaishya R, Patel A, Agrahari V, Pal D, Mitra AK. Optimization of novel pentablock copolymer based composite formulation for sustained delivery of peptide/protein in the treatment of ocular diseases. J. Microencapsul. 2016;33(2):103–113. doi: 10.3109/02652048.2015.1134685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrahari V, Zhang C, Zhang T, et al. Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro . AAPS J. 2014;16(2):181–193. doi: 10.1208/s12248-013-9546-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr. Eye Res. 2000;20(5):347–350. [PubMed] [Google Scholar]
- 26.Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr. Eye Res. 1995;14(7):611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]; • This study has modified an extracellular matrix digestion technique for the isolation of microvascular endothelial cells to isolate human trabecular meshwork cells. The procedure was both efficient and rapid for isolating large numbers of trabecular meshwork cells and resulted in the availability of trabecular meshwork cells in sufficient quantities for subsequent experimentation.
- 27.Li G, Cui G, Dismuke WM, et al. Differential response and withdrawal profile of glucocorticoid-treated human trabecular meshwork cells. Exp. Eye Res. 2016;155:38–46. doi: 10.1016/j.exer.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Examined the secreted protein response and withdrawal profiles from cultured human trabecular meshwork cells following short- and long-term glucocorticoid treatment.
- 28.Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008;86(4):543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Changes in extracellular matrix associated with open-angle glaucoma have been identified.
- 29.Chuang TD, Pearce WJ, Khorram O. miR-29c induction contributes to downregulation of vascular extracellular matrix proteins by glucocorticoids. Am. J. Physiol. Cell Physiol. 2015;309(2):C117–C125. doi: 10.1152/ajpcell.00254.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 1998;273(11):6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 31.Stamer WD, Hoffman EA, Kurali E, Krauss AH. Unique response profile of trabecular meshwork cells to the novel selective glucocorticoid receptor agonist, GW870086X. Invest. Ophthalmol. Vis. Sci. 2013;54(3):2100–2107. doi: 10.1167/iovs.12-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Evaluated a novel selective GC receptor agonist (SEGRA), GW870086X, in different in vitro models of the human conventional outflow pathway.
- 32.Wordinger RJ, Fleenor DL, Hellberg PE, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest. Ophthalmol. Vis. Sci. 2007;48(3):1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 33.Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp. Eye Res. 2009;88:752–759. doi: 10.1016/j.exer.2008.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.