Despite the lack of nuclei and regulated transcription, platelets actively participate in multiple physiological processes, including hemostasis and immunity. Li et al. discuss aspects of platelet design that optimize its functions and argue that platelets may be best conceived as automated, fully equipped surveillance vehicles.
Abstract
Platelets participate in many important physiological processes, including hemostasis and immunity. However, despite their broad participation in these evolutionarily critical roles, the anucleate platelet is uniquely mammalian. In contrast with the large nucleated equivalents in lower vertebrates, we find that the design template for the evolutionary specialization of platelets shares remarkable similarities with human-engineered unmanned aerial vehicles in terms of overall autonomy, maneuverability, and expendability. Here, we review evidence illustrating how platelets are uniquely suited for surveillance and the manner in which they consequently provide various types of support to other cell types.
Introduction
In mammals, platelets are small, anucleate circulating elements best known for their capacity to rapidly aggregate and prevent blood loss during trauma. In the 19th century, the visionary pioneers Schulze and Bizzozero were rapidly drawn to the study of platelets in hemostasis given the efficiency and prominence of the process. Decades of continued studies have identified many of the components and mechanisms that make platelets so sensitive to stimulation but, at the same time, have recognized the many ways in which their uncontrolled activation compromises vascular integrity, as seen in several of the most prevalent and deadly syndromes, from strokes and heart attacks to venous thromboses. These early studies found comforting consistency between the “passive” formation of blood clots and the lack of “transcriptional intelligence” in platelets.
Many decades after these observations were made, however, researchers began to notice striking correlations between platelet numbers and activation states with the onset of immune and inflammatory responses. Further studies discovered that platelet contribution extends to angiogenic and developmental processes, to the direct killing of microorganisms, and even to tumor metastasis. Thus, although hemostasis remains their best characterized function, we now know that platelets are used for many additional tasks in the organism. It follows that the vast array of proteins and transcriptional and translational machinery left within them might have largely unknown purposes. In this review, we focus on the seemingly contradictory well-orchestrated, multitasking functions of platelets and their lack of regulated transcription. We discuss here aspects of platelet biology not usually described in textbooks and other recent reviews, specifically how platelets appear to be designed for their hemostatic and immune functions. We argue that platelets may be best conceived as automated, fully equipped vehicles in which trade-offs were made during evolution to enhance their surveillance and effector functions.
Analogy between platelets and drones
Platelet evolution in mammals and equivalents in other vertebrates
In lower vertebrates such as birds, reptiles, amphibians, and fish, hemostatic functions are generally performed by large, nucleated thrombocytes (Claver and Quaglia, 2009) that also carry out important immune processes such as phagocytosis (Nagasawa et al., 2014). These cells are widely regarded as the functional equivalents of mammalian platelets and may be evolutionarily related. Even in nonvertebrate arthropods, coagulation usually involves nucleated cells (e.g., coagulocytes in insects; Theopold et al., 2004). The most obvious morphological difference between the mammalian platelet and the nonmammalian thrombocyte is the lack of a nucleus in the platelet. As we have learned from textbooks, the eukaryotic cell, as opposed to the prokaryote, is defined largely by the presence of the genome-containing nucleus that directs the whole organization of the cell. Thus, the lack of a nucleus in the platelet, together with other factors, such as its humble size and production method, has led to controversy over its formal recognition as a cell (Garraud and Cognasse, 2015). Although platelets have traditionally been termed “cell fragments,” which misleadingly implies a passivity and nonliving status, they are now increasingly referred to as “anucleate cells.”
As we shall discuss later, the absence of the nucleus in the platelet is a profound change that accords novel advantageous capabilities in a trade-off against the associated disadvantages. For the purpose of our discussion, it might be instructive to compare platelets with another cell type that has not attracted as much controversy: the hemoglobin-rich, oxygen-carrying erythrocyte. Although we may be used to thinking that erythrocytes eventually extrude their nuclei upon maturity (i.e., enucleation) on the basis of our understanding of mammalian biology, this is actually not the case for all species. In fact, most nonmammals retain nucleated erythrocytes, and enucleated erythrocytes are the exceptions rather than the rule. Despite this, among the salamander family Plethodontidae, a few interesting species were discovered to possess enucleated erythrocytes, whereas the others retained nuclei in their erythrocytes. The commonalities shared by these salamanders with enucleated erythrocytes were their larger genome sizes and greatly reduced body sizes, relative to the other salamanders. Phylogenetic comparisons among various salamander species demonstrated positive direct correlations among genome size, nucleus size, and erythrocyte cell size in both enucleated and nucleated species (Mueller et al., 2008). Therefore, it appeared that the greater the DNA content, the more space the nuclei had to occupy, and the larger the erythrocytes had to be. From this study, a logical conjecture was that getting rid of the bulky nucleus afforded the salamander erythrocytes an evolutionary advantage in maneuverability, consistent with the selection pressures on these salamanders toward petite body sizes.
Interesting insights on platelet design can be drawn by comparing the clotting efficiency among different species, for example, between birds and mammals (Schmaier et al., 2011). Although thrombotic cardiovascular diseases such as stroke and heart attacks occur often in humans and can be experimentally induced in mammals, birds have not been reported to suffer from the same. Schmaier et al. (2011) found that although both platelets in mice and thrombocytes in birds reacted similarly to activating stimuli, thrombocytes were less efficient in spreading on collagen surfaces and expressed fewer adhesion molecules on their surfaces, and the aggregates formed were unable to resist the shear forces of arterial blood. Consequently, birds do not form arterial thromboses but may be less efficient at hemostasis than mammals. On the other hand, in lower arthropods such as insects, which have virtually zero risk for thrombosis thanks to their open hemolymph systems (i.e., tissues are directly in contact with hemolymph), enzymatic hemostasis mechanisms, analogous to mammalian thrombin-fibrin cascades, create aggregates that are directly held by strong covalent bonds, unlike the comparatively weak protein-protein interactions in vertebrates (Theopold et al., 2004). Thus, platelet design may be influenced by the differing selection pressures in the different clades, with the mammals favoring effective hemostasis over thrombosis risks.
Design similarities with unmanned aerial vehicles (UAVs)
A popular adage in biology is that “form follows function.” From our previous erythrocyte example, it would seem that removing nuclei from cells that do not require full access to the genome for their functions is efficient and logical. However, platelets perform functions much more complex than erythrocytes and, as we describe below, appear to have many similarities with human-engineered UAVs, or “drones” in common parlance (see Table 1).
Table 1. Similarities between the UAV and the platelet.
| UAV | Platelet |
|---|---|
| Definition | |
| Militarya: “A powered, aerial vehicle that does not carry a human operator, uses aerodynamic forces to provide vehicle lift, can fly autonomously or be piloted remotely, can be expendable or recoverable, and can carry a lethal or nonlethal payload.”b | Medicalc: “A minute, non-nucleated, disk-like cytoplasmic body found in the blood plasma of mammals that is derived from a megakaryocyte and functions to promote blood clotting.”d |
| Overall autonomy | |
| Physical location of “intelligence” | |
| Does not carry a human operator.e | Does not contain a nucleus. |
| Sensors | |
| Radars, sonars, cameras, temperature, etc. | Multitude of surface and internal receptors. |
| Maneuverability | |
| Size | |
| Smaller than conventional aircraft. No space considerations necessary for human operator. | Diameter 2–4 µm. Small size possible because of the lack of a nucleus, which spans at least 5 µm. |
| Access | |
| Provides wide coverage over large areas. Small size provides access to spaces not available to conventional aircraft. | Full coverage of the vascular system. Upon activation, chemotaxis and transmigration has been proposed.f Small size permits rapid access to even the narrowest capillaries. |
| Expendability | |
| Cost-effectiveness | |
| Significant cost and space savings when human operator is left out of its design (e.g., oxygen tanks, cockpits, canopy, interfaces, temperature controls), resulting in higher payload–to–dead weight ratios. | Does not require space or other resources otherwise dedicated for maintaining the nucleus (e.g., nucleotides, phospholipids, nuclear transporters, DNA repair enzymes). |
| Production | |
| Higher maximum production rate than with conventional aircraft. Can be mass-produced on factory lines. | Large quantities of platelets can be mass-produced by megakaryocytes.g Benefits from economy of scale. |
| Payloads | |
| Carries lethal or nonlethal payloads. | Carries molecules with cytotoxic, proinflammatory, or thrombotic functions that may harm both pathogens and tissues as well as nontoxic bioactive mediators that mediate other functions, such as intercellular communication. |
| Functions (during conflicts) | |
| Surveillance and reconnaissance, weapon strikes, delivery of ammunition and supplies. | Detection of pathogens/breached vasculature and signaling for leukocyte activation and/or recruitment; direct pathogen killing/thrombosis; delivery of support proinflammatory/thrombotic factors, as well as angiogenic and growth factors. |
Dictionary of Military and Associated Terms, US Department of Defense, 2005.
Reflects design concerns.
The American Heritage Medical Dictionary, © 2007, 2004 by Houghton Mifflin Company.
Medical definitions tend to reflect the historical bias of the platelet’s hemostatic function.
Defining trait of UAV versus conventional aircraft.
Pitchford, S.C., S. Momi, S. Baglioni, L. Casali, S. Giannini, R. Rossi, C.P. Page, and P. Gresele. 2008. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am. J. Respir. Crit. Care. Med. 177:604–612.
Estimated at 2,000–4,000 platelets per megakaryocyte.
Trade-offs associated with platelet design
In a similar fashion to the UAV concept, nuclei removal allows platelets to reach sizes much smaller than traditional cells, which grants considerable gains to their expendability and maneuverability. However, a secondary outcome of their humble size is that platelets forgo much of their phagocytic ability, a capability robustly demonstrated by thrombocytes but now mostly taken over by other larger specialized leukocytes. Platelets have high surface-to-volume ratios by virtue of their small sizes, and this ratio is increased even further by their adoption of the unique open canaliculi system (OCS), in which their surface membranes invaginate extensively inward into the platelet body, taking full advantage of the lack of central nuclei. These form a network of narrow channels whereby cell membranes come into contact with the blood plasma, thereby providing a large surface area for platelets to interact with their environment. Attempts to engulf cells or particles larger than themselves are unlikely to be effective, and although possible (e.g., bacteria), the amount of cytoplasm available for the phagolysosome formation would be very limited. A measure of “phagocytic” ability is retained, however, as platelets have been shown to unfold their OCS to incompletely wrap and immobilize bacteria (White, 2005).
A downside of platelets’ lacking nuclei is their reduced autonomy, as having access to the full genome may have been beneficial in certain situations. For example, because platelets contain ribosomes and are fully capable of translating mRNA, they can become highly attractive targets of infection by viruses, especially single-stranded RNA viruses that do not require DNA in their reproduction cycle. The classic antiviral type I interferon responses, which are activated largely at the transcription factor level in the nucleus, are unavailable to platelets. In fact, it was shown that the dengue virus, a well-known single-stranded RNA virus of the Flaviviridae family, can actively infect platelets and hijack their machinery to produce fully active virions (Simon et al., 2015). In this case, the only reasonable defense may be to halt platelet production altogether: megakaryocytes greatly reduce platelet production in response to type I interferons, leading to thrombocytopenia (Wadenvik et al., 1991; Rivadeneyra et al., 2015). It is perhaps no coincidence, then, that the dengue virus is notorious for its ability to cause the dreaded life-threatening dengue hemorrhagic fever, in which bleeding and blood plasma leakage accompany extreme thrombocytopenia.
Other than the obvious reductions in energy and material production costs attributed directly to forgoing the nuclei, another major reason why platelets become so cost effective lies in their production mechanism. Megakaryocytes in the bone marrow undergo multiple rounds of programmed endomitosis, eventually forming large polyploid cells (4N to 64N; Foudi et al., 2014) about 50–100 µm in diameter, followed by the mass production of platelet components in the cytoplasm. This process avoids the expensive energy costs associated with nuclei segregation and occurs rarely in adult mammals, although hepatocytes and certain muscle cell types also undergo polyploidy at some point in their lifespan (Zimmet and Ravid, 2000). Megakaryocytes then form cytoplasmic extensions (termed proplatelets) that extend through the bone marrow into the sinusoids, and directed microtubule transport allocates platelet proteins into the tips to form cytoplasm fragments like “beads on a chain” that eventually break off to form individual preplatelets (Machlus and Italiano, 2013). Finally, preplatelets fragment further in the circulation to form individual platelets. This platelet production strategy in megakaryocytes is made possible only because nuclei have been omitted from the platelet design, allowing very large numbers of individual platelets to be produced rapidly and efficiently; a single microliter of blood typically contains at least a few hundred thousand platelets, with the turnover rate estimated at 1011 per day in humans. Interestingly, the demands of the process seem to be relatively flexible, as recent work demonstrated that part of this process also occurs in the lung (accounting for up to 50% of total daily platelet turnover; Lefrançais et al., 2017).
Environmental and danger sensing by platelets
Platelets as surveillance drones
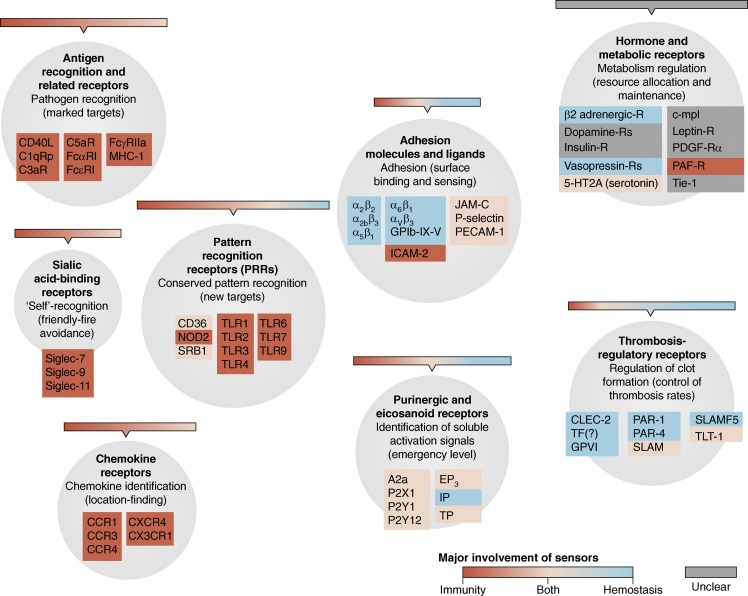
Their ubiquity and high maneuverability enable platelets to obtain virtually full coverage of the entire vascular system at any given point in time. Combined with their expendability, platelets possess all the characteristics to act as the ideal surveillance automatons. Indeed, for such a small package, platelets contain a surprisingly large array of detectors for monitoring threats, augmented by their large OCS surfaces available for environmental interaction. To give readers a sense of the receptors present on platelets, a nonexhaustive list of the types of known receptors is presented in Fig. 1, and their associated ligands are additionally described in Table S1. Because we are not able to fully explore this exciting topic in full detail, we instead refer readers to excellent and dedicated reviews on platelet receptors cited here (Clemetson and Clemetson, 2013; Saboor et al., 2013; Cognasse et al., 2015).
Figure 1.
Platelet receptors. List of receptors in human platelets categorized by their major functional types. The areas of circles correspond proportionally to the number of members shown here. The major involvement of each molecule in hemostasis, immunity, or both is color-coded. Receptors of unclear contributions to hemostasis or immunity are shown in gray. Examples of the corresponding ligands of these receptors are listed in Table S1.
Hemostatic sensing
For detecting breaches of vascular integrity, platelets express several cell surface receptors that not only perform cell signaling but often double as adhesion molecules. Normally, the luminal walls of the blood vessels are coated by protective glycocalyx layers that prevent platelets from coming into direct contact with the endothelium or the basal layers. Any exposure to the structural components of the basal layers would signify that a breach has occurred and thus trigger platelet activation and clot formation to physically seal the gaps. For this purpose, platelets express several receptors that can detect these as well as other components of the coagulation cascade. For example, platelet integrin α2β1 (GPIa-IIa, CD49b/CD29) can bind collagen, while activated αIIbβ3 (GPIIb-IIIa, CD41/CD61) binds fibrinogen. Other activation receptors may also detect soluble markers of damage, the most important being the purinergic receptors P2X1, P2Y1, and P2Y12, which detect ADP and ATP released from damaged cells (Eltzschig et al., 2012).
It is increasingly being appreciated that platelets integrate biophysical cues from the environment. For example, platelets may adjust their activation and apoptotic status based on their mechanosensing of the stiffness of the underlying matrix (Qiu et al., 2014; Kee et al., 2015). Conversely, they may also adjust their membrane stiffness in response to their activation status (Nguyen et al., 2016). Platelets can additionally sense shear flow rates such that diminishing shear rates can signal the appropriate timing to undergo clot retraction (Muthard and Diamond, 2012). GPIb-IX, the major platelet receptor for the von Willebrand factor (VWF), has been implicated as a major receptor for the mechanotransduction of shear forces (Deng et al., 2016; Ju et al., 2016).
Although the exact mechanisms remain unclear, it also appears that platelets can respond to changes in temperature. For example, in vitro experiments indicated that platelet exposure to elevated temperatures (>42°C) could increase platelet aggregation responses to ADP, and the effect persisted even after cooling to normal body temperature (Gader et al., 1990). In the opposite example, platelet exposure to brief periods of low temperatures (0–4°C) can induce the “cold storage lesion” phenomenon. The phenomenon is so termed because it was discovered early on that platelets isolated from blood donors underwent shape changes and had drastically reduced life spans from 8 to 3 days in transfusion recipients, if they were stored by refrigeration, compared with those kept at room temperature (Rumjantseva and Hoffmeister, 2010), even though the platelets were warmed up to body temperature before transfusion. Therefore, counterintuitively, many current hospital guidelines recommend room temperature storage of platelet products despite the increased risk of bacterial growth. An explanation for this phenomenon is that the exposure of platelets to low temperatures leads to the irreversible clustering of surface GPIb receptors, resulting in the recognition of clustered β-N-acetylglucosamine residues by β2 integrins on hepatic Kupffer cells. During extended cold storage, desialylation further leads to the exposure of galactose residues, which are recognized by Ashwell-Morrell receptors of hepatocytes that also perform platelet clearance (Rumjantseva et al., 2009). In any case, platelets appear responsive to temperature fluctuations, and although both of the phenomena described here were demonstrated with nonphysiological extremes, it is tempting to speculate that they represent in-built mechanisms for the promotion or prevention of thrombosis.
Immune sensing
Previously, the prevailing view was that platelets had few, if any, roles in immunity. This started changing, however, when it became apparent that platelets express a plethora of immune-related receptors and ligands (Morrell et al., 2014). About two decades ago, CD40L (CD154), an important ligand used by T cells to activate B cells, was one of the earliest such “purely immune” markers to be discovered on platelets (Henn et al., 1998; Blumberg et al., 2009). Since then, many other molecules present on platelets were also discovered to play important roles in immunity, including integrins and their ligands, P-selectin, Toll-like receptors (e.g., TLR4), scavenger receptors, Siglecs, complement receptors, and immunoglobulin receptors such as FcεRI and FcγRIIa in human (but not mice) platelets, among others.
Platelet payload
Major modes of storage
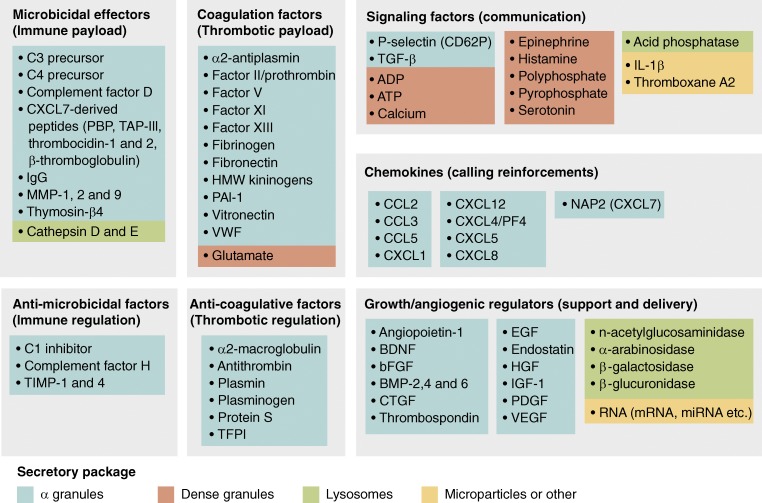
Platelets compartmentalize their payloads (Fig. 2) into a few main types of secretory packages, namely, the α granules, dense granules, and lysosomes (Blair and Flaumenhaft, 2009). They can also deliver cargo via platelet microparticles (Hargett and Bauer, 2013). Platelet secretory contents include not only those that are presynthesized by megakaryocytes and stored in granules, such as PF4, but also those that may be synthesized de novo from preexisting mRNA, as in the case of IL-1b. In addition, platelets also actively undergo receptor-mediated endocytosis to incorporate and concentrate certain proteins from the blood plasma into their granules, as reported for fibrinogen, which is sequestered using αIIbβ3. Proteomic studies have estimated that platelet releasates contain at least 300 distinct mediators (Pagel et al., 2017), while genomic analyses of the platelets identified approximately 9,500 mRNA reads with known protein-coding loci (Rowley et al., 2011; Bray et al., 2013; Schubert et al., 2014), not inclusive of numerous noncoding RNA types.
Figure 2.
Platelet payloads. List of bioactive mediators released by human platelets categorized by their major functional roles. Many of these mediators play multiple roles but are categorized only once. CXCL7 is unique among chemokines in that it is cleaved into multiple distinct peptides with varying functions.
The platelet arsenal
α granules are the most abundant type, with 50–80 per platelet, accounting for 10% of the platelet volume, 10 times more than the dense granules (Blair and Flaumenhaft, 2009). α granules contain most of the protein mediators and are endowed with diverse roles, such as clotting factors (e.g., factor V, VWF, fibrinogen), chemokines (e.g., PF4, CXCL7), and adhesion molecules (e.g., P-selectin, αIIbβ3). When stimulated, the α granules fuse with the nearby membranes of the OCS, releasing their soluble contents to the plasma while translocating membrane-spanning proteins, such as P-selectin, to the platelet surface. It is estimated that more than half of a platelet’s total αIIbβ3 is stored within α granules and displayed only when activated. The α granule membranes occupy almost as much area as the OCS, and thus when stimulated, the fused membranes may increase platelets’ surface area by up to fourfold. Dense granules (three to eight per platelet), on the other hand, contain mostly small molecules such as ADP, serotonin, epinephrine, histamine, and ionic calcium bound by acidic polyphosphates, while lysosomes contain mainly proteases such as cathepsins.
From the functional point of view, platelets can thus potentially secrete a very large repertoire of active mediators. These include those potentially lethal to pathogens such as the antimicrobial factors PF4 and CCL5, and proteases such as elastase. They can also secrete nonlethal mediators, such as platelet activators (e.g., ADP), endothelial modulators (e.g., nitric oxide, histamine), cytokines, chemokines, growth factors, and clotting factors. Granules also contain factors such as adhesion molecules and cell surface receptors that get transferred to the cell surface upon membrane fusion. By releasing these factors or expressing different adhesion receptors, platelets are able to interact with different cell types and activate them.
In addition to these “traditional” mediators, platelets may also modulate the cellular activity of other cell types by the unconventional transfer of its RNA load via microparticles (Risitano et al., 2012; Laffont et al., 2013). Microparticles are small (0.1–1 µm) fragments of cellular plasma membranes, also produced by many other cell types, but are mostly platelet derived (90%; Italiano et al., 2010). They can transfer various peptides, lipids, RNA, and DNA from one cell to another without direct cell-to-cell contact (Barteneva et al., 2013). The RNA content in platelets consist not only of mRNA but also many other types of noncoding RNA, such as micro-RNA and viral-like repeat elements (Bray et al., 2013; Provost, 2017). The exact roles of these platelet microparticles remain currently unclear.
The vast array of bioactive mediators stored in platelets argue that these anucleated cells are in fact optimally equipped to sense, make decisions, and deploy an arsenal of molecules in response to environmental demands. Thus, platelets are remarkably autonomous despite their reduced size and inability to mount complex transcriptional responses.
Platelet programming (functions)
Coordination of sensor input and payload delivery
Akin to UAVs, sensor input must be integrated with their responses to generate effective and meaningful outcomes. Given their wide array of multifunctional receptors and payloads, both “lethal” (i.e., cytotoxic, thrombotic, or proinflammatory) and “nonlethal,” platelets must carefully regulate their responses or risk inadvertent thrombosis or tissue damage. A complex regulatory mechanism, via purinergic signaling by CD39 that converts inflammatory ATP or ADP to AMP, and CD73 that converts AMP to anti-inflammatory adenosine, may regulate the extent of platelet activation (Eltzschig et al., 2012; Antonioli et al., 2013). It is also likely that the abundant and unique noncoding RNA in platelets may somehow coordinate these processes (Provost, 2017). Other undiscovered mechanisms of signal transduction and integration likely exist. For example, platelets appear to distinguish between different types of bacterial LPS, resulting in different secretion responses, even though signaling occurs through the same receptors (Berthet et al., 2012).
Intriguingly, platelet granules contain payloads with paradoxical and mutually antagonistic functions. This is prominent in α granules, which simultaneously contain coagulants and anticoagulants, angiogenic and antiangiogenic factors, proteases and protease inhibitors, and proinflammatory and anti-inflammatory mediators (Fitch-Tewfik and Flaumenhaft, 2013). Given this observation, it is a mystery as to how platelets accomplish their intended effects. An attractive explanation would be the existence of granule subsets organized by functional themes. Indeed, it was observed that VWF and fibrinogen were packaged differentially (Sehgal and Storrie, 2007). However, mapping of the various cargo contents by superresolution microscopy suggested otherwise, as the allocation of the cargo types to individual granules appeared stochastic and nonthematic (Kamykowski et al., 2011). Yet within each granule, individual cargo was observed to spatially segregate into zones, affording the possibility of partial release that is somehow controlled at the subgranule level. Interesting hypotheses have been put forward that await confirmation (Heijnen and van der Sluijs, 2015).
Hemostatic surveillance and support programs
Platelets are responsible for the maintenance of the blood vessel walls (i.e., hemostasis; Fig. 3). This statement may seem obvious in light of their well-known role in thrombosis, but what may be less appreciated is that they also reinforce vessel resistance to leakage through other mechanisms to support endothelial function (Ho-Tin-Noé et al., 2011). For example, depletion of platelets resulted in the ultrastructural thinning of blood vessel endothelia detectable from as early as 6 h onward, but this effect was reversed by platelet replenishment (Haselton and Alexander, 1992), highlighting their role in maintaining the integrity of the resting, intact endothelium. After the occurrence of a thrombotic event, platelets are also additionally involved in the support of multiple processes that restore vessel integrity, including wound healing, angiogenesis, and remodeling (Golebiewska and Poole, 2015).
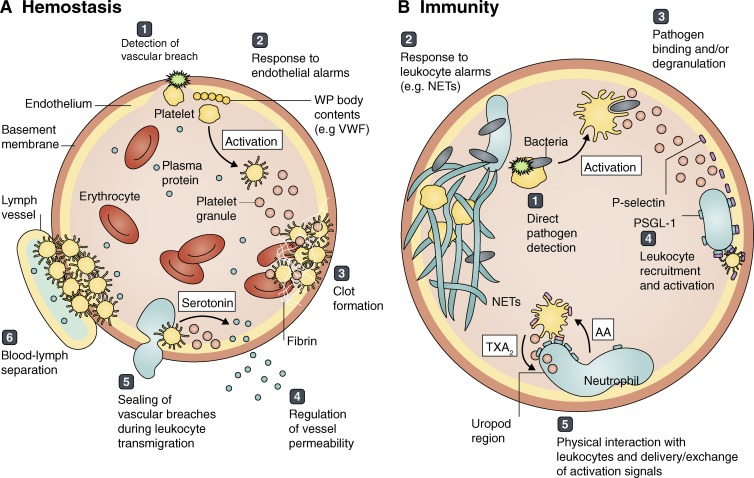
Figure 3.
Major platelet tasks in hemostasis and immunity. Platelets circulate in blood, surveying the vasculature for (A) hemostatic and (B) immune threats. (A) Platelets detect vascular breaches using a variety of receptors, such as those binding exposed collagen (1). They respond to danger signals such as ADP or the contents of Weibel-Palade (WP) bodies, released by damaged or activated endothelial cells (2). Upon activation, platelets can initiate thrombosis (3) while regulating vessel permeability (4). They also act as gatekeepers, physically preventing erythrocyte loss during leukocyte transmigration (5), and also at the lymphovenous junction at steady state or during lymphangiogenesis (6). (B) Platelets may recognize immune threats directly using evolutionarily conserved pattern receptors (1) or indirectly via leukocyte signals, such as neutrophil extracellular traps (NETs) or cytokines (2). Platelets can bind and wrap around pathogens, triggering degranulation to effect killing (3) and direct/indirect leukocyte recruitment (4). Additionally, platelets often physically interact with leukocytes to deliver or exchange signals that result in fully active inflammation, for example by taking up arachidonic acid (AA) from neutrophils to synthesize thromboxane A2 (TXA2; 5).
Depending on the circumstances, however, platelets can also achieve the reverse. In the presence of a hemostatic insult, one might imagine that a tight endothelial seal may be beneficial to reduce fluid loss. However, if the insult is of an immune nature, a tight endothelium would instead impede the timely recruitment of leukocytes to the tissue. Many pathogens have evolved mechanisms that can subvert the immune system (Wilson et al., 2002; e.g., toxin release), and the amount of time that the pathogen is left unchallenged to mount these defenses can determine the outcome of an invasion. Proinflammatory mediators found in platelet granules thus aid leukocyte transmigration as well as enhance delivery of their antipathogenic payload to the tissues by selectively increasing vessel permeability. Indeed, platelet serotonin was responsible for increasing vessel permeability in immune complex–mediated inflammation (Cloutier et al., 2012). Paradoxically, in these cases, even though platelets may promote vascular permeability, they are also simultaneously required to seal the vascular breaches after leukocyte transmigration; otherwise they risk the loss of erythrocytes into the tissues (Gros et al., 2015). During vessel damage and subsequent thrombosis, the entry of plasma proteins into tissues persist for extended durations even after clot formation that halts erythrocyte losses (Welsh et al., 2016). Thus, holistically, platelets are not purely hemostatic, as they preserve tissue integrity through mechanisms that do not always support the integrity of the endothelial barrier.
Surprisingly, platelets are also responsible for the development and maintenance of the lymphatic vessels, even though their presence there is generally undetected. Platelets use the C-type lectin receptor CLEC-2 to interact with lymphatic vessels via binding podoplanin. During embryonic development, this interaction is critical for maintaining the separation of blood and lymph vessels (Osada et al., 2012). This is true even in adults, as perturbation of this interaction results in the backflow of blood into the lymphatic network. Interestingly, platelet thrombi formation was necessary to perform a gatekeeping role at the lymphovenous junction of the thoracic duct (Hess et al., 2014). A similar process preserves the integrity of lymph nodes, which are constitutively infiltrated by lymphocytes, by regulating the expression of vascular endothelial cadherin and sealing off the lymph node vasculature (Herzog et al., 2013). Although podoplanin is normally associated only with lymphatic endothelia, it was recently discovered that venular blood vessels may also gradually express podoplanin under stenosis-mediated hypoxia (which mimics deep vein thrombosis in humans), resulting in CLEC-2–mediated platelet activation (Payne et al., 2017). Hence, this may represent the default on-site thrombosis mechanism in the absence of direct blood loss.
Immune surveillance and support programs
Platelets may interact directly with their targets and perform its killing function. For example, platelets may bind and wrap bacteria (Youssefian et al., 2002) or induce their aggregation (O’Brien et al., 2002), leading to degranulation. During malaria infection, platelets have also been described to perform the direct killing of plasmodium parasites in their blood stage forms in a PF4-dependent manner (McMorran et al., 2009, 2012), leading to the general perception that platelets play protective roles during an infection. However, a recent in vivo study in mice paradoxically found that platelet depletion did not lead to higher parasitemia levels (Gramaglia et al., 2017). Instead, links were found between the presence of platelets and malarial pathogenesis via CD40 interactions. Because about two thirds (Jadhav et al., 2004) of malarial infections are accompanied by thrombocytopenia, it thus remains a quandary for clinicians to decide if they should be boosting or inhibiting platelet function in these patients.
A recurrent observation in immunity and inflammation is that platelets do not act in isolation. They may activate in response to pathogens already marked for destruction by opsonins (e.g., complement or immunoglobulins) from other cell types. Vascular endothelial cells may also signal for their help by expressing activated forms of adhesion molecules or ligands and by enzymatic thinning of their glycocalyx. In some organs, such as the lung, both endothelial cells and leukocytes can directly signal platelet activation through ADP production via CD39 activity (Antonioli et al., 2013). Conversely, platelet granules contain cytokines and chemokines that can activate and recruit leukocytes to the site of activation, and platelets bound to the vascular wall additionally present P-selectin to support leukocyte capture (Schmidtke and Diamond, 2000).
A unique and notable feature of platelets is their tendency to form physical aggregates with other leukocytes, including lymphocytes (Li et al., 2006), dendritic cells (Czapiga et al., 2004), monocytes (Sarma et al., 2002), eosinophils (Pitchford et al., 2005), basophils (Liso and Bonomo, 1982), and neutrophils (Mauler et al., 2016). P-selectin (CD62P) on platelets and PSGL-1 (CD162) on leukocytes are thought to be the most important interaction providing the adhesion (de Bruijne-Admiraal et al., 1992). Additionally, CD40/CD40L interactions and integrin αMβ2 (Mac-1, CD11b/CD18, CR3) interactions (for myeloid cells) also appear important. Aggregate formation may be a secondary outcome of the various cell-cell physical interactions between platelets and leukocytes, as this would leave the platelets “stuck” on the leukocytes for an extended period of time. Although the existence of these leukocyte-platelet aggregates has long been known, its biological significance is only beginning to emerge. Circulating platelet-leukocyte aggregates are increased in sepsis patients, but those that develop multiple-organ failure actually display decreased numbers, likely because of enhanced peripheral sequestration (Gawaz et al., 1995).
Several examples illustrate the diverse support roles that platelets play on immune cells. In the lymph node, platelets were shown to help guide lymphocytes into the high endothelial venules by bridging their interactions (Diacovo et al., 1996), whereas platelets could deliver co-stimulatory signals to immature dendritic cells (Czapiga et al., 2004). In the case of the myeloid cells, platelet binding typically leads to the proinflammatory activation of the leukocyte, for example, in monocytes (Passacquale et al., 2011) and eosinophils (Pitchford et al., 2005). The significance of platelet binding to basophils (Liso and Bonomo, 1982) has not been well studied, but interestingly, mouse basophils also express the fibrinogen receptor αIIbβ3, being the only mature leukocyte to do so, and they up-regulate this integrin upon activation (Bakocevic et al., 2014).
The functional relationships between platelets and neutrophils have been more extensively studied. Platelet-neutrophil complexes occur in many acute and chronic inflammatory diseases (Mauler et al., 2016), and disruption of their formation by antibody blockade greatly reduces the severity of acute inflammation (Zarbock et al., 2006). Upon immune activation, neutrophils polarize and extrude their PSGL-1-containing uropods into the vascular lumen, which allows active scanning of activated platelets in the bloodstream (Sreeramkumar et al., 2014). Notably, this interplay is important for full neutrophil activation and inflammatory reactions, because mice in which neutrophils are unable to polarize or to transduce PSGL-1 signaling display aberrant crawling on the vasculature and are protected from inflammatory injury (Sreeramkumar et al., 2014). In the context of an infection, platelet interactions with neutrophils are initially mediated by P-selectin and PSGL-1, and subsequently interactions with platelet-borne GPIb induce neutrophils to secrete extracellular vesicles containing arachidonic acid, which are taken up by platelets. Platelet cyclooxygenase-1 (COX-1) can then use this lipid as a substrate to synthesize thromboxane A2, ultimately eliciting an inflammatory response needed to clear the infectious agent (Rossaint et al., 2016). Other studies have further described roles for CD40/CD40L interactions (Zuchtriegel et al., 2016) downstream of P-selectin. Thus, nucleated neutrophils appear to rely on anucleated platelets for both instruction and support to fully achieve its own activation status. In these scenarios, platelets first use their own receptors to sense danger and then transmit the information to neutrophils. This is best illustrated in the context of sepsis, in which endotoxin-activated platelets bind neutrophils and induce the formation of neutrophil extracellular traps (NETs), which consist of unpacked extracellular genomic DNA coated with histones and other antimicrobial peptides that form net-like structures within vessels that trap and facilitate bacterial elimination (Clark et al., 2007). Neutrophils have to integrate integrin-mediated outside-in and G-protein coupled receptor signaling to induce NET formation (Rossaint et al., 2014). On the other hand, NETs can also capture circulating platelets and cause the release of polyphosphates and thrombin, resulting in the polymerization of fibrin threads (McDonald et al., 2017), ultimately triggering thrombosis (Fuchs et al., 2010).
Other modalities of platelet-leukocyte cooperation have been described. Under conditions of inflammation, small proteins released by both neutrophils and platelets can heteromerize to elicit responses on a third cell type, such as monocytes (Alard et al., 2015). This type of mechanism may account for the causative role of platelet activation during atherosclerosis, during which release of platelet-borne chemokines onto the vasculature or leukocyte surfaces drive monocyte accumulation on atherosclerotic plaques (Huo et al., 2003). Somewhat contradictorily, a recent study suggests that platelets are also involved in the resolution phase of inflammation (Slaba et al., 2015). Overall, these studies suggest that although platelets do function as classical hemostatic effectors, their large numbers, small size, and vast array of sensors and bioactive molecules enable surveillance of all irrigated tissues, integration of environmental signals, and instruction of leukocyte responses, even when the immune cells are far from the source of danger (Fig. 3).
Concluding remarks
Platelets appear uniquely designed to function without nuclei, foregoing a measure of autonomy, in exchange for substantial advantages in maneuverability and expendability. Despite this, the importance and complexity of platelets do not seem to be diminished. Abundantly filled with sensors and payloads, platelets efficiently survey every nook and cranny of the mammalian vasculature. This, in turn, allows efficient coordination with multiple cell types, from endothelial cells to leukocytes, to participate in many direct and support roles in hemostasis and immunity (Fig. 3). We hope that this review will spark a conceptual shift in how platelets are perceived, from an inert clot-forming “cell fragment” to an entity that displays autonomy, motility, and a remarkable ability to sense and respond to environmental challenges. Future research will likely expand the paradigms discussed here and surely reveal even more surprises in nature’s design for these mammalian surveillance drones.
Supplementary Material
Acknowledgments
J.L. Li is supported by Agency for Science, Technology and Research funding. A. Zarbock is supported by Deutsche Forschungsgemeinschaft (ZA428/13-1 and INST 211/604-2 A05). A. Hidalgo is supported by Plan Estatal de Investigación Científica y Técnica y de Innovación 2013–2016 (SAF2015-65607-R and PCIN-2014-103), Programa Estatal de I+D+i Orientada a los Retos de la Sociedad Retos Investigación I+D+i from MECI, and cofunding from Fondo Europeo de Desarrollo Regional. Centro Nacional de Investigaciones Cardiovasculares Carlos III is supported by the MECI and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (MECI award SEV-2015-0505).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- NET
- neutrophil extracellular trap
- OCS
- open canaliculi system
- PF
- platelet factor
- PSGL
- P-selectin glycoprotein ligand
- UAV
- unmanned aerial vehicle
- VWF
- von Willebrand factor
References
- Alard J.E., Ortega-Gomez A., Wichapong K., Bongiovanni D., Horckmans M., Megens R.T., Leoni G., Ferraro B., Rossaint J., Paulin N., et al. . 2015. Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil HNP1 and platelet CCL5. Sci. Transl. Med. 7:317ra196 10.1126/scitranslmed.aad5330 [DOI] [PubMed] [Google Scholar]
- Antonioli L., Pacher P., Vizi E.S., and Haskó G.. 2013. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 19:355–367. 10.1016/j.molmed.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakocevic N., Claser C., Yoshikawa S., Jones L.A., Chew S., Goh C.C., Malleret B., Larbi A., Ginhoux F., de Lafaille M.C., et al. . 2014. CD41 is a reliable identification and activation marker for murine basophils in the steady state and during helminth and malarial infections. Eur. J. Immunol. 44:1823–1834. 10.1002/eji.201344254 [DOI] [PubMed] [Google Scholar]
- Barteneva N.S., Fasler-Kan E., Bernimoulin M., Stern J.N., Ponomarev E.D., Duckett L., and Vorobjev I.A.. 2013. Circulating microparticles: square the circle. BMC Cell Biol. 14:23 10.1186/1471-2121-14-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet J., Damien P., Hamzeh-Cognasse H., Arthaud C.A., Eyraud M.A., Zéni F., Pozzetto B., McNicol A., Garraud O., and Cognasse F.. 2012. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 145:189–200. 10.1016/j.clim.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Blair P., and Flaumenhaft R.. 2009. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 23:177–189. 10.1016/j.blre.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg N., Spinelli S.L., Francis C.W., Taubman M.B., and Phipps R.P.. 2009. The platelet as an immune cell-CD40 ligand and transfusion immunomodulation. Immunol. Res. 45:251–260. 10.1007/s12026-009-8106-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray P.F., McKenzie S.E., Edelstein L.C., Nagalla S., Delgrosso K., Ertel A., Kupper J., Jing Y., Londin E., Loher P., et al. . 2013. The complex transcriptional landscape of the anucleate human platelet. BMC Genomics. 14:1 10.1186/1471-2164-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., Patel K.D., Chakrabarti S., McAvoy E., Sinclair G.D., et al. . 2007. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13:463–469. 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- Claver J.A., and Quaglia A.I.E.. 2009. Comparative morphology, development, and function of blood cells in nonmammalian vertebrates. J. Exot. Pet Med. 18:87–97. 10.1053/j.jepm.2009.04.006 [DOI] [Google Scholar]
- Clemetson K.J., and Clemetson J.M.. 2013. Platelet receptors. In Platelets. Michaelson A., editor. Academic Press, London: pp. 169–194. 10.1016/B978-0-12-387837-3.00009-2 [DOI] [Google Scholar]
- Cloutier N., Paré A., Farndale R.W., Schumacher H.R., Nigrovic P.A., Lacroix S., and Boilard E.. 2012. Platelets can enhance vascular permeability. Blood. 120:1334–1343. 10.1182/blood-2012-02-413047 [DOI] [PubMed] [Google Scholar]
- Cognasse F., Nguyen K.A., Damien P., McNicol A., Pozzetto B., Hamzeh-Cognasse H., and Garraud O.. 2015. The inflammatory role of platelets via their TLRs and Siglec receptors. Front. Immunol. 6:83 10.3389/fimmu.2015.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapiga M., Kirk A.D., and Lekstrom-Himes J.. 2004. Platelets deliver costimulatory signals to antigen-presenting cells: a potential bridge between injury and immune activation. Exp. Hematol. 32:135–139. 10.1016/j.exphem.2003.11.004 [DOI] [PubMed] [Google Scholar]
- de Bruijne-Admiraal L.G., Modderman P.W., Von dem Borne A.E., and Sonnenberg A.. 1992. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood. 80:134–142. [PubMed] [Google Scholar]
- Deng W., Xu Y., Chen W., Paul D.S., Syed A.K., Dragovich M.A., Liang X., Zakas P., Berndt M.C., Di Paola J., et al. . 2016. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nat. Commun. 7:12863 10.1038/ncomms12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovo T.G., Puri K.D., Warnock R.A., Springer T.A., and von Andrian U.H.. 1996. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 273:252–255. 10.1126/science.273.5272.252 [DOI] [PubMed] [Google Scholar]
- Eltzschig H.K., Sitkovsky M.V., and Robson S.C.. 2012. Purinergic signaling during inflammation. N. Engl. J. Med. 367:2322–2333. 10.1056/NEJMra1205750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch-Tewfik J.L., and Flaumenhaft R.. 2013. Platelet granule exocytosis: a comparison with chromaffin cells. Front. Endocrinol. (Lausanne). 4:77 10.3389/fendo.2013.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foudi A., Kramer D.J., Qin J., Ye D., Behlich A.S., Mordecai S., Preffer F.I., Amzallag A., Ramaswamy S., Hochedlinger K., et al. . 2014. Distinct, strict requirements for Gfi-1b in adult bone marrow red cell and platelet generation. J. Exp. Med. 211:909–927. 10.1084/jem.20131065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D. Jr., Wrobleski S.K., Wakefield T.W., Hartwig J.H., and Wagner D.D.. 2010. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA. 107:15880–15885. 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gader A.M., al-Mashhadani S.A., and al-Harthy S.S.. 1990. Direct activation of platelets by heat is the possible trigger of the coagulopathy of heat stroke. Br. J. Haematol. 74:86–92. 10.1111/j.1365-2141.1990.tb02543.x [DOI] [PubMed] [Google Scholar]
- Garraud O., and Cognasse F.. 2015. Are platelets cells? And if yes, are they immune cells? Front. Immunol. 6:70 10.3389/fimmu.2015.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawaz M., Fateh-Moghadam S., Pilz G., Gurland H.J., and Werdan K.. 1995. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur. J. Clin. Invest. 25:843–851. 10.1111/j.1365-2362.1995.tb01694.x [DOI] [PubMed] [Google Scholar]
- Golebiewska E.M., and Poole A.W.. 2015. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 29:153–162. 10.1016/j.blre.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I., Velez J., Combes V., Grau G.E., Wree M., and van der Heyde H.C.. 2017. Platelets activate a pathogenic response to blood-stage Plasmodium infection but not a protective immune response. Blood. 129:1669–1679. 10.1182/blood-2016-08-733519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros A., Syvannarath V., Lamrani L., Ollivier V., Loyau S., Goerge T., Nieswandt B., Jandrot-Perrus M., and Ho-Tin-Noé B.. 2015. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood. 126:1017–1026. 10.1182/blood-2014-12-617159 [DOI] [PubMed] [Google Scholar]
- Hargett L.A., and Bauer N.N.. 2013. On the origin of microparticles: from “platelet dust” to mediators of intercellular communication. Pulm. Circ. 3:329–340. 10.4103/2045-8932.114760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton F.R., and Alexander J.S.. 1992. Platelets and a platelet-released factor enhance endothelial barrier. Am. J. Physiol. 263:L670–L678. [DOI] [PubMed] [Google Scholar]
- Heijnen H., and van der Sluijs P.. 2015. Platelet secretory behaviour: as diverse as the granules…or not? J. Thromb. Haemost. 13:2141–2151. 10.1111/jth.13147 [DOI] [PubMed] [Google Scholar]
- Henn V., Slupsky J.R., Gräfe M., Anagnostopoulos I., Förster R., Müller-Berghaus G., and Kroczek R.A.. 1998. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 391:591–594. 10.1038/35393 [DOI] [PubMed] [Google Scholar]
- Herzog B.H., Fu J., Wilson S.J., Hess P.R., Sen A., McDaniel J.M., Pan Y., Sheng M., Yago T., Silasi-Mansat R., et al. . 2013. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 502:105–109. 10.1038/nature12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P.R., Rawnsley D.R., Jakus Z., Yang Y., Sweet D.T., Fu J., Herzog B., Lu M., Nieswandt B., Oliver G., et al. . 2014. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Invest. 124:273–284. 10.1172/JCI70422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Tin-Noé B., Demers M., and Wagner D.D.. 2011. How platelets safeguard vascular integrity. J. Thromb. Haemost. 9:56–65. 10.1111/j.1538-7836.2011.04317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y., Schober A., Forlow S.B., Smith D.F., Hyman M.C., Jung S., Littman D.R., Weber C., and Ley K.. 2003. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat. Med. 9:61–67. 10.1038/nm810 [DOI] [PubMed] [Google Scholar]
- Italiano J.E. Jr., Mairuhu A.T., and Flaumenhaft R.. 2010. Clinical relevance of microparticles from platelets and megakaryocytes. Curr. Opin. Hematol. 17:578–584. 10.1097/MOH.0b013e32833e77ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav U.M., Patkar V.S., and Kadam N.N.. 2004. Thrombocytopenia in malaria—correlation with type and severity of malaria. J. Assoc. Physicians India. 52:615–618. [PubMed] [Google Scholar]
- Ju L., Chen Y., Xue L., Du X., and Zhu C.. 2016. Cooperative unfolding of distinctive mechanoreceptor domains transduces force into signals. eLife. 5:5 10.7554/eLife.15447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamykowski J., Carlton P., Sehgal S., and Storrie B.. 2011. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet α-granules. Blood. 118:1370–1373. 10.1182/blood-2011-01-330910 [DOI] [PubMed] [Google Scholar]
- Kee M.F., Myers D.R., Sakurai Y., Lam W.A., and Qiu Y.. 2015. Platelet mechanosensing of collagen matrices. PLoS One. 10:e0126624 10.1371/journal.pone.0126624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont B., Corduan A., Plé H., Duchez A.C., Cloutier N., Boilard E., and Provost P.. 2013. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 122:253–261. 10.1182/blood-2013-03-492801 [DOI] [PubMed] [Google Scholar]
- Lefrançais E., Ortiz-Muñoz G., Caudrillier A., Mallavia B., Liu F., Sayah D.M., Thornton E.E., Headley M.B., David T., Coughlin S.R., et al. . 2017. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 544:105–109. 10.1038/nature21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Ji Q., and Hjemdahl P.. 2006. Platelet-lymphocyte conjugation differs between lymphocyte subpopulations. J. Thromb. Haemost. 4:874–881. 10.1111/j.1538-7836.2006.01817.x [DOI] [PubMed] [Google Scholar]
- Liso V., and Bonomo L.. 1982. Platelet satellitism to basophils in a patient with chronic myelocytic leukaemia. Blut. 45:347–350. 10.1007/BF00319529 [DOI] [PubMed] [Google Scholar]
- Machlus K.R., and Italiano J.E. Jr. 2013. The incredible journey: from megakaryocyte development to platelet formation. J. Cell Biol. 201:785–796. 10.1083/jcb.201304054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauler M., Seyfert J., Haenel D., Seeba H., Guenther J., Stallmann D., Schoenichen C., Hilgendorf I., Bode C., Ahrens I., and Duerschmied D.. 2016. Platelet-neutrophil complex formation-a detailed in vitro analysis of murine and human blood samples. J. Leukoc. Biol. 99:781–789. 10.1189/jlb.3TA0315-082R [DOI] [PubMed] [Google Scholar]
- McDonald B., Davis R.P., Kim S.J., Tse M., Esmon C.T., Kolaczkowska E., and Jenne C.N.. 2017. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 129:1357–1367. 10.1182/blood-2016-09-741298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorran B.J., Marshall V.M., de Graaf C., Drysdale K.E., Shabbar M., Smyth G.K., Corbin J.E., Alexander W.S., and Foote S.J.. 2009. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 323:797–800. 10.1126/science.1166296 [DOI] [PubMed] [Google Scholar]
- McMorran B.J., Wieczorski L., Drysdale K.E., Chan J.A., Huang H.M., Smith C., Mitiku C., Beeson J.G., Burgio G., and Foote S.J.. 2012. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science. 338:1348–1351. 10.1126/science.1228892 [DOI] [PubMed] [Google Scholar]
- Morrell C.N., Aggrey A.A., Chapman L.M., and Modjeski K.L.. 2014. Emerging roles for platelets as immune and inflammatory cells. Blood. 123:2759–2767. 10.1182/blood-2013-11-462432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R.L., Gregory T.R., Gregory S.M., Hsieh A., and Boore J.L.. 2008. Genome size, cell size, and the evolution of enucleated erythrocytes in attenuate salamanders. Zoology (Jena). 111:218–230. 10.1016/j.zool.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthard R.W., and Diamond S.L.. 2012. Blood clots are rapidly assembled hemodynamic sensors: flow arrest triggers intraluminal thrombus contraction. Arterioscler. Thromb. Vasc. Biol. 32:2938–2945. 10.1161/ATVBAHA.112.300312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T., Nakayasu C., Rieger A.M., Barreda D.R., Somamoto T., and Nakao M.. 2014. Phagocytosis by thrombocytes is a conserved innate immune mechanism in lower vertebrates. Front. Immunol. 5:445 10.3389/fimmu.2014.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.H., Palankar R., Bui V.C., Medvedev N., Greinacher A., and Delcea M.. 2016. Rupture forces among human blood platelets at different degrees of activation. Sci. Rep. 6:25402 10.1038/srep25402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien L., Kerrigan S.W., Kaw G., Hogan M., Penadés J., Litt D., Fitzgerald D.J., Foster T.J., and Cox D.. 2002. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol. Microbiol. 44:1033–1044. 10.1046/j.1365-2958.2002.02935.x [DOI] [PubMed] [Google Scholar]
- Osada M., Inoue O., Ding G., Shirai T., Ichise H., Hirayama K., Takano K., Yatomi Y., Hirashima M., Fujii H., et al. . 2012. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J. Biol. Chem. 287:22241–22252. 10.1074/jbc.M111.329987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel O., Walter E., Jurk K., and Zahedi R.P.. 2017. Taking the stock of granule cargo: platelet releasate proteomics. Platelets. 28:119–128. 10.1080/09537104.2016.1254762 [DOI] [PubMed] [Google Scholar]
- Passacquale G., Vamadevan P., Pereira L., Hamid C., Corrigall V., and Ferro A.. 2011. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One. 6:e25595 10.1371/journal.pone.0025595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne H., Ponomaryov T., Watson S.P., and Brill A.. 2017. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. 129:2013–2020. 10.1182/blood-2016-09-742999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchford S.C., Momi S., Giannini S., Casali L., Spina D., Page C.P., and Gresele P.. 2005. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 105:2074–2081. 10.1182/blood-2004-06-2282 [DOI] [PubMed] [Google Scholar]
- Provost P. 2017. The clinical significance of platelet microparticle-associated microRNAs. Clin. Chem. Lab. Med. 55:657–666. 10.1515/cclm-2016-0895 [DOI] [PubMed] [Google Scholar]
- Qiu Y., Brown A.C., Myers D.R., Sakurai Y., Mannino R.G., Tran R., Ahn B., Hardy E.T., Kee M.F., Kumar S., et al. . 2014. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. USA. 111:14430–14435. 10.1073/pnas.1322917111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano A., Beaulieu L.M., Vitseva O., and Freedman J.E.. 2012. Platelets and platelet-like particles mediate intercellular RNA transfer. Blood. 119:6288–6295. 10.1182/blood-2011-12-396440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivadeneyra L., Pozner R.G., Meiss R., Fondevila C., Gómez R.M., and Schattner M.. 2015. Poly (I:C) downregulates platelet production and function through type I interferon. Thromb. Haemost. 114:982–993. 10.1160/TH14-11-0951 [DOI] [PubMed] [Google Scholar]
- Rossaint J., Herter J.M., Van Aken H., Napirei M., Döring Y., Weber C., Soehnlein O., and Zarbock A.. 2014. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood. 123:2573–2584. 10.1182/blood-2013-07-516484 [DOI] [PubMed] [Google Scholar]
- Rossaint J., Kühne K., Skupski J., Van Aken H., Looney M.R., Hidalgo A., and Zarbock A.. 2016. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 7:13464 10.1038/ncomms13464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J.W., Oler A.J., Tolley N.D., Hunter B.N., Low E.N., Nix D.A., Yost C.C., Zimmerman G.A., and Weyrich A.S.. 2011. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 118:e101–e111. 10.1182/blood-2011-03-339705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumjantseva V., and Hoffmeister K.M.. 2010. Novel and unexpected clearance mechanisms for cold platelets. Transfus. Apheresis Sci. 42:63–70. 10.1016/j.transci.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumjantseva V., Grewal P.K., Wandall H.H., Josefsson E.C., Sørensen A.L., Larson G., Marth J.D., Hartwig J.H., and Hoffmeister K.M.. 2009. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat. Med. 15:1273–1280. 10.1038/nm.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboor M., Ayub Q., Ilyas S., and Moinuddin. 2013. Platelet receptors; an instrumental of platelet physiology. Pak. J. Med. Sci. 29:891–896. 10.12669/pjms.293.3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma J., Laan C.A., Alam S., Jha A., Fox K.A., and Dransfield I.. 2002. Increased platelet binding to circulating monocytes in acute coronary syndromes. Circulation. 105:2166–2171. 10.1161/01.CIR.0000015700.27754.6F [DOI] [PubMed] [Google Scholar]
- Schmaier A.A., Stalker T.J., Runge J.J., Lee D., Nagaswami C., Mericko P., Chen M., Cliché S., Gariépy C., Brass L.F., et al. . 2011. Occlusive thrombi arise in mammals but not birds in response to arterial injury: evolutionary insight into human cardiovascular disease. Blood. 118:3661–3669. 10.1182/blood-2011-02-338244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke D.W., and Diamond S.L.. 2000. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J. Cell Biol. 149:719–730. 10.1083/jcb.149.3.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S., Weyrich A.S., and Rowley J.W.. 2014. A tour through the transcriptional landscape of platelets. Blood. 124:493–502. 10.1182/blood-2014-04-512756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal S., and Storrie B.. 2007. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J. Thromb. Haemost. 5:2009–2016. 10.1111/j.1538-7836.2007.02698.x [DOI] [PubMed] [Google Scholar]
- Simon A.Y., Sutherland M.R., and Pryzdial E.L.. 2015. Dengue virus binding and replication by platelets. Blood. 126:378–385. 10.1182/blood-2014-09-598029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaba I., Wang J., Kolaczkowska E., McDonald B., Lee W.Y., and Kubes P.. 2015. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology. 62:1593–1605. 10.1002/hep.28003 [DOI] [PubMed] [Google Scholar]
- Sreeramkumar V., Adrover J.M., Ballesteros I., Cuartero M.I., Rossaint J., Bilbao I., Nácher M., Pitaval C., Radovanovic I., Fukui Y., et al. . 2014. Neutrophils scan for activated platelets to initiate inflammation. Science. 346:1234–1238. 10.1126/science.1256478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theopold U., Schmidt O., Söderhäll K., and Dushay M.S.. 2004. Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 25:289–294. 10.1016/j.it.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Wadenvik H., Kutti J., Ridell B., Revesz P., Jacobsson S., Magnusson B., Westin J., and Vilén L.. 1991. The effect of alpha-interferon on bone marrow megakaryocytes and platelet production rate in essential thrombocythemia. Blood. 77:2103–2108. [PubMed] [Google Scholar]
- Welsh J.D., Muthard R.W., Stalker T.J., Taliaferro J.P., Diamond S.L., and Brass L.F.. 2016. A systems approach to hemostasis: 4. How hemostatic thrombi limit the loss of plasma-borne molecules from the microvasculature. Blood. 127:1598–1605. 10.1182/blood-2015-09-672188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.G. 2005. Platelets are covercytes, not phagocytes: uptake of bacteria involves channels of the open canalicular system. Platelets. 16:121–131. 10.1080/09537100400007390 [DOI] [PubMed] [Google Scholar]
- Wilson J.W., Schurr M.J., LeBlanc C.L., Ramamurthy R., Buchanan K.L., and Nickerson C.A.. 2002. Mechanisms of bacterial pathogenicity. Postgrad. Med. J. 78:216–224. 10.1136/pmj.78.918.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssefian T., Drouin A., Massé J.M., Guichard J., and Cramer E.M.. 2002. Host defense role of platelets: engulfment of HIV and Staphylococcus aureus occurs in a specific subcellular compartment and is enhanced by platelet activation. Blood. 99:4021–4029. 10.1182/blood-2001-12-0191 [DOI] [PubMed] [Google Scholar]
- Zarbock A., Singbartl K., and Ley K.. 2006. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J. Clin. Invest. 116:3211–3219. 10.1172/JCI29499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet J., and Ravid K.. 2000. Polyploidy: occurrence in nature, mechanisms, and significance for the megakaryocyte-platelet system. Exp. Hematol. 28:3–16. 10.1016/S0301-472X(99)00124-1 [DOI] [PubMed] [Google Scholar]
- Zuchtriegel G., Uhl B., Puhr-Westerheide D., Pörnbacher M., Lauber K., Krombach F., and Reichel C.A.. 2016. Platelets guide leukocytes to their sites of extravasation. PLoS Biol. 14:e1002459 10.1371/journal.pbio.1002459 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.