Abstract
Objectives. To test the effectiveness of a preclinical, telephone-based patient navigation intervention to encourage colorectal cancer (CRC) screening among older Black men.
Methods. We conducted a 3-parallel-arm, randomized trial among 731 self-identified Black men recruited at barbershops between 2010 and 2013 in New York City. Participants had to be aged 50 years or older, not be up-to-date on CRC screening, have uncontrolled high blood pressure, and have a working telephone. We randomized participants to 1 of 3 groups: (1) patient navigation by a community health worker for CRC screening (PN), (2) motivational interviewing for blood pressure control by a trained counselor (MINT), or (3) both interventions (PLUS). We assessed CRC screening completion at 6-month follow-up.
Results. Intent-to-treat analysis revealed that participants in the navigation interventions were significantly more likely than those in the MINT-only group to be screened for CRC during the 6-month study period (17.5% of participants in PN, 17.8% in PLUS, 8.4% in MINT; P < .01).
Conclusions. Telephone-based preclinical patient navigation has the potential to be effective for older Black men. Our results indicate the importance of community-based health interventions for improving health among minority men.
Black men suffer disproportionately from the effects of chronic diseases compared with other demographic groups. In the United States, Black men have the highest incidence of colorectal cancer (CRC) and the highest CRC mortality,1–3 yet Blacks have signficiantly lower screening rates than Whites nationally.4 One explanation for the disproportionate CRC mortality may be that Black men are less likely than are White men to be diagnosed at an early stage of the disease, leading to decreased survival rates.2 Lower rates of early-stage diagnosis may be attributable in part to lack of timely screening, because CRC screening leads to identification and, often, curative excision of precancerous polyps and early cancers. Even in New York City, where disparities in overall CRC screening have been largely reduced, racial differences in age at screening, early-stage diagnosis, and CRC mortality persist.3,5,6 Thus, for Black men, a focus on timely CRC screening is particularly important.
Several approaches have been shown to increase CRC screening rates.7,8 One such intervention is patient navigation (PN), defined as “assistance offered to patients, survivors, families, and caregivers to help them access and chart a course through the healthcare system” and overcome barriers to health care.9(p71) PN has demonstrated efficacy in increasing CRC screening rates when delivered in practice-based settings,9–15 particularly among minority groups. Several of these studies have implemented PN for patients who have already received a doctor’s recommendation for screening,10–12,15 potentially missing individuals who are least likely to be screened. However, the effectiveness of PN programs for Black men has not been tested in nonclinical, community-based settings.
In contrast with the traditional navigation model, in which patients are navigated from the primary care doctor’s office to the colonoscopy suite, a model of navigation from community settings may be of particular importance for Black men.13 Black men are less likely to receive regular health care or to have a personal doctor than are Whites,16 because of barriers including cost, lower rates of insurance coverage, lack of trust, experiences of discrimination in health care, and multilevel societal racism.16–21 Physician-level factors further complicate these complex patient- and system-level factors. A recent study showed that being Black, having a lower income, or having a lower education level was associated with being less likely to receive a recommendation for CRC screening from a physician.22 Thus, a traditional PN model is likely not to reach those men who may need navigation the most. In this context, the translation of evidence-based navigation approaches to community-based settings is necessary for reducing disparities in CRC mortality in Black men.
Our past research suggests that nonclinical places such as barbershops may be a promising setting for reaching Black men, regardless of their education, income, or health care–seeking behavior.23 Prior research has demonstrated the efficacy of barbershop interventions for addressing cardiovascular disease in Black men,24,25 but no CRC screening interventions have been tested in the barbershop setting. The Multi-Intervention Study to Improve CRC Screening and to Enhance Risk Reduction in Black Men (MISTER B) aimed to determine whether a PN intervention to encourage CRC screening would improve screening rates among middle-aged and older Black men recruited from barbershops in New York City.
METHODS
The MISTER B trial was a 3-arm randomized control trial of Black men aged 50 years and older recruited at barbershops throughout New York City. We used a cross-randomized design in which all participants received at least 1 intervention. In this design, all groups get assigned an intervention, but the focus of each single intervention (blood pressure and CRC screening in this case) is different and without overlap, allowing us to use each single intervention arm as a control group for the other intervention. This analysis focuses on the effectiveness of the PN intervention only, or in combination with the blood pressure control intervention, compared with the group receiving only the intervention for blood pressure control. The study protocol is described in detail elsewhere.26
Setting and Study Population
The inclusion criteria included
being male,
self-identifying as Black or of African descent,
not having up-to-date CRC screening,
having high blood pressure reading at the time of eligibility screening (defined as 135/85 mmHg or higher, or 130/80 mmHg or higher for those with diabetes or kidney disease),
having a working telephone, and
being able to speak English.
Exclusion criteria included being unable to provide informed consent. Participants were recruited at barbershops identified by study staff and situated in neighborhoods with large populations of Black men. Study staff selected specific barbershops on the basis of the owners’ interest in participating. In most cases, study staff approached owners during neighborhood tours. Random selection of barbershops was not possible because of a lack of complete listing of existing barbershops in the study area. Participants were customers at the barbershop where the screening event was held or men who were residents of the neighborhood near the barbershop. Recruitment commenced in 2010 and follow-up in-person interviews were concluded in 2014.
Sample Size
To test the effectiveness of the PN intervention, we estimated that a sample size of 127 participants per group would provide 90% power to detect a group difference in completed CRC screening at 6 months from an expected rate of 35% to a desired level of 60%. However, since the study was designed to test the effectiveness of 2 distinct interventions, our ultimate sample size estimate was larger because of our calculations for testing change in blood pressure at 6 months, which would require approximately 200 participants. Consequently, we recruited 240 participants per intervention group to allow for attrition.
Randomization
After participants completed the consent process and baseline interview, we immediately randomized them to 1 of 3 conditions: (1) PN to support CRC screening, (2) motivational interviewing for blood pressure control (MINT), or (3) both interventions (PLUS). The study statistician made an ordered list of randomized assignments, including the randomly assigned order in which those assigned to PLUS (i.e., both interventions) would receive their first intervention session (either motivational interviewing or CRC session first). Detailed randomization procedures have been previously described.26
Interventions
Participants in all groups received printed health education materials published by the American Cancer Society and the National Heart, Lung, and Blood Institute addressing CRC screening and hypertension self-management, respectively. Interventionists reviewed these materials with all participants at the initial intervention session.
The PN intervention consisted of 2 or more telephone sessions. The first session included a brief educational session followed by assessment of readiness for screening and potential logistic and psychosocial barriers. Taking into account their insurance status, location, and preferences, navigators encouraged participants who expressed an interest in colonoscopy to make an appointment within 2 weeks. PN participants received follow-up calls from the navigator within 2 weeks and periodically throughout the 6 months, with frequency depending on participants’ needs. Participants who preferred fecal immunochemical test (FIT) screening were sent a FIT kit and instructions by mail, and received follow-up calls from the navigator.
Participants randomized to MINT received 4 sessions scheduled at specific intervals. The first session consisted of brief education followed by motivational interviewing–driven goal setting. In subsequent sessions, motivational interviewing was used to follow up or to refine the initial goals. For participants receiving only the MINT intervention, receipt and review of the published materials about CRC during the first session was the only CRC-related content received throughout the intervention.
Participants randomized to the PLUS arm received the initial intervention call from their first assigned interventionist (depending on randomly assigned order, as described in “Randomization” section) prior to receiving the initial call from their second assigned interventionist. The interventions then proceeded concurrently. Interventionists initiated intervention sessions within 2 weeks of completing the baseline intervention. They followed a telephone calling protocol that included a minimum of 3 call attempts per session and 1 mailed reminder to ensure that all participants received adequate opportunity to participate in each session.
Measurements and Outcomes
At 6 months, participants completed an in-person follow-up interview at the barbershop where the participant was recruited or at a nearby location that was convenient for the participant. The primary outcome was CRC screening completion as determined by self-report. Whenever possible, a project coordinator verified self-reported CRC screenings by requesting colonoscopy reports from providers.
Baseline and follow-up interviews assessed demographics and psychosocial and physiological measurements to address the mechanisms of intervention effects and provide context for study findings. These included self-reported measures on access to care, race-based medical suspicion and perceived discrimination, attitudes toward colonoscopies, and health literacy. We also assessed behavioral intention for CRC screening at baseline.27,28
Statistical Analysis
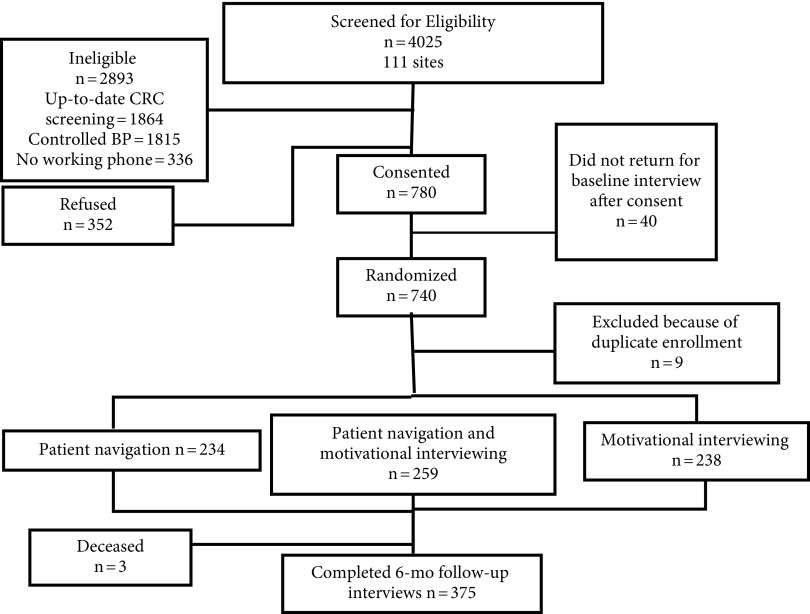
We conducted all analyses using SAS version 9.4 (SAS Institute, Cary, NC). To protect against potential selection bias, we tested age, education level, self-rated health, and awareness of having hypertension, diabetes, cholesterol, and kidney failure as potential covariates, and we selected those in which there was a significant difference between enrolled participants and eligible but not enrolled participants. To test our hypotheses, we adhered to an intent-to-treat design taking attrition into account (Figure 1). To determine whether attrition presented additional biases, we assessed differences in baseline characteristics between those who completed the study and those who did not. As with the procedure for selection bias, we included as covariates any variables for which completers and noncompleters differed significantly.
FIGURE 1—
CONSORT Diagram: The Multi-Intervention Study to Improve Colorectal Screening and to Enhance Risk Reduction in Black Men (MISTER B) Trial, New York City, 2010–2013
Note. BP = blood pressure; CRC = colorectal cancer.
Primary outcome.
We used mixed-effects regression analysis (SAS, PROC GLIMMIX) to test the hypothesis that those receiving the PN intervention would have higher screening rates at 6 months than those in the MINT-only intervention, including the barbershop as a nested effect. We took a conservative, intent-to-treat approach, assuming that those for whom data were not available at 6 months did not get screened for cancer. In model 1, we included covariates in the model (as described in “Statistical Analysis”) to account for selection and attrition biases. To further test the relationship between receiving the intervention and the outcome utilizing a per protocol scheme, we tested the association between completing the PN intervention (≥ 2 intervention sessions) and completing CRC screening at 6 months, and between completing the intervention and completing the 6-month follow-up interview. Finally, we tested for interactions between the 2 intervention groups receiving the PN intervention to determine whether being in the group that received both interventions had a differential effect on the outcome.
Post hoc analyses.
We noticed substantial variation in access to care among participants in the study (namely, in having health insurance and a personal doctor). We also noted low health literacy among the study sample. In addition, past literature documents that our study population (Black men) may be likely to experience discrimination in interactions with the health care system, and such experiences may affect men’s likelihood of seeking care. Thus, we conducted post hoc sensitivity analyses to determine whether results varied by access to care, discrimination (assessed as race-based medical suspicion), or health literacy. In models 2 through 5, we added these variables of interest, one at a time, to model 1, to determine whether adjusting for health literacy (model 2), having health insurance (model 3), having a personal doctor (model 4), or level of race-based medical suspicion (model 5) altered the relationship between group assignment and CRC screening.
RESULTS
A total of 4025 Black men aged 50 years and older were screened by study staff for eligibility at 111 sites across New York City (Figure 1) between December 2009 and December 2013, of whom approximately 51% reported up-to-date CRC screenings. A total of 1092 men were eligible for the study, of whom 71.4% agreed to participate. Of those, 740 completed a baseline interview and were subsequently enrolled and randomized to 1 of the 3 conditions. We excluded duplicate participants who enrolled more than once (n = 9), retaining only their first set of study data for analyses.
Baseline characteristics of the final sample (n = 731) are presented in Table 1 by intervention group. The average age was 57.4 years (SD = 6.6 years). There was a large range of annual incomes among participants ($0–$180 000), with a mean of $16 726 (SD = $18 007). Although many participants were missing income data, this did not differ by completion status. Almost one third had less than a high school education. Nearly one half were unemployed. Only 59.6% reported having a personal doctor and 22.8% were uninsured. Limited health literacy was found among 73.6% of participants. The average baseline race-based medical suspicion subscale score, measured on a scale of 1 to 5 with 5 indicating greater suspicion, was 2.3 (SD = 0.8). Table 1 also includes baseline attitudes toward colonoscopy.
TABLE 1—
Baseline Characteristics of Black Male Participants of a Randomized Control Trial Testing a Colorectal Cancer–Screening Intervention, by Randomization Group: New York City, 2010–2013
| Total |
PN Only |
PLUS |
MINT Only |
||||||
| Baseline Characteristics | No. | % or Mean ±SD | No. | % or Mean ±SD | No. | % or Mean ±SD | No. | % or Mean ±SD | Pa |
| Demographics | |||||||||
| Age, y | 707 | 57.4 ±6.6 | 225 | 57.2 ±6.5 | 250 | 56.9 ±6.0 | 232 | 58.2 ±7.1 | .07 |
| Foreign born | 718 | 27.0 | 230 | 20.9 | 253 | 28.5 | 235 | 31.5 | .029 |
| Highest level of education | 722 | 233 | 253 | 236 | .18 | ||||
| Less than high school | 30.1 | 31.3 | 25.3 | 33.9 | |||||
| High school graduate | 39.9 | 39.0 | 45.1 | 35.1 | |||||
| Some college | 17.5 | 15.9 | 19.4 | 17.0 | |||||
| College graduate or higher | 12.6 | 13.7 | 10.3 | 14.0 | |||||
| Household income, $ | 581 | 16 726 ±18 007 | 186 | 17 387 ±17 569 | 201 | 15 986 ±17 217 | 194 | 16 860 ±19 244 | .73 |
| Employment status | 718 | 229 | 254 | 235 | .36 | ||||
| Employed | 31.2 | 35.3 | 28.3 | 30.2 | |||||
| Unemployed | 45.8 | 40.6 | 51.6 | 44.7 | |||||
| Retired | 11.7 | 11.8 | 10.6 | 12.8 | |||||
| Unable to work | 11.1 | 12.2 | 9.5 | 11.9 | |||||
| Other employment situation | 0.1 | … | … | 0.4 | |||||
| Marital status | 713 | 229 | 252 | 232 | .19 | ||||
| Married or living with partner | 25.8 | 21.8 | 24.2 | 31.4 | |||||
| Divorced or separated | 28.8 | 22.8 | 32.5 | 24.6 | |||||
| Widowed | 6.6 | 7.4 | 6.8 | 5.6 | |||||
| Never married | 38.9 | 41.9 | 36.5 | 38.3 | |||||
| Access to care | |||||||||
| Health insurance | 705 | 226 | 253 | 226 | .38 | ||||
| Medicaid | 48.2 | 46.0 | 48.6 | 50.0 | |||||
| Medicare | 14.2 | 14.6 | 13.8 | 14.2 | |||||
| Private insurance | 10.2 | 12.4 | 11.9 | 6.2 | |||||
| Other insurance | 4.5 | 3.1 | 4.4 | 6.2 | |||||
| No insurance | 22.8 | 23.9 | 21.3 | 23.5 | |||||
| Has a regular place of care | 700 | 72.3 | 227 | 72.7 | 246 | 72.8 | 227 | 71.4 | .93 |
| Has a personal doctor | 728 | 59.6 | 233 | 61.8 | 258 | 56.6 | 237 | 60.8 | .46 |
| Health literacy | 679 | 213 | 245 | 221 | .64 | ||||
| Limited health literacy | 73.6 | 71.8 | 73.1 | 76.0 | |||||
| Possible limitations | 17.8 | 18.8 | 19.6 | 14.9 | |||||
| Adequate health literacy | 8.5 | 9.4 | 7.4 | 9.1 | |||||
| Group-based medical mistrust- suspicion subscale | 703 | 2.3 ±0.8 | 225 | 2.3 ±0.8 | 250 | 2.3 ±0.9 | 228 | 2.2 ±0.8 | .12 |
| Health status | |||||||||
| Self-rated general health | 715 | 232 | 248 | 235 | .75 | ||||
| Excellent | 6.6 | 5.2 | 6.9 | 7.7 | |||||
| Very good | 15.4 | 16.0 | 17.3 | 12.8 | |||||
| Good | 38.2 | 37.1 | 36.2 | 41.3 | |||||
| Fair | 31.2 | 31.5 | 31.1 | 31.1 | |||||
| Poor | 8.7 | 10.3 | 8.5 | 7.2 | |||||
| Family history of CRC | 702 | 6.8 | 222 | 9.0 | 249 | 6.4 | 231 | 5.2 | .71 |
| Systolic blood pressure | 728 | 146.9 ±16.1 | 233 | 147.3 ±17.5 | 257 | 146.6 ±14.8 | 238 | 146.9 ±16.0 | .91 |
| Diastolic blood pressure | 728 | 92.4 ±11.1 | 233 | 94.2 ±11.4 | 257 | 92.1 ±10.2 | 238 | 91.1 ±11.6 | .013 |
| Comorbidity (Charlson score) | 630 | 2.1 ±2.5 | 202 | 2.3 ±2.7 | 228 | 1.9 ±2.4 | 200 | 2.0 ±2.4 | .27 |
| Health behaviors | |||||||||
| Fruit and vegetable intake | 685 | 3.6 ±2.2 | 219 | 3.6 ±2.1 | 243 | 3.7 ±2.4 | 223 | 3.7 ±2.2 | .86 |
| Physical activity level (IPAQ-short) | 707 | 224 | 254 | 229 | .92 | ||||
| Low | 30.7 | 30.4 | 29.1 | 32.8 | |||||
| Moderate | 22.4 | 23.2 | 22.8 | 21.0 | |||||
| High | 47.0 | 46.4 | 48.0 | 46.2 | |||||
| Smoking status | 712 | 229 | 251 | 232 | .81 | ||||
| Never | 20.4 | 20.1 | 19.1 | 22.0 | |||||
| Former smoker | 25.0 | 24.0 | 24.3 | 26.7 | |||||
| Current smoker | 54.6 | 55.9 | 56.6 | 51.3 | |||||
| Received checkup in past y | 729 | 40.2 | 233 | 39.1 | 258 | 39.2 | 238 | 42.4 | .69 |
| Attitudes toward colonoscopies | |||||||||
| Fear of overall colonoscopy procedureb | 724 | 16.7 | 229 | 14.9 | 258 | 17.8 | 237 | 17.3 | .55 |
| Colonoscopies are a part of good health carec | 720 | 86.8 | 230 | 83.0 | 254 | 88.6 | 236 | 88.6 | .63 |
| Can find a way to pay for a colonoscopyc | 722 | 51.3 | 231 | 48.9 | 255 | 54.5 | 236 | 50.0 | .83 |
| Plan to get a colonoscopy in next 6 mo (agree or strongly agree) | 713 | 69.7 | 225 | 64.0 | 256 | 71.9 | 232 | 72.9 | .51 |
Note. CRC = colorectal cancer; IPAQ = International Physical Activity Questionnaire; MINT = motivational interviewing for blood pressure control; PLUS = both patient navigation and motivational interviewing for blood pressure control; PN = patient navigation. The sample size was n = 731.
P values calculated by χ2 test for categorical variables and ANOVA test for continuous variables.
Those answering “very fearful” or “extremely fearful.”
Those answering “strongly agree” or “agree.”
Intervention Adherence
In the PN intervention, the number of intervention sessions varied, with additional sessions included when individuals needed more assistance. The MINT intervention was designed to include 4 distinct sessions. However, to standardize measurement of intervention dosage, we considered either intervention (PN or MINT) to be completed when 2 or more sessions were completed. Completion of 2 or more sessions indicated that the participant was engaged in the intervention beyond the initial session, which included “standard care” for each condition—that is, those attending the first session received standard written materials and education regarding both disease conditions (i.e., hypertension and CRC) and standard behavioral recommendations, in addition to more in-depth counseling specialized to their intervention assignment. The majority of participants received the first session of their assigned interventions, and 36.7% of the PN group and 59.2% of the MINT group completed at least 2 intervention sessions. Intervention completion rates are summarized in Table A (available as a supplement to the online version of this article at http://www.ajph.org).
We defined study completion as having completed 6-month interviews regardless of intervention participation. There were no significant differences in socioeconomic status, health status, or psychosocial characteristics by study completion status. Study completers were slightly more likely to have a personal doctor at baseline than those who did not complete (62.9% vs 56.5%; P < .08). Completers were significantly more likely to be unable to work than noncompleters (13.4% vs 9.0%; P = .02).
Completion of Colorectal Cancer Screening
At 6 months, 8.4% of the participants randomized to the MINT-only group had completed CRC screening, compared with 17.5% of those randomized to receive the PN-only intervention and 17.8% of those in the PLUS group. After adjustment, compared with those in the MINT-only group, participants in the PN-only (adjusted odds ratio [AOR] = 2.28; 95% confidence interval [CI] = 1.28, 4.06) and PLUS (AOR = 2.44; 95% CI = 1.38, 4.34) groups were more than twice as likely to have been screened for CRC at 6 months (intraclass correlation coefficient = 0.039).
Among those receiving the PN intervention, completing the intervention was significantly associated with completing the study (P < .001). Furthermore, in a per protocol analysis, completing the PN intervention was significantly associated with completing CRC screening at 6 months (AOR = 16.04; 95% CI = 8.32, 30.93). In sensitivity analyses, we adjusted for health literacy and separately for insurance status and having a personal doctor (Table 2). In each model, participants in the PLUS and PN-only groups were significantly more likely to have been screened for CRC at 6-month follow-up.
TABLE 2—
Odds Ratios and 95% Confidence Intervals for Completion of Colorectal Cancer Screening at 6-Month Follow-Up, by Intervention Group: New York City, 2010–2013
| PN only | PLUS | MINT Only | |
| No. | 234 | 259 | 238 |
| Screened | 41 | 46 | 20 |
| Unadjusted OR | 2.32 (1.55, 3.46) | 2.35 (1.59, 3.49) | 1 (Ref) |
| Model 1, OR (95% CI) | 2.28 (1.28, 4.06) | 2.44 (1.38, 4.34) | 1 (Ref) |
| Model 2, OR (95% CI) | 2.43 (1.32, 4.46) | 2.51 (1.37, 4.60) | 1 (Ref) |
| Model 3, OR (95% CI) | 3.86 (2.03, 7.32) | 3.11 (1.67, 5.76) | 1 (Ref) |
| Model 4, OR (95% CI) | 3.89 (2.04, 7.40) | 3.17 (1.70, 5.90) | 1 (Ref) |
| Model 5, OR (95% CI) | 3.96 (2.03, 7.70) | 3.30 (1.74, 6.28) | 1 (Ref) |
Note. CI = confidence interval; CRC = colorectal cancer; IPAQ = International Physical Activity Questionnaire; MINT = motivational interviewing for blood pressure control; OR = odds ratio; PLUS = both patient navigation and motivational interviewing for blood pressure control; PN = patient navigation. Model 1 adjusted for education, hypertension awareness, and self-reported diabetes. Model 2 is model 1 plus adjustment for health literacy. Model 3 is model 1 plus adjustment for insurance status. Model 4 is model 1 plus adjustment for having a personal doctor. Model 5 is model 1 plus adjustment for the Group-Based Medical Mistrust Scale suspicion subscale.
DISCUSSION
Using a 3-arm, cross-randomized design, we evaluated the effects of a telephone-based PN intervention to promote CRC screening for low-income, urban Black men 50 and older who were eligible for CRC screening and who had uncontrolled hypertension. At 6 months, navigation resulted in a doubling of CRC screening for both active PN groups. Despite intervention dropout, we found that participating in the intervention was associated with a 16-fold increase in the odds of completing CRC screening by the 6-month follow-up.
Previous studies have shown that PN improves CRC screening among ethnic minorities in clinical settings.29 Our findings extend evidence of the benefits of CRC screening phone navigation to a community sample of largely low-income Black men. Although navigation resulted in twice the rate of screening over 6 months, the absolute increase was a relatively modest 15%. Baseline data from the sample suggest potential explanations. Nearly 70% of all men expressed intention to obtain colonoscopy screening in the next 6 months, yet only 8% did so in the control group and 17% in the navigation groups. The gap between intention and behavior may reflect a combination of low engagement with the health care system, logistical barriers, and a favorable response bias. Although only 60% of participants reported having their own personal physician and only 40% reported having a checkup in the past year, this middle-aged-and-older sample had significant health needs. Most reported comorbidity and low self-rated health. Furthermore, only about 1 of 4 men reported being married or living with a partner, which suggests low social support and may be associated with lower utilization of preventive services.
We found no significant interactions between intervention groups, indicating that participating in multiple interventions had no more effect on getting screened than participating in only the PN intervention. Furthermore, the PN intervention was effective even for men who did not have insurance and had limited health literacy, 2 characteristics that may significantly impede timely screening.30 With an average age of 57 years, our sample represented those in the age range most in need of screening to increase early detection and decrease CRC mortality through screening.
Our study is the first to test PN for CRC screening in a nonclinical setting among Black men, regardless of prior engagement with the health care system. Our results highlight the importance of intervention setting, and of extending public health interventions to nontraditional community-based settings, to address racial and socioeconomic disparities in chronic disease outcomes. One past study tested a community-based intervention for CRC screening and found similar success.30 However, participants were limited to those on a list of Medicare beneficiaries, indicating that they (1) had access to care and (2) were likely to be older than 65 years. Furthermore, the study found that the intervention was less effective for those with low health literacy, which was not the case in our study. In post hoc analyses, we found that adjusting for health literacy and access to care (operationalized by having health insurance, having a personal doctor, and race-based suspicion of health care), our intervention significantly increased the likelihood of being screened for CRC within 6 months. These results further emphasize the importance of introducing interventions to encourage screening in nonclinical settings.
Black-owned barbershops are rapidly gaining traction as potential community partners for health promotion programs targeting Black men.31–33 Barbershops hold special appeal for community-based intervention trials, as they are cultural institutions that draw large and loyal male clienteles and provide an open forum for discussion of numerous topics, including health, with influential peers, attracting a relatively diverse population representative of those in the community at large. The first randomized barbershop-based hypertension trial in the peer-reviewed literature (the BARBER-1 trial) was promising, demonstrating that a program of continuous blood pressure monitoring and peer-based health messaging in a barbershop can be successfully implemented by lay health workers rather than research personnel, leading to improvements in outcomes.24,25 Similarly, our results indicate that a cancer-screening navigation program focused on increasing engagement among Black men is possible in a barbershop setting.
Limitations
Our study was not without challenges. One key challenge was a high attrition rate in all intervention groups. Attrition was particularly high among participants receiving the PN intervention, despite the sociodemographic homogeneity of the intervention groups at baseline. Across all intervention groups, inconsistent telephone access was the most frequent challenge to reaching participants. Our interventionists found that many participants were unreachable after the first session because of disconnected telephone numbers or calls left unanswered. In some cases, we were able to reach these participants by trying at different times of the month or day. Many participants were uninterested in participating in the PN intervention, perhaps because of the sensitive nature of the content. The fact that the PN intervention content was notably more sensitive in nature than the MINT content may have contributed to the uneven attrition between groups. Despite these issues, using an intent-to-treat approach, our study showed a significant effect of the PN intervention on the likelihood of CRC screening at 6 months.
As our study recruitment was conducted in a community-based setting, which is quite different from past studies testing PN conducted in clinical sites, our procedures were also affected by neighborhood dynamics, barbers’ reception of the study, and business flows at partnering barbershops. For example, a few barbershops moved or closed over the course of the study, potentially reducing our ability to follow up with customers at these shops. Also, because of the individual-level randomization scheme, we did not track barbershop characteristics over time. These aspects of community dynamics may have introduced unmeasured structural bias affecting our results. Despite the challenges of conducting a clinical intervention outside of the clinical setting, this very aspect of the study was also a strength, because it allowed us to reach an underserved population that may not have otherwise benefited from interventions to encourage CRC screening.
Conclusions
The results of our study provide support for the use of behavioral interventions initiated in community-based settings to improve health-related behavior among older Black men, particularly for cancer screening. Such interventions, in turn, could decrease racial disparities in morbidity and mortality caused by CRC. Our PN intervention was effective despite low health literacy levels and lack of insurance, 2 key barriers to obtaining timely CRC screening. Our results highlight the potential for preclinical PN to improve cancer screening among vulnerable populations, including those with low health literacy and those who lack access to regular health care.
ACKNOWLEDGMENTS
This study was funded by the National Institute on Minority Health and Health Disparities, National Institutes of Health (1P60MD003421) and the Centers for Disease Control and Prevention (1U48DP002671). The ClinicalTrials.Gov registry number is NCT01092078.
We dedicate this article to the memory of our friend and colleague, Theodore Hickman, who served as a community health worker with our team for the duration of this study and beyond, touching the lives of many along the way with his innate ability to connect with others and his passion for helping those in need.
Many thanks to the MISTER B research team for all their hard work in recruitment, retention, and implementation. Our warmest gratitude to the many barbers and barbershop customers who participated in the project.
HUMAN PARTICIPANT PROTECTION
The study was reviewed and approved by the New York University School of Medicine institutional review board.
Footnotes
See also Naylor, p. 1356.
REFERENCES
- 1.Group UCSW. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-Based Report. Atlanta, GA: Dept of Health and Human Services, Centers for Disease Control and Prevention, National Cancer Institute; 2015. [Google Scholar]
- 2.Colorectal Cancer Facts & Figures 2011–2012. Atlanta, GA: American Cancer Society; 2011. [Google Scholar]
- 3.Williams R, White P, Nieto J, Vieira D, Francois F, Hamilton F. Colorectal cancer in African Americans: an update. Clin Transl Gastroenterol. 2016;7(7):e185. doi: 10.1038/ctg.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(3):41–45. [PubMed] [Google Scholar]
- 5.New York City health indicators by race/ethnicity, 2012–2014. 2015. Available at: https://www.health.ny.gov/statistics/community/minority/county/newyorkcity.htm. Accessed May 19, 2017.
- 6.Carlesimo M, Huang K. New York City colorectal cancer screening data and trends. Paper presented at: Ninth Annual New York Citywide Colon Cancer Control Coalition (C5) Summit; June 4, 2014; New York, NY.
- 7.Lewis CL, Brenner AT, Griffith JM, Pignone MP. The uptake and effect of a mailed multi-modal colon cancer screening intervention: a pilot controlled trial. Implement Sci. 2008;3:32. doi: 10.1186/1748-5908-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basch CE, Wolf RL, Brouse CH et al. Telephone outreach to increase colorectal cancer screening in an urban minority population. Am J Public Health. 2006;96(12):2246–2253. doi: 10.2105/AJPH.2005.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The NCI Strategic Plan for Leading the Nation to Eliminate the Suffering and Death Due to Cancer. Washington, DC: National Cancer Institute, US Dept of Health and Human Services; 2006. [Google Scholar]
- 10.Nash D, Azeez S, Vlahov D, Schori M. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83(2):231–243. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LA, Santos S, Jandorf L et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6(4):443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100(3):278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 13.Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer. 2005;104(4):848–855. doi: 10.1002/cncr.21214. [DOI] [PubMed] [Google Scholar]
- 14.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3(1):19–30. [PubMed] [Google Scholar]
- 15.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancer screening in an urban neighborhood health clinic. J Urban Health. 2005;82(2):216–224. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones DJ, Crump AD, Lloyd JJ. Health disparities in boys and men of color. Am J Public Health. 2012;102(suppl 2):S170–S172. doi: 10.2105/AJPH.2011.300646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaVeist TA. Minority Populations and Health: An Introduction to Health Disparities in the United States. San Francisco, CA: Jossey-Bass; 2005. [Google Scholar]
- 18.Smedley BD, Stith AY, editors. Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 19.Williams DR. The health of men: structured inequalities and opportunities. Am J Public Health. 2008;98(9 suppl):S150–S157. doi: 10.2105/ajph.98.supplement_1.s150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahalt C, Binswanger IA, Steinman M, Tulsky J, Williams BA. Confined to ignorance: the absence of prisoner information from nationally representative health data sets. J Gen Intern Med. 2012;27(2):160–166. doi: 10.1007/s11606-011-1858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol. 2015;41:311–330. [Google Scholar]
- 22.Coleman Wallace DA, Baltrus PT, Wallace TC, Blumenthal DS, Rust GS. Black white disparities in receiving a physician recommendation for colorectal cancer screening and reasons for not undergoing screening. J Health Care Poor Underserved. 2013;24(3):1115–1124. doi: 10.1353/hpu.2013.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole H, Schoenthaler A, Braithwaite RS et al. Community-based settings and sampling strategies: implications for reducing racial health disparities among black men, New York City, 2010–2013. Prev Chronic Dis. 2014;11:E105. doi: 10.5888/pcd11.140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hess PL, Reingold JS, Jones J et al. Barbershops as hypertension detection, referral, and follow-up centers for black men. Hypertension. 2007;49(5):1040–1046. doi: 10.1161/HYPERTENSIONAHA.106.080432. [DOI] [PubMed] [Google Scholar]
- 25.Victor RG, Ravenell JE, Freeman A et al. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med. 2011;171(4):342–350. doi: 10.1001/archinternmed.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravenell J, Thompson H, Cole H et al. A novel community-based study to address disparities in hypertension and colorectal cancer: a study protocol for a randomized control trial. Trials. 2013;14:287. doi: 10.1186/1745-6215-14-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shelton RC, Winkel G, Davis SN et al. Validation of the group-based medical mistrust scale among urban black men. J Gen Intern Med. 2010;25(6):549–555. doi: 10.1007/s11606-010-1288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson HS, Valdimarsdottir HB, Winkel G, Jandorf L, Redd W. The Group-Based Medical Mistrust Scale: psychometric properties and association with breast cancer screening. Prev Med. 2004;38(2):209–218. doi: 10.1016/j.ypmed.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Naylor K, Ward J, Polite BN. Interventions to improve care related to colorectal cancer among racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012;27(8):1033–1046. doi: 10.1007/s11606-012-2044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horne HN, Phelan-Emrick DF, Pollack CE et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015;26(2):239–246. doi: 10.1007/s10552-014-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitka M. Efforts needed to foster participation of blacks in stroke studies. JAMA. 2004;291(11):1311–1312. doi: 10.1001/jama.291.11.1311. [DOI] [PubMed] [Google Scholar]
- 32.Kong BW. Community-based hypertension control programs that work. J Health Care Poor Underserved. 1997;8(4):409–415. doi: 10.1353/hpu.2010.0031. [DOI] [PubMed] [Google Scholar]
- 33.Ferdinand KC. The Healthy Heart Community Prevention Project: a model for primary cardiovascular risk reduction in the African-American population. J Natl Med Assoc. 1995;87(8 suppl):638–641. [PMC free article] [PubMed] [Google Scholar]