Abstract
Objectives. To assess the relationships between childhood lead exposure and 3 domains of later adolescent health: mental, physical, and behavioral.
Methods. We followed a random sample of birth cohort members from the Project on Human Development in Chicago Neighborhoods, recruited in 1995 to 1997, to age 17 years and matched to childhood blood test results from the Department of Public Health. We used ordinary least squares regression, coarsened exact matching, and instrumental variables to assess the relationship between average blood lead levels in childhood and impulsivity, anxiety or depression, and body mass index in adolescence. All models adjusted for relevant individual, household, and neighborhood characteristics.
Results. After adjustment, a 1 microgram per deciliter increase in average childhood blood lead level significantly predicts 0.06 (95% confidence interval [CI] = 0.01, 0.12) and 0.09 (95% CI = 0.03, 0.16) SD increases and a 0.37 (95% CI = 0.11, 0.64) point increase in adolescent impulsivity, anxiety or depression, and body mass index, respectively, following ordinary least squares regression. Results following matching and instrumental variable strategies are very similar.
Conclusions. Childhood lead exposure undermines adolescent well-being, with implications for the persistence of racial and class inequalities, considering structural patterns of initial exposure.
The health crises in Flint, Michigan, and East Chicago, Indiana, brought the dangers of lead exposure to widespread public attention.1,2 The news was surprising because lead toxicity is widely considered a bygone hazard rather than a contemporary environmental threat. Yet there is evidence of continued lead contamination in many other US cities and nationally.3,4 Hidden contamination is alarming, because it is well established that lead exposure, even at very low levels, is detrimental to cognitive functioning.5,6
Once in the body, lead exerts harm through biological pathways that bear implications for behavioral, emotional, and physical health problems. Many of lead’s toxic properties stem from its ability to mimic calcium in the body7 and damage multiple areas of the brain as well as neurotransmitter systems associated with executive function and mood regulation, potentially increasing distractibility and hyperactive behavior as well as emotional response.8 Medical theories also link immunotoxicity, to which lead is a contributor, with noncommunicable diseases, including childhood asthma and obesity.9
Epidemiological research to date has linked childhood lead exposure with parent and teacher reports of problem behaviors in childhood,10–12 as well as attention-deficit–hyperactivity disorder.13 Epidemiological evidence for a positive relationship between lead exposure and symptoms of anxiety or depression in childhood11 is more limited, and findings with respect to physical health are mixed.14,15 Evidence of lead’s effects on these noncognitive outcomes relies on mainly cross-sectional13,14 or nonrepresentative longitudinal samples among children10–12 or cross-sectional or retrospective measures of exposure among adults.15 Evidence from prospective samples in the United States does not extend beyond outcomes observed in elementary school, when children’s development is still dynamic, or relies on estimated measures of childhood lead exposure rather than direct biological assays.16
Furthermore, because of structural inequality and institutional disinvestment,17,18 rates of lead exposure vary sharply by racial segregation, neighborhood poverty, and housing quality.19–21 Although previous studies generally control for individual-level sociodemographic characteristics, they rarely include neighborhood-level characteristics that influence both lead exposure and developmental well-being, undermining confidence in causal claims.
We assessed the relationships between childhood lead exposure and impulsivity, mental health, and obesity, building on previous research in 4 ways. First, our prospective birth cohort sample is representative of children born in Chicago, Illinois, in the 1990s and includes direct biological measures of lead exposure in early childhood. Second, we assessed long-term developmental health measured in adolescence. Third, we accounted for the structural inequality of lead exposure at both the individual and neighborhood levels. Fourth, we employed multiple analytic strategies to examine the plausibility of a causal relationship between childhood lead exposure and adolescent outcomes beyond cognitive function.
METHODS
We drew on an original follow-up of the birth cohort from the Project on Human Development in Chicago Neighborhoods (PHDCN). The PHDCN began as a representative, longitudinal, multicohort study of children and their caregivers living in 80 neighborhoods in the city of Chicago in the mid-1990s.22,23 Extensive in-home interviews and assessments were conducted with the primary caregivers of the sampled birth cohort members (n = 1255) 3 times over a 7-year period, with wave 1 occurring in 1995 to 1997. In 2012 to 2013, a random sample of birth cohort members who participated in wave 3, conducted in 1999 to 2002, was selected for a wave 4 follow-up. Cohort participants were followed no matter where they moved in the United States. Participation at baseline and retention at waves 3 and 4 were 78%, 75%, and 67%, respectively, which is comparatively high for urban samples. By wave 4, birth cohort members were aged 16 to 18 years. As in waves 1 to 3, interviews were carried out with the caretakers of the 378 located birth cohort members.
Childhood Lead Exposure
The blood lead levels (BLL) of children in Chicago are tested following the Chicago Department of Public Health’s (CDPH) recommended schedule of 4 tests by age 36 months, with additional testing until age 6 years if children move to a new address or if test results are high per contemporaneous guidelines from the Centers for Disease Control and Prevention. The majority of tests are conducted using venous blood samples, and the remainder using capillary blood samples. In 2015, identifiable CDPH blood test results were matched to birth cohort members present at wave 4 using exact as well as “fuzzy” matching on first name, last name, and date of birth. Of the 378 eligible birth cohort members, 254 were successfully matched with blood lead test results. These matched records comprise our analytic sample.
Consistent with the CDPH’s focus on children at high risk for lead exposure, children with matching test results are disproportionately Black or Hispanic and from lower socioeconomic backgrounds, relative to all wave 4 birth cohort members. In addition to accounting for these risk factors at both the individual and neighborhood levels, we directly accounted for selection into the analytic sample by adjusting all analyses for the CDPH’s lead testing coverage rate for each child’s neighborhood. We calculated coverage rates for 1995, 1996, and 1997 by dividing the total number of children tested (n = 54 703, 82 222, and 79 874, respectively) in each neighborhood (defined by census block groups) in each year (obtained from CDPH) by the total number of children aged 1 to 3 years residing in each block group in each year (obtained from the 1990 and 2000 decennial censuses and then linearly interpolated). Most blood lead tests are conducted in this age range. In calculating these coverage rates, we first subtracted the number of children in wave 4 of the PHDCN with a blood lead test in 1995, 1996, or 1997 from the appropriate neighborhoods’ counts in the appropriate years, so that the lead testing coverage rates we employed were not endogenous with our individual-level measure of lead exposure. We then averaged the coverage rates from 1995 to 1997, the period of wave 1 data collection.
To minimize potential measurement error among blood lead tests, we used children’s average BLL (in µg/dL) across test results as our measure of exposure. Because lead is absorbed most efficiently when ingested at the earliest stages of development,24 we excluded blood lead tests conducted at age 6 years or older before calculating each child’s average BLL. As the CDPH’s testing recommendations are not always strictly followed, children in our sample had an average of 2.6 (SD = 1.9) blood lead tests before age 6 years. The average ages at first and last blood tests were 2.08 (SD = 1.46) and 3.92 (SD = 1.48) years, respectively. These ages align with the ideal window for capturing lead exposure.25
Lead’s harmful effects have been detected at very low levels,26 and the Centers for Disease Control and Prevention maintains that there is no safe level of exposure. We therefore used children’s average BLL as a continuous variable in our analyses.
Demographic, Household, and Neighborhood Characteristics
We accounted for individual-, household-, and neighborhood-level characteristics that relate to both childhood lead exposure and developmental well-being. We derived these measures from the first wave of the PHDCN, soon after the children in our sample were born (mean age = 0.64 years; SD = 0.32). At the individual level, we included indicators of gender and race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, other).
At the household level, we included indicators of the primary caregiver’s immigrant generational status (first generation, second generation, third generation or greater), marital status (married, single, cohabiting), education level (< high school, high school or general equivalency diploma, > high school, ≥ bachelor’s degree), and receipt of Temporary Assistance for Needy Families (TANF). Results are robust to the inclusion of household income as a measure of socioeconomic status. We used TANF receipt because it is a direct measure of household deprivation and has less missing data.
At the neighborhood level, we used data from the 1990 decennial census to calculate the proportion of individuals in each child’s neighborhood (census block group) who identified as non-Hispanic White, non-Hispanic Black, or Hispanic, as well as the proportion of individuals below the poverty line. All neighborhood-level characteristics we employed were standardized to 2000 block group boundaries for consistency across measures.
Adolescent Well-Being
We examined 3 domains of health-related development: behavioral, mental, and physical. We derived all 3 outcome measures from wave 4 of the PHDCN, 15 years on average after the CDPH measured BLL.
Our behavioral outcome is the impulsivity scale of the Child Behavior Checklist, a widely used, reliable, and valid reporting measure for identifying emotional and behavioral problems.27 We measured impulsivity by asking the primary caregivers whether, now or within the past 6 months, each of the following items were true of the focal adolescent: can’t concentrate, can’t pay attention for long; can’t sit still, restless, or hyperactive; acts confused or seems to be in a fog; and is impulsive or acts without thinking. Caregivers responded whether each item was often (2), sometimes (1), or not (0) true. For ease of interpretation, we then summed their answers and standardized (mean = 0; SD = 1).
Our indicator of mental health comes from the anxiety or depression Child Behavior Checklist scale. We used the same process to construct the scale, which we measured by asking the primary caregiver whether, now or within the past 6 months, each of the following items were true of his or her adolescent: sudden changes in mood or feelings; feels or complains that no one loves him or her; feels worthless or inferior; is too fearful or anxious; and acts unhappy, sad, or depressed.
Our indicator of physical health is body mass index (BMI; defined as weight in kilograms divided by the square of height in meters), a widely used indicator of potential health problems28,29 that we calculated on the basis of primary caregivers’ reports of the sampled adolescents’ heights and weights without shoes.
Analytic Strategy
We restricted all analyses to the individuals for whom we had complete information on all the measures we employed (n = 208). No significant differences were revealed by t test for the means of our outcome measures between this sample with complete information and the full wave 4 birth cohort. Because of the small number of individuals missing PHDCN measures among children tested for BLL, we did not use imputed missing data. However, our results were very similar when we used multiple imputation of missing data. We weighted all analyses to account for the PHDCN’s multilevel survey design, response rate, and subsequent attrition. Appendix A (available as a supplement to the online version of this article at http://www.ajph.org) provides details on the construction of the weights.
We first estimated 3 ordinary least squares (OLS) regression models with each of our outcome measures as the dependent variables and average BLL as the key independent variable, accounting for individual, household, and neighborhood characteristics. Because development closely relates to age, we also controlled for age of the adolescents at wave 4, when our developmental health outcomes were measured.
Despite our set of control variables, the results may not represent unbiased estimates of lead’s average effects if childhood lead exposure is not evenly distributed across social demographic groups. We used coarsened exact matching (CEM) to address this concern of potential imbalance in our covariates. CEM is nonparametric, is relatively unaffected by measurement error, and strictly bounds the degree of model dependence.30 CEM uses an automatic binning algorithm to coarsen values of specified covariates and then exact match observations on the basis of these coarsened values. It then drops observations in both the treatment and control groups without an exact match.31
To implement CEM, we specified the treatment group as those with an average BLL of 6 micrograms per deciliter or greater and matched our observations on a core set of individual-, household-, and neighborhood-level social indicators. At the individual and household levels, we included children’s gender and race/ethnicity and primary caregivers’ immigrant generational status, education level, and TANF receipt at wave 1. At the neighborhood level, guided by previous research on concentrated poverty and ecological lead exposure,17,19,32 we included meaningful dichotomous indicators of children’s neighborhood environments: whether children’s neighborhoods at wave 1 were predominantly (≥ 70%) Black and whether children’s neighborhoods at wave 1 were characterized by high poverty rates (≥ 30% below the poverty line).
The ℒ1 statistic is a measure of imbalance that ranges from 0 to 1, with larger values indicating greater imbalance between the treatment and control groups.31 When ℒ1 is calculated on the basis of the measures we employed in our match, implementing CEM reduces imbalance in our sample from 0.71 to nearly 0. This results in 81 matched observations with all covariates available (33 of which have an average BLL ≥ 6µg/dL). The matched sample is, on average, similar to our sample with complete information in relation to BLL test results but is composed of higher proportions of girls, children who are Black, children with single parents, children whose parents received TANF at wave 1, and children who live in neighborhoods with higher percentages of Blacks.
We ran the OLS models on this matched sample and used weights produced by the CEM algorithm that account for the differential sizes of the matched groups of observations. The results of these models constitute estimates of the relationship between lead exposure and health for the matched subsample of our data for which there is common support. The CEM results we have presented were relatively unaffected by alternate specifications of our matching algorithm that were both more and less parsimonious.
We adjusted all 95% confidence intervals (CIs) to account for the multilevel sampling design of the PHDCN through Stata’s version 14 “svy” procedure (StataCorp LP, College Station, TX).
We conducted sensitivity analyses to address the possibility of omitted variable bias or the possibility that the relationships we observed in the OLS and CEM models arose from omitted factors that influenced both lead exposure and the 3 domains of adolescent health. These analyses and an instrumental variable approach are described in detail in Appendix B (available as a supplement to the online version of this article at http://www.ajph.org).
RESULTS
Underscoring the magnitude of the lead problem, the mean average BLL in our sample was 6.14 micrograms per deciliter (SD = 4.58; Table 1), which is higher than the Centers for Disease Control and Prevention ’s monitoring threshold of 5 micrograms per deciliter. Our sample was nearly evenly split with respect to gender and was quite diverse, as the majority of the sample was either Black or Hispanic. About 10% of primary caregivers had a college degree and 45% were receiving TANF at wave 1. Large SDs in our measures of neighborhoods’ demographic compositions reflect the segregated nature of Chicago’s neighborhoods.
TABLE 1—
Weighted Individual-, Household-, and Neighborhood-Level Characteristics: Birth Cohort of the Project on Human Development in Chicago Neighborhoods, Chicago, IL, 1995–2013
| Characteristic | Unweighted No. | Weighted Mean or Proportion (SD) |
| Average BLL (µg/dL) when younger than 6 y | 208 | 6.14 (4.58) |
| Age, wave 4 | 208 | 17.00 (0.50) |
| Gender | ||
| Female | 111 | 0.54 |
| Male | 97 | 0.46 |
| Race/ethnicity | ||
| White | 26 | 0.12 |
| Black | 78 | 0.49 |
| Hispanic | 93 | 0.34 |
| Other | 11 | 0.05 |
| Caregiver immigrant generation | ||
| ≥ third generation | 106 | 0.63 |
| Second generation | 25 | 0.07 |
| First generation | 77 | 0.30 |
| Caregiver marital status, wave 1 | ||
| Married | 95 | 0.42 |
| Single | 75 | 0.40 |
| Cohabiting | 38 | 0.19 |
| Caregiver education level, wave 1 | ||
| < high school | 81 | 0.42 |
| High school or GED | 30 | 0.17 |
| > high school | 73 | 0.32 |
| ≥ bachelor’s degree | 24 | 0.09 |
| Caregiver receives TANF, wave 1 | ||
| No | 117 | 0.55 |
| Yes | 91 | 0.45 |
| % block group non-Hispanic Black, 1990 | 208 | 0.47 (0.46) |
| % block group Hispanic, 1990 | 208 | 0.22 (0.29) |
| % block group below poverty level, 1990 | 208 | 0.25 (0.22) |
| % block group with BLL test, 1995–1997 average (centered) | 208 | 0.02 (0.36) |
| Impulsivity, wave 4 (standardized) | 208 | 0.05 (1.04) |
| Anxiety or depression, wave 4 (standardized) | 208 | 0.08 (1.10) |
| BMI, wave 4, kg/m2 | 208 | 23.68 (5.33) |
Note. BLL = blood lead level; BMI = body mass index; GED = general equivalency diploma; TANF = Temporary Assistance for Needy Families.
Social Gradient of Lead Exposure
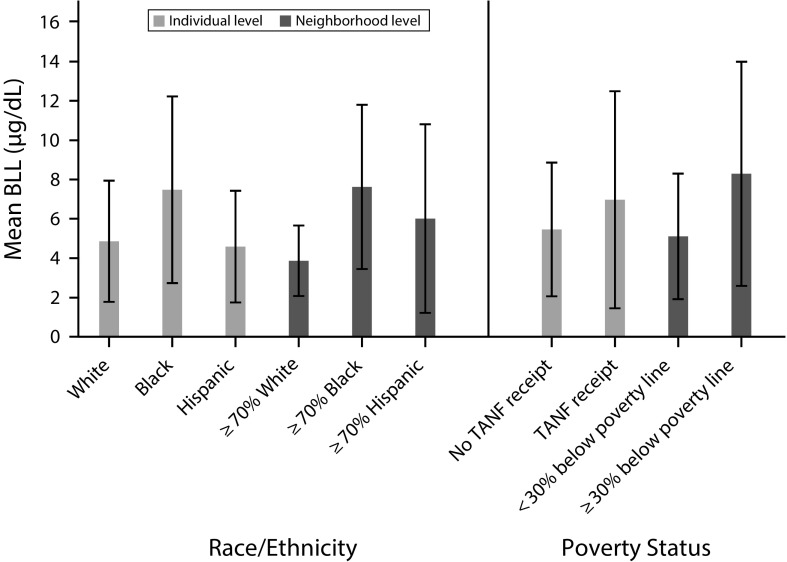
Figure 1 confirms that minority and poor children in our sample were disproportionately exposed to lead toxicity relative to White and less poor children, respectively, at the individual and neighborhood levels. At the individual level, the mean average BLL for Black children was 7.48 micrograms per deciliter (SD = 4.75) compared with 4.86 micrograms per deciliter (SD = 3.08) for White children. Hispanic children were also under the Centers for Disease Control and Prevention monitoring threshold, with a mean of 4.59 micrograms per deciliter (SD = 2.84).
FIGURE 1—
Childhood Lead Exposure by Individual- and Neighborhood-Level Race/Ethnicity and Poverty Status: Birth Cohort of the Project on Human Development in Chicago Neighborhoods, Chicago, IL, 1995–2013
Note. TANF = Temporary Assistance for Needy Families. The figure shows the average blood lead level in µg/dL when children were younger than 6 years. Means are weighted. Whiskers indicate SDs.
However, children who lived in both predominantly (≥ 70%) Black (mean = 7.62 µg/dL; SD = 4.18) and predominantly Hispanic neighborhoods (mean = 6.01 µg/dL; SD = 4.80) had higher average BLLs than did children who lived in predominantly White neighborhoods (mean = 3.87 µg/dL; SD = 1.79).
Similarly, children whose primary caregivers were receiving TANF when they were born (mean = 6.97 µg/dL; SD = 5.52) and who lived in neighborhoods characterized by high poverty rates (mean = 8.29 µg/dL; SD = 5.70) had higher lead exposure than did children in less poor homes (mean = 5.46 µg/dL; SD = 3.40) and neighborhoods (mean = 5.11 µg/dL; SD = 3.19).
The t test revealed that these mean differences are statistically significant for Blacks relative to Whites at the individual and neighborhood levels, for Hispanics relative to Whites at the neighborhood level, and for children from poor relative to less poor homes at the neighborhood level.
Childhood Lead Exposure and Adolescent Health
The OLS and CEM models provide consistent evidence of a positive relationship between childhood lead exposure and later impulsivity, anxiety or depression, and BMI in adolescence, after accounting for individual, household, and neighborhood characteristics (Table 2). A 1 microgram per deciliter increase in average childhood BLL is associated with a 0.06 (95% CI = 0.01, 0.12) SD increase in the Child Behavior Checklist impulsivity scale at approximately age 17 years according to the OLS model and a 0.08 (95% CI = 0.01, 0.16) SD increase in the matched subsample of children with better balanced covariates. There is a similar consistency in relation to anxiety or depression and BMI. A 1 microgram per deciliter increase in average BLL is associated with a 0.09 (95% CI = 0.03, 0.16) and a 0.11 (95% CI = 0.01, 0.21) SD increase in the Child Behavior Checklist anxiety or depression scale, following OLS and CEM, respectively. Each 1 microgram per deciliter increase of early childhood blood lead is linked to a gain of 0.37 (95% CI = 0.11, 0.64) in the BMI index following OLS, and 0.53 (95% CI = 0.23, 0.84) following the CEM strategy. All coefficients on BLL for the 3 outcomes are significant at P < .05. Moreover, our instrumental variable models yield similarly consistent estimates across all 3 outcomes (Appendix B).
TABLE 2—
Weighted OLS and OLS Following CEM Models for Associations of Childhood Lead Exposure with Behavioral, Mental, and Physical Health Indicators at Wave 4: Birth Cohort of the Project on Human Development in Chicago Neighborhoods, Chicago, Illinois, 1995–2013
| Characteristic | OLS (95% CI) | CEM (95% CI) |
| Impulsivity (standardized) | 0.06 (0.01, 0.12) | 0.08 (0.01, 0.16) |
| R2 | 0.24 | 0.40 |
| Anxiety or depression (standardized) | 0.09 (0.03, 0.16) | 0.11 (0.01, 0.21) |
| R2 | 0.32 | 0.45 |
| BMI (kg/m2) | 0.37 (0.11, 0.64) | 0.53 (0.23, 0.84) |
| R2 | 0.24 | 0.55 |
| No. | 208 | 81 |
Note. BMI = body mass index; CEM = coarsened exact matching; CI = confidence interval; OLS = ordinary least square. All coefficients significant at P < .05. All models were adjusted for child’s age at wave 4, gender, and race/ethnicity; primary caregiver’s immigrant generational status, marital status, education level, and Temporary Assistance for Needy Families receipt; the proportion of the child’s residential neighborhood that is non-Hispanic Black, Hispanic, and below the poverty line; and the proportion of the child’s residential neighborhood that was tested for lead exposure.
Returning to our OLS estimates, we used the coefficients generated by the models to calculate predicted changes in our outcome measures associated with an increase in average childhood BLL from 1 to 5 micrograms per deciliter after accounting for all the observed covariates. Such an increase in early childhood lead exposure is associated with 0.24 and 0.37 SD increases in the impulsivity and anxiety or depression scales, respectively, and a 1.49 increase in BMI. With respect to the former, these SD increases correspond to 0.33 and 0.57 increases on the raw impulsivity (range = 0–5) and anxiety or depression (range = 0–10) scales, respectively.
DISCUSSION
Capitalizing on prospective longitudinal data matched to childhood BLL and comprehensive measures of family background, neighborhood context, and individual outcomes, we advanced previous research by estimating the consequences of childhood lead exposure for long-term behavior, mental health, and physical health outcomes. We found that the estimated consequences of childhood lead exposure extend beyond cognitive function and into later adolescence across these 3 domains.
Exposure to lead is unevenly distributed in US society and in our sample, with children who are minorities and who are poor experiencing higher rates of exposure than do children who are White and less poor, respectively. Inequality in lead exposure is structural in origin and becomes embodied within individuals,19 generating long-term consequences for health and, as shown in other studies, cognitive development. Aizer et al.33 found that reductions in racial disparities in lead exposure resulted in reductions in the Black–White test score gap among children in third grade attending public school in Rhode Island, controlling for birth characteristics as well as individual- and neighborhood-level sociodemographics.
Limitations
Our birth cohort sample is representative of children born in Chicago in the mid-1990s but is relatively small, and lead testing was not completed for all children. Although our results are relatively unaffected by the additional inclusion of neighborhood-level indicators of violence and deteriorated buildings (see Appendix B), future research should replicate our analyses in larger samples and in different locations. We would not expect caregivers’ knowledge of their children’s lead exposure to systematically affect their reports of their children’s heights and weights in adolescence, but such knowledge may lead to increased awareness of and, in turn, reporting of their children’s impulsivity or anxiety or depression, potentially biasing our estimates upward. Future research on lead’s consequences should incorporate self-reported measures of behavior and mental health as well as additional measures of children’s early social environments to elucidate the social pathways through which lead’s effects might be exacerbated or alleviated.
Meanwhile, causality is difficult to assess in nonexperimental research. Our study is no exception. We approached this challenge using extensive multilevel adjustments for confounding and multiple analytic tools, including CEM on key predictors of lead exposure and plausibly exogenous instrumental variables. Each of these methods makes different assumptions, yet they yield convergent point estimates, giving us increased confidence in the underlying pattern. Although causality cannot be definitively established, the overall weight of the evidence points to an important link between lead exposure in early childhood and multiple domains of adolescent health.
Public Health Implications
Lead exposure is a policy-modifiable mechanism through which racial and class inequalities are maintained. Our results call for increased attention to racial and class inequalities in housing conditions in the United States,34 increased efforts at lead abatement and quicker elimination of exposure in our most high-risk communities,4,19 and enhanced monitoring of the link between social inequalities and lead exposure.32
Regulators and inspectors are charged with developing and enforcing safe building codes, and, among those who rent, landlords are rightfully tasked with meeting these standards of environmental safety, including lead abatement. The existence of dozens of smelting plants in Chicago and many more around the country—even if shuttered—also point to the need for soil remediation.35
It is thus cause for concern when federal funding for the lead testing of children is slated to be cut and sharp reductions in environmental regulations are proposed. Indeed, the stratification of resources at the individual level in the United States means that not all individuals or families have the time or money to address these hazards if the responsible regulatory parties fall short.
ACKNOWLEDGMENTS
Funding for this article was provided in part by grants from the Hymen Milgrom Supporting Organization at the University of Chicago and the Project on Race, Class and Cumulative Adversity at Harvard University funded by the Ford Foundation and the Hutchins Family Foundation.
We thank Emile Jorgensen, Christopher Muller, and the reviewers and associate editor Michael Greenberg of the American Journal of Public Health for helpful comments on a previous version.
HUMAN PARTICIPANT PROTECTION
This study was approved by the institutional review board at Harvard University and all participants provided written informed consent.
Footnotes
See also Vaughan and Galea, p. 1367.
REFERENCES
- 1.Goodnough A. Flint weighs scope of harm to children caused by lead in water. New York Times. January 30, 2016:A1. [Google Scholar]
- 2.Goodnough A. Their soil toxic, 1,100 Indiana residents scramble to find new homes. New York Times. August 30, 2016:A1. [Google Scholar]
- 3.Pell MB, Schneyer J. The thousands of U.S. locales where lead poisoning is worse than in Flint. 2016. Available at: http://www.reuters.com/investigates/special-report/usa-lead-testing. Accessed May 26, 2017.
- 4.Ahrens KA, Haley BA, Rossen LM, Lloyd PC, Aoki Y. Housing assistance and blood lead levels: children in the United States, 2005–2012. Am J Public Health. 2016;106(11):2049–2056. doi: 10.2105/AJPH.2016.303432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evens A, Hryhorczuk D, Lanphear BP et al. The impact of low-level lead toxicity on school performance among children in the Chicago public schools: a population-based retrospective cohort study. Environ Health. 2015;14:21. doi: 10.1186/s12940-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reuben A, Caspi A, Belsky DW et al. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA. 2017;317(12):1244–1251. doi: 10.1001/jama.2017.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Needleman H. Lead poisoning. Annu Rev Med. 2004;55:209–222. doi: 10.1146/annurev.med.55.091902.103653. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein Y, Markowitz ME, Rosen JF. Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Brain Res Rev. 1998;27(2):168–176. doi: 10.1016/s0165-0173(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 9.Dietert RR. Developmental immunotoxicity, perinatal programming, and noncommunicable diseases: focus on human studies. Adv Med. 2014;2014:867805. doi: 10.1155/2014/867805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellinger D, Leviton A, Allred E, Rabinowitz M. Pre- and postnatal lead exposure and behavior problems in school-aged children. Environ Res. 1994;66(1):12–30. doi: 10.1006/enrs.1994.1041. [DOI] [PubMed] [Google Scholar]
- 11.Wasserman GA, Staghezza-Jaramillo B, Shrout P, Popovac D, Graziano J. The effect of lead exposure on behavior problems in preschool children. Am J Public Health. 1998;88(3):481–486. doi: 10.2105/ajph.88.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen A, Cai B, Dietrich KN, Radcliffe J, Rogan WJ. Lead exposure, IQ, and behavior in urban 5- to 7-year-olds: does lead affect behavior only by lowering IQ? Pediatrics. 2007;119(3):e650–e658. doi: 10.1542/peds.2006-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froehlich TE, Lanphear BP, Auinger P et al. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124(6):e1054–e1063. doi: 10.1542/peds.2009-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scinicariello F, Buser MC, Mevissen M, Portier CJ. Blood lead level association with lower body weight in NHANES 1999–2006. Toxicol Appl Pharmacol. 2013;273(3):516–523. doi: 10.1016/j.taap.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease: a systematic review. Environ Health Perspect. 2007;115(3):472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes JW. Lead exposure and behavior: effects on antisocial and risky behavior among children and adolescents. Econ Inq. 2015;53(3):1580–1605. [Google Scholar]
- 17.Sharkey PT. Stuck in Place: Urban Neighborhoods and the End of Progress Toward Racial Equality. Chicago, IL: University of Chicago Press; 2013. [Google Scholar]
- 18.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson RJ, Winter AS. The racial ecology of lead poisoning: toxic inequality in Chicago neighborhoods, 1995–2013. Du Bois Rev. 2016;13(2):261–283. [Google Scholar]
- 20.Hanna-Attisha M, LaChance J, Sadler RC, Schnepp AC. Elevated blood lead levels in children associated with the Flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. 2016;106(2):283–290. doi: 10.2105/AJPH.2015.303003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanphear BP, Byrd RS, Auinger P, Schaffer SJ. Community characteristics associated with elevated blood lead levels in children. Pediatrics. 1998;101(2):264–271. doi: 10.1542/peds.101.2.264. [DOI] [PubMed] [Google Scholar]
- 22.Sampson RJ. Great American City: Chicago and the Enduring Neighborhood Effect. Chicago, IL: University of Chicago Press; 2012. [Google Scholar]
- 23.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler EE, Edwards BB, Jense RL, Mahaffey KR, Fomon SJ. Absorption and retention of lead by infants. Pediatr Res. 1978;12(1):29–34. doi: 10.1203/00006450-197801000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Lanphear BP, Hornung R, Ho M, Howard CR, Eberly S, Knauf K. Environmental lead exposure during early childhood. J Pediatr. 2002;140(1):40–47. doi: 10.1067/mpd.2002.120513. [Erratum in J Pediatr 2002;140(4):490. Eberle Shirley corrected to Eberly Shirley] [DOI] [PubMed] [Google Scholar]
- 26.Lanphear BP, Hornung R, Khoury J et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achenbach TM. Manual for the Young Adult Self-Report and the Young Adult Behavior Checklist. Burlington, VT: University of Vermont, Department of Psychiatry; 1997. [Google Scholar]
- 28.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 30.Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20(1):1–24. [Google Scholar]
- 31.Blackwell M, Iacus S, King G, Porro G. CEM: coarsened exact matching in Stata. Stata J. 2009;9(4):524–546. [Google Scholar]
- 32.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: the public health disparities geocoding project (US) J Epidemiol Community Health. 2003;57(3):186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aizer A, Currie J, Simon P, Vivier P. Do Low Levels of Blood Lead Reduce Children’s Future Test Scores? Cambridge, MA: National Bureau of Economic Research; 2016. NBER Working Paper 22558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pattillo M. Housing: commodity versus right. Annu Rev Sociol. 2013;39:509–531. [Google Scholar]
- 35.Eckel WP, Rabinowitz MB, Foster GD. Discovering unrecognized lead smelting sites by historical methods. Am J Public Health. 2001;91(4):625–627. doi: 10.2105/ajph.91.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]