Abstract
Background: Underreporting of adverse drug reactions (ADRs) has placed a heavy financial burden on health care resources worldwide. Realizing the importance of proper ADR reporting is paramount for implementing better patient care. Objective: This study was designed to assess knowledge, attitude, and practice (KAP) of ADR reporting among United Arab Emirates (UAE) health care professionals to clarify their present strategies and identify steps to avoid underreporting. Methods: A self-administered cross-sectional questionnaire was designed and randomly distributed to different health care personnel (n = 150). All participants were briefly informed about the aim of the study and given sufficient time to respond. The responses were collected over 6 months. The data were statistically analyzed for each reporter category (community pharmacist, hospital pharmacist, and doctors) using the chi-square test. Results: We found that 81%, 83%, and 83.3% of doctors, community pharmacists, and hospital pharmacists, respectively, were not aware of the existence of a reporting center and 56%, 60%, and 72% were not aware of a reporting procedure. Poor ADR reporting practices were shown by responders; only 19%, 14%, and 12.1% of doctors, community pharmacists, and hospital pharmacists reported ADRs. Conclusion: This study showed poor KAP results among health care professionals. Proper educational intervention strategies should be established in different health care settings for better patient care. With proper guidance, objectives in all health care settings should be targeted to positively change the concept of health care to consider ADR reporting as a common accepted daily routine practice.
Keywords: adverse drug reactions, attitude, community pharmacists, hospital pharmacists, knowledge, pharmacovigilance, practice
According to the World Health Organization (WHO) definition, an adverse drug reaction (ADR) is any noxious, unintended, and undesired effect of a drug, which occurs at doses used in humans for prophylaxis, diagnosis, or therapy.1 This description highlights the importance of the roles played by individual patient responses in determining an ADR. The use of any medication can create unwanted consequences. As stated by the Center for Health Policy Research, more than 50% of approved drugs in the United States are associated with some type of an adverse effect that was not detected prior to approval.1 At this point, the role of pharmacovigilance in addressing drug safety issues becomes crucial.
Pharmacovigilance is defined as “the science and the activities which relate to the detection, assessment, understanding and the prevention of adverse effects or any other drug-related problems.”2(p7) Good pharmacovigilance programs are an effective means to identify risks in the shortest possible time so that harm can be avoided or minimized. A proper spontaneous ADR reporting system is the basis for sound and comprehensive postmarketing surveillance drug studies. These studies compensate for any inadequacy in drug safety data resulting from the many limitations of premarketing clinical trials.2,3
The WHO has recommended that every country initiate pharmacovigilance programs to identify drugs that cause ADRs.1,4 However, underreporting of ADRs in various health care settings still represents a considerable challenge that needs to be faced, especially in developed countries.1 The significant impact of ADR reporting by health care workers on establishing an effective worldwide ADR database has been emphasized in several previous studies.5,6 The presence of ADRs is an important public health issue that has a significant impact on patient morbidity and mortality and has caused up to 11% of hospital admissions.7-9 A previous meta-analysis reported that the overall incidence of serious and even fatal ADRs worldwide was 6.7% and 0.32% of hospitalized patients, respectively.1,2 Many hospitalizations and emergency visits caused by ADRs are preventable.10-12
In addition to compromised patient health, ADRs place a significant economic burden on health care budgets. The WHO reports that the costs of ADRs, including hospitalizations, surgery, and lost productivity, exceeded the cost of actual therapeutic treatment in some countries.3 Up to 15% to 20% of the health care budgets of several countries is used to treat drug-related problems.6 Correct ADR reporting would be cost-effective by minimizing ADRs incidence and hospital admissions.
The aim of this study was to evaluate the knowledge, attitude, and practice (KAP) strategies of ADR reporting among United Arab Emirates (UAE) health care workers (pharmacists and practitioners) in different health care settings. Highlighting this problem and targeting its causes should enable us to initiate essential steps to improve awareness of ADR reporting in UAE and devise more practical means for ADR reporting so that it becomes a routine part of the professional obligation of UAE health care professionals.
All procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration and its later amendments or comparable ethical standards. The ethics committee approval of each involved health care setting was obtained prior to starting the study. The subjects involved in the study gave informed consent.
Methods
A self-administered cross-sectional questionnaire was designed and randomly distributed to different UAE hospitals and pharmacies to assess KAP strategies of ADR reporting in different health care settings. The study involved health care professionals, including medical doctors, community pharmacists, and hospital pharmacists who were working in different health care settings in different areas.
A KAP questionnaire of 14 questions was designed similar to those in previous studies.11,13-15 The additional 6 questions at the beginning of the survey aimed to collect demographic details such as age, gender, profession (community pharmacist, hospital pharmacist, physician), nationality, emirate of residence, and type of working sector (private or government). The last question aimed at collecting suggestions for improving ADR reporting. The validity of the questionnaire was measured by pretesting it with a sample of health care professionals, and modifications were carried out as per their suggestions.
Each study subject was approached, the study purpose was briefly explained, and informed consent was obtained. The questionnaire was made available to different health care personnel (N = 150) by e-mail and by personal interview. Each participating subject was given sufficient time to fill out and submit the questionnaire.
Statistical Analysis
The questionnaire results were coded and analyzed using the Statistical Package for Social Science (SPSS) version 20 (SPSS Inc, Chicago, Illinois). The data were analyzed descriptively for each reporter category (community pharmacist, hospital pharmacist, and medical doctors). Descriptive statistics (mean ± SD, counts, and percentages) were used for responses to identify KAP of ADR reporting. The relationship between the different professional classes and their knowledge and practice of ADR reporting were determined using chi-square test at P < .05.
Results
Of the 150 survey questionnaires circulated, 91 responded. The overall response rate was 60.7% (45.1% male and 55% female). The mean ± SD age of responders was 30.8 ± 7.6 years. The response rate of medical doctors, community pharmacists, and hospital pharmacists was 17.6%, 46.2%, and 36.3%, respectively. The response rate from the private sector and government sector health care settings was 25.2% and 74.7%, respectively. The full demographic details that were collected are summarized in Table 1.
Table 1.
Demographic Details of Health Care Personnel Sample (N = 91).
| Demographic details | Mean ± SD or n (%) |
|---|---|
| Age (Yrs) | 30.8 ± 7.6 |
| Sex | |
| Female | 41 (45.1%) |
| Male | 50 (55%) |
| Profession | |
| Doctor | 16 (17.6%) |
| Community pharmacist | 42 (46.2) |
| Hospital pharmacist | 33 (36.3%) |
| Sector | |
| Private | 23 (25.2%) |
| Government | 68 (74.7%) |
| Emirate | |
| Abu Dhabi | 14 (15.4%) |
| Ajman | 4 (4.4%) |
| Dubai | 25 (27.5%) |
| Fujairah | 11 (12.1%) |
| Ras Al Khaima | 5 (5.5%) |
| Sharjah | 24 (26.4%) |
| Umm Al Quwain | 8 (8.8%) |
Response results of the questions assessing the knowledge of pharmacists/practitioners are illustrated in the Figure 1. Eight-one percent, 83%, and 83.3% and of medical doctors, community pharmacists, and hospital pharmacists, respectively, were not aware of the existence of a reporting center in UAE and 56%, 60%, and 72% were not aware of a reporting procedure. Their responses were nonsignificant. Poor ADR reporting practices were shown by responders; only 19%, 14%, and 12.1% of medical doctors, community pharmacists, and hospital pharmacists reported ADRs. Our results have shown a high frequency percent of encountered ADRs in practice per week in all professional groups. The results were 81%, 79%, and 83% for medical doctors, community pharmacists, and hospital pharmacists, respectively, during weeks 0 to 5. The frequency percent of response for questions assessing attitude and practice adopted by each professional class are summarized in Table 2. As shown in Table 2, the attitude and practice strategies adopted by each professional class were found to be not significant. Regarding the most qualified person to report an ADR (where more than one answer was allowed), the response rates were highest for the pharmacists (87.9%) followed by medical practitioners (68.1%) and nurses (32.9%).
Figure 1.
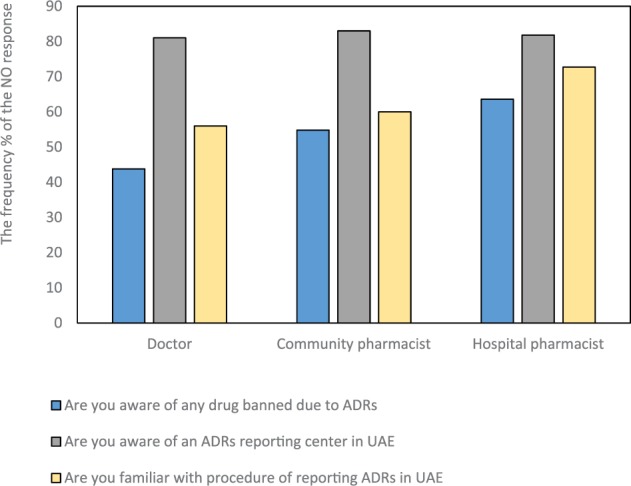
The frequency percent of the NO response of health care workers to knowledge assessed questions of ADRs reporting by professional class.
Note. ADR = adverse drug reaction; UAE = United Arab Emirates.
Table 2.
The Responses for Assessing Attitude and Practice Strategies Adopted by Health Care Personnel.
| Question | Doctor | Community pharmacist | Hospital pharmacist | P |
|---|---|---|---|---|
| Have you ever reported an ADR? | ||||
| Yes | 19% | 14% | 12.1% | NS |
| No | 81% | 86% | 87.8% | |
| Does ADR reporting damage the professional image? | ||||
| Yes | 18.7% | 16.7% | 12.1% | NS |
| No | 81.3% | 83.3% | 87.8% | |
| Factors that encourage ADR reporting | ||||
| Seriousness of the ADRs | 16.0% | 85.7% | 81.8% | NS |
| Unusualness of the reaction | 43.8% | 42.9% | 33.3% | |
| Involvement of a new drug | 50.8% | 26.2% | 51.5% | |
| Confidence in diagnosis an ADR | 31.3% | 21.4% | 42.4% | |
| Factors that discourage ADR reporting | ||||
| Did not know how or where to report an ADR | 100% | 78.6% | 100% | NS |
| Did not think it important | 0.6% | 4.8% | 6.1% | |
| Lack of access to ADR reporting forms | 43.8% | 31% | 45.5% | |
| Managing patient was more important than ADR reporting | 25% | 47.6% | 27.3% | |
| Patient confidentiality issues | 12.5% | 16.6% | 21.2% | |
| Concerns about legal and professional liability | 6.3% | 19% | 24.2% | |
| To detect an ADR | ||||
| Only ask patient | 25% | 16.7% | 12.1% | NS |
| Only monitor patient report | 6.3% | 7.1% | 3.0% | |
| All of the above | 68.8% | 78.6% | 84.8% | |
| Action taken when an ADR is suspected | ||||
| Stop medicine | 50% | 35.7% | 39.4% | NS |
| Prescribe appropriate medicine to control the ADR | 37.5% | 31% | 15.2% | |
| Record all details and report to the governmental health ministry | 6.3% | 9.5% | 9.1% | |
| Record all the details and report to the manufacturer | 0% | 4.8% | 9.1% | |
| If the ADR is well known for medicine, tell the patient not to worry | 6.3% | 19% | 27.3% | |
| Which ADR to report | ||||
| All ADRs | 62.5% | 26.2% | 45.5% | NS |
| Serious ADRs | 18.8% | 50% | 39.4% | |
| ADRs to new drugs | 6.3% | 11.9% | 9.1% | |
| Unknown ADRs to old drugs | 12.5% | 7.1% | 3.0% | |
| None | 0% | 4.8% | 3.0% | |
Note. Significance, p < .05. ADR = adverse drug reaction; NS = nonsignificant.
The seriousness of the ADR was the most important factor for reporting an ADR, whereas confidence in ADR diagnosis was the least important issue to consider. The necessary actions taken by health care professionals to deal with an ADR showed great variation. The majority of responders (40%) would stop the medicine, while 26% and 20% would prescribe medicine to control the ADR and would tell the patient not to worry if the ADR is known, respectively. Only a small sector of responders would record all details and report to the ministry of health or the manufacturer of the medicine.
Suggestions made by responders to improve ADR reporting are summarized in Figure 2. Increasing reporting system awareness and easier report submission were suggested by 73.6% and 64.8%, respectively. Other suggestions were making reporting mandatory (31%), electronic submission of the reports (37.4%), increasing awareness of health care personnel reporting (47.3%), increasing public awareness of these issues (37.4%), ensuring confidentiality (26.4%), and providing financial compensation for report submission (9.9%).
Figure 2.
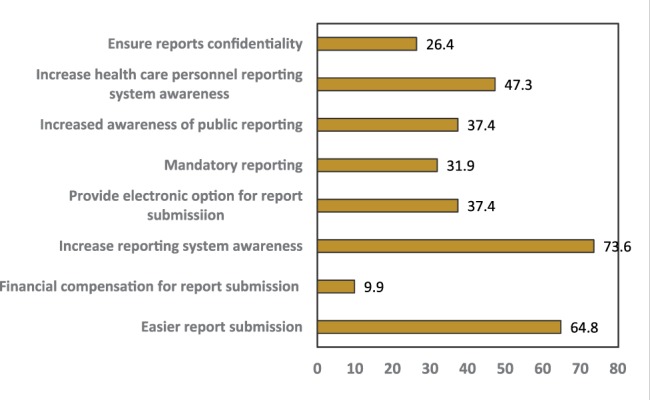
Suggestion to improve adverse drug reaction reporting by all responders.
Discussion
According to our knowledge, this is the first study in the UAE to investigate KAP responses of different health care professionals, including medical doctors, community pharmacists, and hospital pharmacists in different health care settings. A previous UAE study by Qassim et al16 investigated KAP of ADR reporting; however, its findings were restricted to community pharmacists working in 2 cities in the UAE: Sharjah and Ajman.
Our results showed that 81% and 56% of medical doctors, 83% and 60% of community pharmacists, and 83.3% and 60% of hospital pharmacists did not know how or where to report an ADR, respectively. This was consistent with a previous UAE study reporting 83.4% and 55.9% of UAE community pharmacists were unaware of how to or where to report an ADR, respectively.17 Most health care professionals were unaware of the existence of the Health Authority of Abu Dhabi (HAAD) pharmacovigilance program for ADR detection and monitoring. These results suggest that the lack of ADR reporting is a major issue in the health care industry of UAE.18,19
Underreporting in UAE is not an isolated phenomenon. Other areas of the world such as China,17 India,20 Malaysia,7 Nigeria,21 and Saudi Arabia21 also face similar issues. Countries, such as the United Kingdom, with well-established pharmacovigilance programs report a high level of underreporting.22,23
It is interesting to note that in this study, the poor ADR reporting practices did not match the high frequency percentage of ADRs encountered in practice per week in all professional groups. Results showed that 81%, 79%, and 83% of medical doctors, community pharmacists, and hospital pharmacists encountered ADRs in practice during weeks 0 to 5.
In this study, responders were not even sure which ADR should be reported. Although 60% agreed that all ADRs should be reported, 18.8% found that only reporting serious ADRs was important, while 6.3% and 12.5% would report ADRs to new drugs and new ADRs to old drugs only. In addition, 31.3% of responders reported that their confidence in the ADR identification would encourage their reporting.
This clearly does not match the HAAD pharmacovigilance policy of mandating all health care providers to report any suspected ADRs despite the uncertainty about causal relationship. This is also not in line with the HAAD pharmacovigilance policy that mandates all ADRs be reported by health care providers. Emphasis lies on reporting ADRs of a serious nature, those that are unexpected with product, or ADRs to new drugs.
Consequently, inadequate knowledge has led to poor practices by all professional groups sampled in the present study. Only 19%, 14%, and 12.1% of medical doctors, community pharmacists, and hospital pharmacists have ever reported an ADR. There is an urgent need to educate health care personnel about proper reporting guidelines imposed by HAAD pharmacovigilance policy. A positive correlation between knowledge and attitudes toward ADRs reporting was identified in an earlier UAE study.16
Regarding the most qualified person to report an ADR (where more than one answer was allowed), the response rates were highest for the pharmacists (87.9%) and then medical practitioners (68.1%) and nurses (32.9%). The majority of responders (87.9%) clearly viewed pharmacists as the most qualified professional group to report an ADR. This result may be biased by the fact that pharmacists were the major responders to this questionnaire, representing 82.5% with only 17.6% doctors involved. It also suggests that there is a strong belief that ADR reporting is a fundamental role of pharmacists.24,25 This was similarly found in previous studies in the UAE16 and Saudi Arabia.19 Higher response rates of 100% were also encountered by previous studies in Malaysia7 and the Netherlands.6
According to American Society of Health-System Pharmacists (ASHP) guidelines, pharmacists are important experts on drugs. They are pivotal in the development, maintenance, and ongoing evaluation of ADR programs and lead the education of health professionals regarding potential ADRs.25
Health care personnel were aware of the problem of poor ADR reporting; 73.6% suggested more education and awareness of ADR reporting system were required and 47% suggested increasing the awareness to health care personnel. Approximately 37.4% suggested increasing the awareness of public reporting. Direct patient ADRs reporting has been the focus of previous studies.22,23 The collaborative role by patients has been found to increase the speed of ADR knowledge acquisition and improve pharmacovigilance. It also encourages the more active role of patients in their treatment management.
Another suggestion by responders included mandatory reporting of ADRs (31.9%). Only 9.9% of responders suggested financial incentives for ADRs reporting. This highlights that most health care professionals recognize ADR reporting is a fundamental rather than optional responsibility.
This study showed that KAP strategies of UAE health care professionals toward ADR and pharmacovigilance policy are very limited. This is probably due to the recent initiation of the pharmacovigilance program by HAAD in UAE, which was established in 2007. Baseline KAP evaluation of UAE health care professionals toward ADR reporting will help pinpoint the proper interventional strategies needed to develop the HAAD pharmacovigilance, which is still in its infancy stage.
The results obtained in this study from different UAE health care settings pointed out 2 important areas requiring improvement. First, there is the need for more integration of the recently established HAAD pharmacovigilance among health care settings and more educational interventions in the form of seminars and workshops to improve ADR reporting. Educational interventions help increase ADR awareness and positively reflect everyday clinical practice.14,25 Second, more efforts are required to incorporate the subject of pharmacovigilance and ADRs in the curricula of medical and pharmacy schools.
There are strong links between improved ADR reporting in clinical practice and decreased ADR incidence and reduction in health care costs.
Limitations of the Study
The main limitation of our study was the relatively small number of responders, especially physicians. In addition, problems of self-reporting studies such as personal bias or recall accuracy may have affected the results. This study did not include nurses, who also play an important role in ADR reporting.
Conclusion
The key for an effective and successful pharmacovigilance system is the fruitful participation of all health care professionals. However, underreporting of ADRs is still a global issue of major concern that needs appropriate attention. Overall, our study showed poor KAP results from both UAE prescribers and pharmacists, indicating there is a big gap between the present health care personnel practice and pharmacovigilance center policy. Indeed a closer relationship between health care professionals and pharmacovigilance centers with continuing medical education (CME) should be actively encouraged to aid in improving spontaneous reporting for the sake of better patient care.
Acknowledging pharmacists as key players in ADR monitoring and reporting, with more emphasis on teaching pharmacovigilance in pharmacy undergraduate courses, should create more informed and better educated pharmacists. KAP in health care should be viewed as potential modifiable factors that will decrease the incidence of ADRs and reduce health care costs in clinical practice.
We recommend that CME about ADR reporting guidelines be provided in the form of booklets and posters that are made available at different locations in health care facilities as a constant reminder. This is in addition to regular emphasis about the paramount importance of ADR reporting with CME workshops or seminars and in undergraduate pharmacy courses.
A successful ADR program should monitor, detect, evaluate, document, and report ADRs. Pharmacovigilance programs should focus on the essential role of pharmacists in initiating proper ADR intervention and giving feedback to other health care settings.
Acknowledgments
We would like to acknowledge all the health care professionals who responded promptly to the questionnaire. We also appreciate the help of Ms Ihcene Benouared, Ms Alaa Saad, and Ms Ranim Almolki in assisting with data acquisition.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. World Health Organization. Safety of medicines: a guide to detecting and reporting adverse drug reactions. http://apps.who.int/iris/bitstream/10665/67378/1/WHO_EDM_QSM_2002.2.pdf. Published 2002. Accessed June 7, 2017.
- 2. Moore TJ, Psaty BM, Furberg CD. Time to act on drug safety. JAMA. 1998;279(19):1571-1573. [DOI] [PubMed] [Google Scholar]
- 3. Saha L. Role of pharmacovigilance in drug development. Enliven Pharmacovigil Drug Saf. 2014;1(1):9-10. [Google Scholar]
- 4. Ponmari SJ, Sivaraman M, Aruna T, Subashree V. Knowledge and awareness of pharmacovigilance among various medical fraternities. Asian J Pharmacol Toxicol. 2015;03(10):45-48. [Google Scholar]
- 5. Vallano A, Cereza G, Pedròs C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60(6):653-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hohl CM, McGrail K, Sobolev B. The effect of pharmacistled medication review in high-risk patients in the emergency department: an evaluation protocol. CMAJ open. 2015. January;3(1):E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aziz Z, Siang TC, Badarudin NS. Reporting of adverse drug reactions: predictors of under-reporting in Malaysia. Pharmacoepidemiol Drug Saf. 2007;16(2):223-228. [DOI] [PubMed] [Google Scholar]
- 8. Herdeiro MT, Figueiras A, Polónia J, Gestal-Otero JJ. Physicians’ attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf. 2005;28(9):825-833. [DOI] [PubMed] [Google Scholar]
- 9. Generali JA, Danish MA, Rosenbaum SE. Knowledge of and attitudes about adverse drug reaction reporting among Rhode Island pharmacists. Ann Pharmacother. 1995;29(4):365-369. [DOI] [PubMed] [Google Scholar]
- 10. Valente S, Murray L, Fisher D. Nurses improve medication safety with medication allergy and adverse drug reports. J Nurs Care Qual. 2007;22(4):322-327. [DOI] [PubMed] [Google Scholar]
- 11. Ahmad A, Patel I, Balkrishnan R, Mohanta GP, Manna PK. An evaluation of knowledge, attitude and practice of Indian pharmacists towards adverse drug reaction reporting: a pilot study. Perspect Clin Res. 2013;4(4):204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taché SV, Sönnichsen A, Ashcroft DM. Prevalence of adverse drug events in ambulatory care: a systematic review. Ann Pharmacother. 2011;45(7-8):977-989. [DOI] [PubMed] [Google Scholar]
- 13. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc. 1996;41(2):192-199. [DOI] [PubMed] [Google Scholar]
- 14. Jericho BG, Tassone RF, Centomani NM, et al. An assessment of an educational intervention on resident physician attitudes, knowledge, and skills related to adverse event reporting. J Grad Med Educ. 2010;2(2):188-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200-1205. [DOI] [PubMed] [Google Scholar]
- 16. Qassim S, Metwaly Z, Shamsain M, Al Hariri Y. Reporting adverse drug reactions: evaluation of knowledge, attitude and practice among community pharmacists in UAE. IOSR J Pharm. 2014;4(4):17-23. [Google Scholar]
- 17. Li Q, Zhang S, Chen H, et al. Awareness and attitudes of healthcare professionals in Wuhan, China to the reporting of adverse drug reactions. Chin Med J (Engl). 2004;117(6):856-861. [PubMed] [Google Scholar]
- 18. Hardeep Bajaj JK, Rakesh K. A survey on the knowledge, attitude and the practice of pharmacovigilance among the health care professionals in a teaching hospital in northern India. J Clin Diagn Res. 2013;7(1):97-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McLernon DJ, Bond CM, Lee AJ, et al. Patient views and experiences of making adverse drug reaction reports to the Yellow Card Scheme in the UK. Pharmacoepidemiol Drug Saf. 2011;20(5):523-531. [DOI] [PubMed] [Google Scholar]
- 20. Okezie EO, Olufunmilayo F. Adverse drug reactions reporting by physicians in Ibadan, Nigeria. Pharmacoepidemiol Drug Saf. 2008;17:517-522. [DOI] [PubMed] [Google Scholar]
- 21. Suyagh M, Farah D, Abu Farha R. Pharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Saudi Pharm J. 2014;23(2):147-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ. How do patients contribute to signal detection? a retrospective analysis of spontaneous reporting of adverse drug reactions in the UK’s yellow card scheme. Drug Saf. 2013;36(3):199-206. [DOI] [PubMed] [Google Scholar]
- 23. Parretta E, Rafaniello C, Magro L, et al. Improvement of patient adverse drug reaction reporting through a community pharmacist-based intervention in the Campania region of Italy. Expert Opin Drug Saf. 2014;13(suppl 1):S21-S29. [DOI] [PubMed] [Google Scholar]
- 24. Vessal G, Mardani Z, Mollai M. Knowledge, attitudes, and perceptions of pharmacists to adverse drug reaction reporting in Iran. Pharm World Sci. 2009;31:183-187. [DOI] [PubMed] [Google Scholar]
- 25. Van Grootheest AC, de Jong-van den Berg LTW. The role of hospital and community pharmacists in pharmacovigilance. Res Social Adm Pharm. 2005;1(1):12-33. [DOI] [PubMed] [Google Scholar]


