Abstract
Objective:
Worldwide, consequences of binge drinking are a major health and policy concern. This article reviews contemporary binge drinking definitions as well as different questionnaires and biomarkers that have been used in research settings to examine binge drinking behavior among young adults.
Method:
A review of electronic databases was conducted for binge drinking definitions, questionnaires, and biomarkers for the measurement of binge drinking in young adults (18–30 years).
Results:
Binge drinking is often defined as four or more drinks for females and five or more drinks for males on an occasion or in one sitting within a designated time frame (2 weeks vs. past 30 days). Several tools and questionnaires are available to identify young adult repeated binge drinkers. Biomarkers have been used to corroborate self-reported alcohol consumption, of which direct biomarkers such as phosphatidylethanol may be useful in confirming recent heavy drinking.
Conclusions:
It is important to measure binge drinking along a continuum and to use questions that allow for assessment of intensity, frequency, duration, and daily versus weekend consumption patterns. Open-ended questions that allow for intensity (number of drinks) and frequency can be used to determine dose-response relationships with respect to specific outcome measures. Direct alcohol biomarkers reflecting alcohol consumption over a period of several days are useful in conjunction with questionnaire data for identifying young adult binge drinkers.
Among world health organization (WHO) regions, consequences of binge are a major health and policy concern (Anderson, 2008; Kanny et al., 2013; WHO, 2014). Over time, this pattern of drinking—which has been called also been called heavy episodic drinking, among other terms—can have a marked impact on alcohol-attributable health outcomes (Rehm et al., 2010).
There are six designated WHO regions, among which the European Union (EU) is the heaviest-drinking region, with more than one fifth of the EU population (≥15 years) reporting heavy episodic drinking (WHO, 2014). EU and U.S. young adults (15–39 years) have high prevalence rates of binge drinking (Anderson, 2008; Kanny et al., 2013). Compared with previous generations, more present-day young adults drink to get drunk and consume 6–7 drinks per binge drinking episode, exceeding the current binge threshold of 4+/5+ drinks per episode (Davoren et al., 2016; Francis et al., 2014; Mundt et al., 2009; Tavolacci et al., 2016; White et al., 2006).
The changes in the intensity and frequency of binge drinking patterns have led researchers to propose new binge drinking definitions and methods (e.g., questionnaires, correlates such as blackouts and biomarkers) for evaluating the adverse consequences of binge drinking among young adults. The aim of this article was to review contemporary and new binge drinking definitions, as well as different questionnaires that have been used and validated to examine binge drinking behavior among young adults. In addition to updating this information, we also summarize the use of biomarkers and other correlates, such as blackouts, to examine alcohol-related harm among young adults.
Method
We searched MEDLINE, PubMed, and the Cochrane Database of Systematic Reviews to identify articles relevant to the measurement of young adult binge drinking and the use of biomarkers in young adults. A combination of search terms was used and included binge drinking, heavy drinking, heavy episodic drinking, alcohol consumption, college students, young adults, students, binge drinking questionnaires, and alcohol consumption biomarkers. Also reviewed were bibliographies of relevant review publications. For binge drinking questionnaire and biomarker studies, inclusion criteria were (a) the inclusion of U.S. or EU young adult (18–30 years) populations, (b) the use of an alcohol screening tool (e.g., Alcohol Use Disorders Identification Test [AUDIT]) or a questionnaire for categorizing hazardous alcohol consumption, and (c) the inclusion of a control or comparative nondrinking group.
Results
Binge drinking terms and definitions
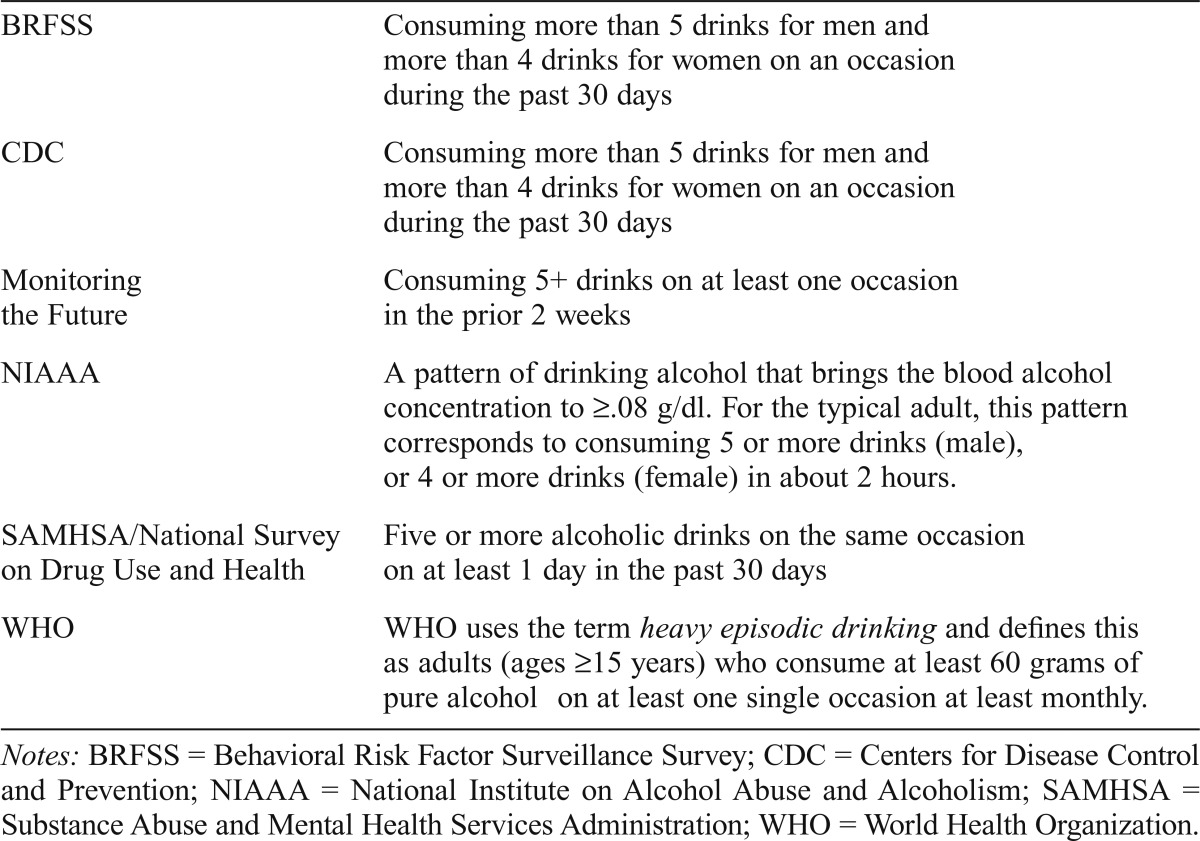
In the young adult literature and other reports, researchers have used different terms to describe binge drinking (Courtney & Polich, 2009). Terms have included heavy episodic drinking, drinking to intoxication, hazardous drinking, atrisk drinking, and heavy drinking days. The College Alcohol Study used the term binge drinking, whereas organizations such as WHO use the term heavy episodic drinking. These patterns of drinking have been defined differently. For example, in the College Alcohol Study, Wechsler and colleagues (1994) defined binge drinking as “consuming 5 drinks or more in a row for men (4 or more drinks for women) per occasion within the past 2 weeks” before the survey. WHO’s definition of heavy episodic drinking is somewhat similar, as are other definitions used by some U.S. national studies/data-bases (e.g., Behavioral Risk Factor Surveillance Survey) and by agencies such as the Substance Abuse and Mental Health Services Administration (Table 1). As noted, definitions are similar, although with variations on drinking quantity for men and women, the terms used to describe the period of consumption (e.g., on an occasion, in about 2 hours), and the time frame of past occurrences of binge drinking episodes (e.g., 2 weeks vs. past 30 days). These definitions, although useful for determining prevalence of binge drinking, do not sufficiently allow investigators to understand dose-response relationships and frequency over an extended or specific period (e.g., every week for 12 months).
Table 1.
Binge drinking definitions
| BRFSS | Consuming more than 5 drinks for men and more than 4 drinks for women on an occasion during the past 30 days |
| CDC | Consuming more than 5 drinks for men and more than 4 drinks for women on an occasion during the past 30 days |
| Monitoring the Future | Consuming 5+ drinks on at least one occasion in the prior 2 weeks |
| NIAAA | A pattern of drinking alcohol that brings the blood alcohol concentration to ≥08 g/dl. For the typical adult, this pattern corresponds to consuming 5 or more drinks (male), or 4 or more drinks (female) in about 2 hours. |
| SAMHSA/National Survey on Drug Use and Health | Five or more alcoholic drinks on the same occasion on at least 1 day in the past 30 days |
| WHO | WHO uses the term heavy episodic drinking and defines this as adults (ages ≥15 years) who consume at least 60 grams of pure alcohol on at least one single occasion at least monthly. |
Notes: BRFSS = Behavioral Risk Factor Surveillance Survey; CDC = Centers for Disease Control and Prevention; NIAAA = National Institute on Alcohol Abuse and Alcoholism; SAMHSA = Substance Abuse and Mental Health Services Administration; WHO = World Health Organization.
In 2004, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) defined binge as a pattern of drinking associated with producing a blood alcohol concentration (BAC) of at least .08 g/dl (Table 1). Although it is frequently used, some researchers have challenged the validity of the .08 g/dl cutoff because those with a larger body mass may not be detected as binge drinkers (Fillmore & Jude, 2011). Among college students (n = 251), Fillmore and Jude compared the two definitions of binge drinking (i.e., 5+/4+ definition vs. .08 g/dl definition) in terms of sensitivity and specificity for detecting “at-risk” drinkers (defined by a 6+/8+ cut score on the total AUDIT). Using the aforementioned AUDIT cutoff scores, among the total sample 56% were classified as at-risk drinkers. The 5+/4+ definition was effective at detecting more than 80% of individuals classified as at risk compared with 52% with the .08 g/dl definition. Those with a larger body mass were less likely to be detected as binge drinkers with the .08 g/dl definition. Importantly, however, this was not because they were drinking within safe limits, as these subjects had an AUDIT score that indicated at-risk drinking. It is not surprising that the estimated BAC demonstrated lower sensitivity in detecting at-risk drinking in individuals with greater body weights because the calculation incorporates body weight within the denominator of the estimated BAC equation.
Questionnaires and tools for the measurement of binge drinking
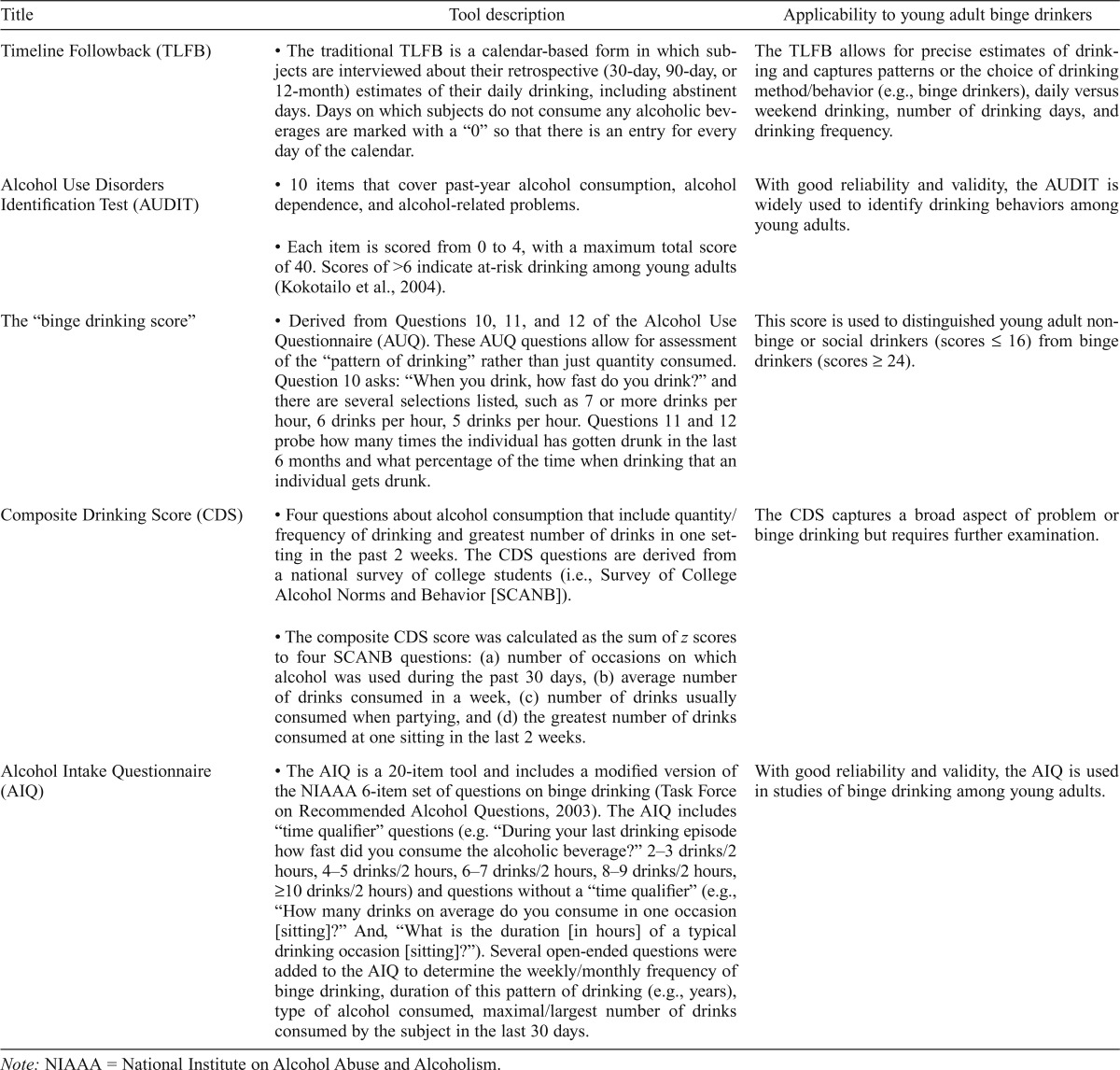
Several alcohol consumption questionnaires and screening tools have been used in young adults (Table 2). Sobell and Sobell (1992) developed the Timeline Followback (TLFB) method, a well-validated and reliable method for evaluating alcohol consumption (Table 2). They established the test–retest reliability of the TLFB in identifying heavy drinking days among Canadian college students (N = 80) as well as daily versus weekend drinking (correlation coefficients ≥ .76) (Sobell et al., 1986). Others have used the TLFB method to distinguish non–binge drinking college students from binge drinking college students (Kokotailo et al., 2004; Luczak et al., 2002).
Table 2.
Alcohol questionnaires and tools
| Title | Tool description | Applicability to young adult binge drinkers |
| Timeline Followback (TLFB) | • The traditional TLFB is a calendar-based form in which subjects are interviewed about their retrospective (30-day, 90-day, or 12-month) estimates of their daily drinking, including abstinent days. Days on which subjects do not consume any alcoholic beverages are marked with a “0” so that there is an entry for every day of the calendar. | The TLFB allows for precise estimates of drinking and captures patterns or the choice of drinking method/behavior (e.g., binge drinkers), daily versus weekend drinking, number of drinking days, and drinking frequency. |
| Alcohol Use Disorders Identification Test (AUDIT) | • 10 items that cover past-year alcohol consumption, alcohol dependence, and alcohol-related problems. | With good reliability and validity, the AUDIT is widely used to identify drinking behaviors among young adults. |
| • Each item is scored from 0 to 4, with a maximum total score of 40. Scores of >6 indicate at-risk drinking among young adults (Kokotailo et al., 2004). | ||
| The “binge drinking score” | • Derived from Questions 10, 11, and 12 of the Alcohol Use Questionnaire (AUQ). These AUQ questions allow for assessment of the “pattern of drinking” rather than just quantity consumed. Question 10 asks: “When you drink, how fast do you drink?” and there are several selections listed, such as 7 or more drinks per hour, 6 drinks per hour, 5 drinks per hour. Questions 11 and 12 probe how many times the individual has gotten drunk in the last 6 months and what percentage of the time when drinking that an individual gets drunk. | This score is used to distinguished young adult nonbinge or social drinkers (scores ≤ 16) from binge drinkers (scores ≥ 24). |
| Composite Drinking Score (CDS) | • Four questions about alcohol consumption that include quantity/ frequency of drinking and greatest number of drinks in one setting in the past 2 weeks. The CDS questions are derived from a national survey of college students (i.e., Survey of College Alcohol Norms and Behavior [SCANB]). | The CDS captures a broad aspect of problem or binge drinking but requires further examination. |
| • The composite CDS score was calculated as the sum of z scores to four SCANB questions: (a) number of occasions on which alcohol was used during the past 30 days, (b) average number of drinks consumed in a week, (c) number of drinks usually consumed when partying, and (d) the greatest number of drinks consumed at one sitting in the last 2 weeks. | ||
| Alcohol Intake Questionnaire (AIQ) | • The AIQ is a 20-item tool and includes a modified version of the NIAAA 6-item set of questions on binge drinking (Task Force on Recommended Alcohol Questions, 2003). The AIQ includes “time qualifier” questions (e.g. “During your last drinking episode how fast did you consume the alcoholic beverage?” 2–3 drinks/2 hours, 4–5 drinks/2 hours, 6–7 drinks/2 hours, 8–9 drinks/2 hours, ≥10 drinks/2 hours) and questions without a “time qualifier” (e.g., “How many drinks on average do you consume in one occasion [sitting]?” And, “What is the duration [in hours] of a typical drinking occasion [sitting]?”). Several open-ended questions were added to the AIQ to determine the weekly/monthly frequency of binge drinking, duration of this pattern of drinking (e.g., years), type of alcohol consumed, maximal/largest number of drinks consumed by the subject in the last 30 days. | With good reliability and validity, the AIQ is used in studies of binge drinking among young adults. |
Note: NIAAA = National Institute on Alcohol Abuse and Alcoholism.
Several brief alcohol use screening tools, such as the AUDIT, CAGE (cut down, annoyed, guilty, eye opener), and TWEAK screen (tolerance, worried, eye opener, amnesia, cut down) have been used to detect alcohol misuse in young adults. In terms of detecting a binge drinking pattern, however, the CAGE and TWEAK are limited in the information or dimensions that reflect drinking patterns, quantity/frequency, mean number of drinks per occasion, and binge drinking duration. In contrast, the AUDIT includes questions related to alcohol consumption frequency and amount and has been used to identify both alcohol dependence and at-risk heavy (binge) drinking among college students (Saunders et al., 1993) (Table 2). Among U.S. college students (N = 391, Mage = 20 years), the receiver operator curves demonstrated that the AUDIT had the highest area under the curve for detecting high-risk alcohol use (.872, 95% confidence interval [.83, .91]) and, at a cutoff score of 6 or more, detected 91% of high-risk drinkers (n = 88) (Kokotailo et al., 2004). High-risk drinking was defined as four or more occasions when 5+/4+ drinks were consumed in one sitting over the past 28 days for males and females, respectively.
Others have also found that an AUDIT score of 8 or more had good sensitivity (.82) and specificity (.78) for detecting at-risk drinking in young adults (Mage =19 years, n = 401) (DeMartini & Carey, 2012). The AUDIT-C (cutoff of 5) also had a high sensitivity (.82) and specificity (.82) for detecting at-risk drinking. However, receiver–operating characteristic curve analysis revealed that, compared with the AUDIT, the AUDIT-C was better at detecting at-risk drinkers. In that study, at-risk drinking was defined as “14 or more drinks in a typical week or at least 4 heavy drinking days in the past month for males and for females more than 7 drinks in a typical week or 4 heavy drinking episodes in the past month” (DeMartini & Carey, 2012).
Piano et al. (2015) compared AUDIT scores among U.S. young adult (M [SD] = 22 [3] years) binge (n = 58) and moderate (n = 22) drinkers and abstainers (n = 23). Binge drinking was defined as consuming 4+/5+ drinks (females/ males) on one occasion or within a 2-hour period within the last 30 days, and moderate drinking was defined as no more than 2–3 drinks per sitting no more than one to two times per week in the last 5 years. AUDIT scores were significantly greater in binge drinkers (M [SD] = 13 [4]) compared with moderate drinkers (M [SD] = 6 [3]) and abstainers (M [SD] = 0.6 [0.89]). Significant correlations were also found between AUDIT scores and whole blood phosphatidylethanol levels, a direct biomarker of heavy alcohol consumption (Spearman’s r = .74, p < .0001). Among Tanzanian young adult college students (18–24 years), Francis et al. (2015) found significant positive correlations between AUDIT-C scores and whole blood phosphatidylethanol levels (Spearman correlation coefficient, rs = .58; p < .001).
Other tools have been developed and tested. Townshend and Duka (2005) examined the validity of using a “binge drinking score” derived from the Alcohol Use Questionnaire (AUQ) developed by Mehrabian and Russell (1978) (Table 2). Using the binge drinking score to distinguish nonbinge or social drinkers from binge drinkers, they found differences in cognitive performance in the binge drinkers compared with nonbinge drinkers (Townshend & Duka, 2005). These authors previously determined that total AUQ scores as well as the individual binge drinking questions (10–12) were significantly correlated with alcohol intake and patterns noted from the completion of weekly diaries by the young adult subjects (Pearson’s r for questions 10, 11, and 12 were .592, .541, and .574, respectively) (Townshend & Duka, 2002).
Huang et al. (2006) examined the utility of calculating a Composite Drinking Scale (CDS) (Table 2) to evaluate problem or binge drinking among young adults. Students were classified as infrequent binge drinkers (binge drinking one or two times in a 2-week period) or frequent binge drinkers (three or more times in a 2-week period). Binge or heavy drinking for males was defined as consuming five or more drinks in one sitting at least once in the last 2 weeks and for females as consuming four or more drinks in one sitting at least once in the last 2 weeks. Among U.S. college students (n = 4,798, Mage = 21.8 years) surveyed, Huang et al. found good reliability: Cronbach’s α = .89 and item-total correlations of the four CDS items ranged between .65 and .81. To determine construct validity, CDS scores and individual CDS questions were compared with measures of alcohol-related problems within the last 30 days (e.g., drove under the influence). Also examined were factors known to be correlated with higher levels of binge drinking, such as being under 24 years of age, being White, and being a member of a fraternity or sorority. All of these measures were found to be strongly associated with the composite CDS score. As the authors acknowledge, however, more research is needed to establish a cutoff or national norm for the CDS.
The Alcohol Intake Questionnaire (AIQ) was developed to examine the adverse cardiovascular effects of repeated binge drinking among young adults (Goslawski et al., 2013; Piano et al., 2015). The AIQ is a 20-item tool and includes a modified version of the NIAAA six-item set of questions on binge drinking (Task Force on Recommended Alcohol Questions, 2003) (Table 2). The AIQ’s test–retest reliability was determined in 27 subjects. The average test–retest interval was 10.8 days (SD = 6.5 days, range: 7–23 days). Except for one item, the estimated test reliability was high (i.e., intra-class correlation for ratio-scale items ranged from .95 to 1.0; Spearman’s p ranged from .792 to .882; and Kendall’s Tau for interval-scale items ranged from .71 to .88). One item addressing the drinks consumed in the last drinking episode showed low test–retest reliability with a low Kendall’s Tau (.44); however, this finding was expected because part of this item includes the “last date/day” of the last binge drinking episode. Strong correlations were found between the maximum number of drinks consumed in the past 30 days and levels of the alcohol biomarker phosphatidylethanol (PEth) (whole blood PEth r = .637, p ≤ .0001) (Piano et al., 2015). There was also a significant correlation between whole blood PEth levels and the number of times subjects had consumed four to five drinks in one sitting within the last 30 days (r = .718, p ≤ .0001).
In summary, there are several brief alcohol screening tools and questionnaires for evaluating binge drinking in young adults (Table 2). An NIAAA Task Force (Task Force on Recommended Alcohol Questions, 2003) and other experts identified the need for more than two questions to measure the intensity and importantly to distinguish between a long-term, repeated binge drinking practice and infrequent or rare binge drinking episodes. Others have also shown that in college populations the AUDIT has good specificity and sensitivity for detecting alcohol abuse (according to criteria from the Diagnostic and Statistical Manual of Mental Disorders, Third Edition [DSM-III; American Psychiatric Association, 1980]). Therefore, the AUDIT may also aid in placing at-risk or binge drinking into the broader context of alcohol use disorders (Kokotailo et al., 2004).
Binge drinking and associated negative consequences
Negative drinking consequences, such as being hurt or injured after drinking, can be used in conjunction with the above questionnaires. Cranford and colleagues (2006) found that the odds of having been hurt or injured as a result of drinking in the past 12 months were nine times higher among past-year binge drinkers compared with nonbinge drinkers. Using the 28-day TLFB in college students, Mundt et al. (2009) found a positive association between heavy drinking days (greater than 4 drinks/day and up to 11 plus drinks/day) and the number of heavy drinking days in the past 28 days and alcohol-associated negative consequences. Alcohol negative consequences included accidents or injuries (e.g., a bad fall) in which alcohol drinking was a factor.
Although not designed to measure binge drinking per se, the Young Adult Alcohol Consequences Questionnaire (YAACQ) was developed to capture a broad spectrum of alcohol-related consequences (Read et al., 2006). Several domains within the YAACQ have questions about blackout drinking and physical dependence. Among young adults (n = 1,311, Mage =19 years), Read and colleagues (2016) examined different YAACQ cutoff values for delineating three levels (or zones) of hazardous drinking risk: low, moderate, and high. A cutoff of 8 or more differentiated those at low risk from those at moderate risk or greater, and a cutoff of 16 differentiated between moderate and high risk. In those with scores of 16 or greater (Zone 3), rates of binge drinking were nearly three times greater compared with Zone 1 (low risk), as were the scores for blackout drinking, impaired control, poor self-care, and physiological dependence (Read et al., 2016). Findings support the use of the YAACQ for assessing different levels of drinking risk.
Binge drinking can lead to a rapid rise in the BAC and therefore the occurrence of blackouts. Data from reports indicate that, among college students who consume alcohol, 20%–50% report having experienced an alcohol-induced blackout (Barnett et al., 2014; Hingson et al., 2016; Mundt et al., 2012). Others have examined whether alcohol-induced blackouts can serve as a marker for binge drinking–related harm. Hingson et al. (2016) found that binge drinking (4+ [females] or 5+ [males] drinks on an occasion at least once in the previous 30 days) was a significant predictor of blackouts. In that study, a blackout was defined as “forgetting where you were or what you did while drinking.” In addition, blackouts in the past 6 months were the strongest independent predictor of numerous negative outcomes, such as missing class or work and seeing a doctor because of an overdose (Hingson et al., 2016).
Wetherill and Fromme (2016) provide an excellent systematic review of the occurrence and measurement of blackouts. Alcohol-induced blackouts are often confused with “passing out from alcohol”; however, a blackout is defined as amnesia, or memory loss, for all or part of a drinking episode (Wetherill & Fromme, 2016). As noted by Wetherill and Fromme, a standardized definition of alcohol-induced blackouts is lacking. Thus, some type of definition should be provided within the questionnaire so that subjects do not confuse “passing out” with a blackout.
Biomarkers of binge drinking
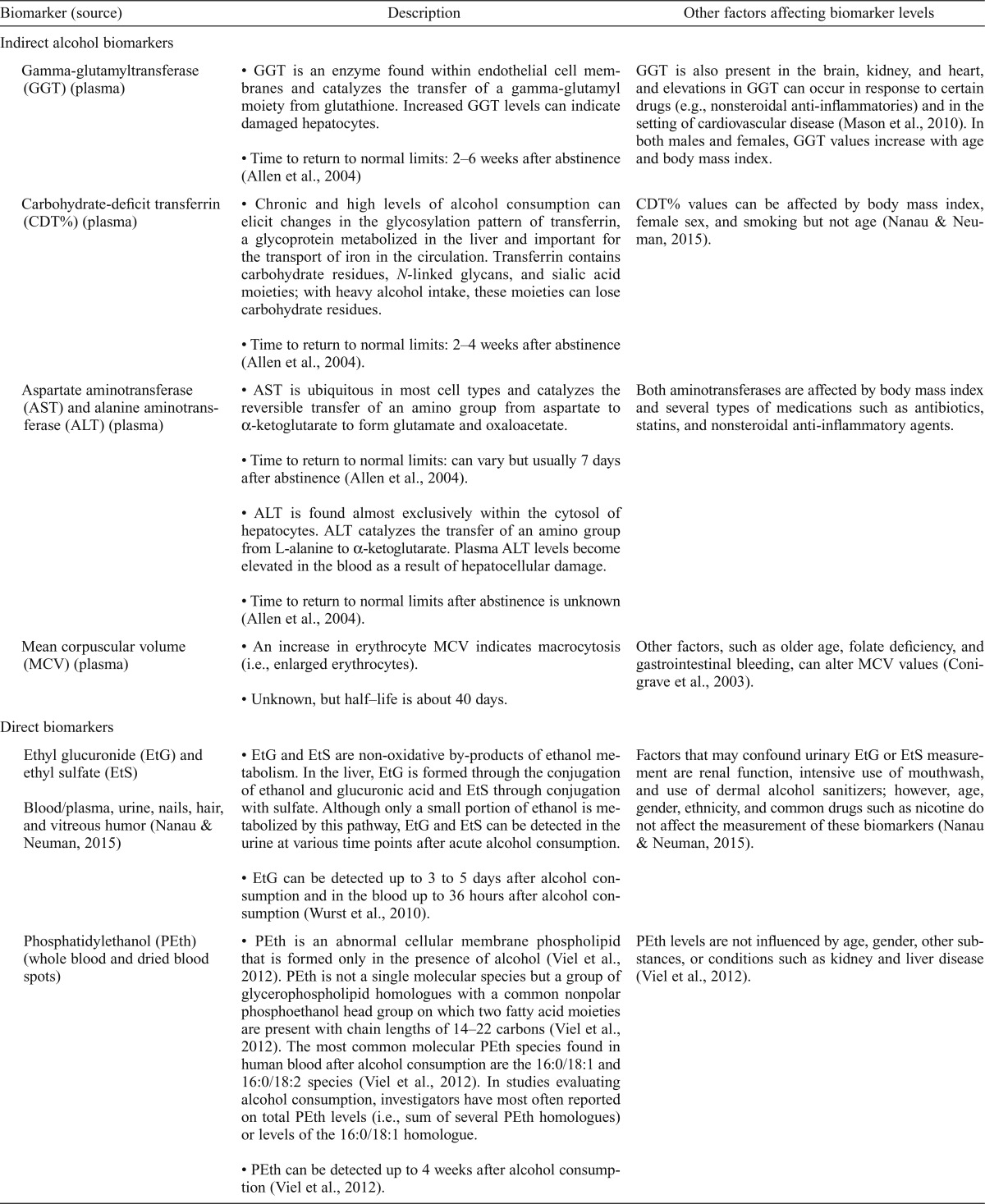
Biomarkers may corroborate the objective classification of nondrinkers, moderate drinkers, and heavy/binge drinkers. A variety of biomarkers are altered by regular excessive alcohol consumption (Conigrave et al., 2003; Gonzalo et al., 2014). Alcohol biomarkers are categorized into indirect or direct biomarkers of alcohol consumption. Indirect biomarkers are gamma-glutamyltransferase (GGT), carbohydrate-deficient transferrin (CDT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and mean corpuscular volume (MCV). Direct markers, which include ethyl glucuronide (EtG), ethyl sulfate (EtS), and PEth, are formed as a consequence of alcohol consumption (Table 3). In the below section, we briefly review these biomarkers and data from population and other studies that have included young adults and compared different levels and patterns of alcohol consumption.
Table 3.
Biomarker description
| Biomarker (source) | Description | Other factors affecting biomarker levels |
| Indirect alcohol biomarkers | ||
| Gamma-glutamyltransferase (GGT) (plasma) | • GGT is an enzyme found within endothelial cell membranes and catalyzes the transfer of a gamma-glutamyl moiety from glutathione. Increased GGT levels can indicate damaged hepatocytes. | GGT is also present in the brain, kidney, and heart, and elevations in GGT can occur in response to certain drugs (e.g., nonsteroidal anti-inflammatories) and in the setting of cardiovascular disease (Mason et al., 2010). In both males and females, GGT values increase with age and body mass index. |
| • Time to return to normal limits: 2–6 weeks after abstinence (Allen et al., 2004) | ||
| Carbohydrate-deficit transferrin (CDT%) (plasma) | • Chronic and high levels of alcohol consumption can elicit changes in the glycosylation pattern of transferrin, a glycoprotein metabolized in the liver and important for the transport of iron in the circulation. Transferrin contains carbohydrate residues, N-linked glycans, and sialic acid moieties; with heavy alcohol intake, these moieties can lose carbohydrate residues. | CDT% values can be affected by body mass index, female sex, and smoking but not age (Nanau & Neuman, 2015). |
| • Time to return to normal limits: 2–4 weeks after abstinence (Allen et al., 2004). | ||
| Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (plasma) | • AST is ubiquitous in most cell types and catalyzes the reversible transfer of an amino group from aspartate to α-ketoglutarate to form glutamate and oxaloacetate. | Both aminotransferases are affected by body mass index and several types of medications such as antibiotics, statins, and nonsteroidal anti-inflammatory agents. |
| • Time to return to normal limits: can vary but usually 7 days after abstinence (Allen et al., 2004). | ||
| • ALT is found almost exclusively within the cytosol of hepatocytes. ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate. Plasma ALT levels become elevated in the blood as a result of hepatocellular damage. | ||
| • Time to return to normal limits after abstinence is unknown (Allen et al., 2004). | ||
| Mean corpuscular volume (MCV) (plasma) | • An increase in erythrocyte MCV indicates macrocytosis (i.e., enlarged erythrocytes). | Other factors, such as older age, folate deficiency, and gastrointestinal bleeding, can alter MCV values (Conigrave et al., 2003). |
| • Unknown, but half–life is about 40 days. | ||
| Direct biomarkers | ||
| Ethyl glucuronide (EtG) and ethyl sulfate (EtS) | • EtG and EtS are non-oxidative by-products of ethanol metabolism. In the liver, EtG is formed through the conjugation of ethanol and glucuronic acid and EtS through conjugation with sulfate. Although only a small portion of ethanol is metabolized by this pathway, EtG and EtS can be detected in the urine at various time points after acute alcohol consumption. | Factors that may confound urinary EtG or EtS measurement are renal function, intensive use of mouthwash, and use of dermal alcohol sanitizers; however, age, gender, ethnicity, and common drugs such as nicotine do not affect the measurement of these biomarkers (Nanau & Neuman, 2015). |
| Blood/plasma, urine, nails, hair, and vitreous humor (Nanau & Neuman, 2015) | ||
| • EtG can be detected up to 3 to 5 days after alcohol consumption and in the blood up to 36 hours after alcohol consumption (Wurst et al., 2010). | ||
| Phosphatidylethanol (PEth) (whole blood and dried blood spots) | • PEth is an abnormal cellular membrane phospholipid that is formed only in the presence of alcohol (Viel et al., 2012). PEth is not a single molecular species but a group of glycerophospholipid homologues with a common nonpolar phosphoethanol head group on which two fatty acid moieties are present with chain lengths of 14–22 carbons (Viel et al., 2012). The most common molecular PEth species found in human blood after alcohol consumption are the 16:0/18:1 and 16:0/18:2 species (Viel et al., 2012). In studies evaluating alcohol consumption, investigators have most often reported on total PEth levels (i.e., sum of several PEth homologues) or levels of the 16:0/18:1 homologue. | PEth levels are not influenced by age, gender, other substances, or conditions such as kidney and liver disease (Vieletal., 2012). |
| • PEth can be detected up to 4 weeks after alcohol consumption (Viel et al., 2012). |
Indirect biomarkers
In a cross-sectional study including individuals between 25 and 74 years (N = 6,962), GGT levels had a low to moderate but significant association with alcohol consumption during the preceding week (r = .28 males, r = .14 females), during the past year (r = .30 males, r = .13 females), and with the frequency of alcohol intoxication (r = .21 males, r = .09 females) (Sillanaukee et al., 2000). In males and females, increasing alcohol consumption thresholds (>73.7 g/week in males and >60.3 g/week in females) increased the risk of having higher GGT levels (Sillanaukee et al., 2000). Meerkerk and colleagues (1999) examined the effects of different drinking patterns (irregular excessive, regular excessive, and very excessive) on changes in GGT, in men (N = 524) between ages 18 and 70 years. Irregular drinking (i.e., which was qualified as binge drinking) was defined as consuming at least six glasses of alcohol per day during the 9 to 20 days/month (on average more than 13 glasses per week). In this study, GGT had a low predictive value (.05) and sensitivity (.29) for detecting binge drinking (Meerkerk et al., 1999). Pirro et al. (2011) examined the potential of various biomarkers for discriminating among active heavy drinkers (n = 59, those who consumed more than 60 g ethanol/day), social drinkers (n = 51, social drinking not defined), and nondrinkers (n = 65, no alcohol for past 6 months) (age range: 22–74 years). In that study, GGT was significantly higher in heavy drinkers and could distinguish heavy drinkers from both social drinkers and nondrinkers. However, Piano et al. (2015) reported that, in young adults (Mage = 22 years), there were no differences in GGT values among abstainers (n = 22), moderate drinkers (n = 23), and binge drinkers (n = 58) (past-month average number of binge episodes was 7.2).
Compared with other indirect biomarkers, CDT% has good sensitivity and specificity for detecting chronic alcohol consumption greater than 60 g/day with no changes at levels lower than 30 g/day (Gonzalo et al., 2014). However, in terms of detecting binge or irregular excessive drinking among men (n = 524) between ages 18 and 70 years, Meerkerk et al. (1999) found that CDT% had low sensitivity (.24), high specificity (.92), and a low positive predictive value (.11). Sillanaukee et al. (2000) found that CDT% had a low to moderate but significant association with alcohol consumption during the preceding week (r = .28 males, r = .19 females), during the past year (r = .29 males, r = .16 females), and with the frequency of alcohol intoxication (r = .24 males, r = .22 females). Pirro et al. (2011) also examined CDT% for discriminating heavy drinkers, social drinkers, and nondrinkers and reported that CDT% was significantly greater in heavy drinkers and distinguished heavy drinkers from both social drinkers and nondrinkers.
The aminotransferases (AST and ALT) have also been used for detecting chronic heavy drinking, but no studies were found that examined aminotransferase levels and binge drinking per se among young adults (Allen, 2004). However, Pirro et al. (2011) found that AST levels were greater in heavy drinkers (men and women combined, consuming >60 g ethanol/day) compared with social drinkers and nondrinkers, whereas ALT levels were similar among all three drinking categories. In that same study, based on cutoff values for AST (40 IU/L) and ALT (50 IU/L), sensitivity and specificity for identifying alcohol abuse (defined by DSM-IV [American Psychiatric Association, 1994] criteria) were low (AST sensitivity = .475, ALT sensitivity = .356; AST [1 – specificity] = .181; ALT [1 – specificity] = .198). Similarly, in a randomized study of alcohol abstinence and alcohol consumption, among men and women (Mage = 33/34 years), the daily consumption of red wine (1.3 standard drinks for women and 2.7 for men) resulted in no change in ALT levels, but a 13% increase in AST values (Kechagias et al., 2015). However, mean AST values (M [SD] = 26 [6] U/L) were within normal range. Area under the receiver–operating curve was .5 and .61 for AST and ALT, respectively, indicating poor discrimination between abstention and moderate alcohol intake (Kechagias et al., 2015). Others have reported that using the ratio of AST/ALT may provide a more meaningful reflection of chronic heavy drinking, with a cutoff value of the ratio greater than 2 indicating chronic heavy alcohol intake (Allen, 2004).
An increase in erythrocyte MCV has been used as a biomarker of long-term heavy alcohol consumption. Others (Conigrave et al., 2003) have reported that MCV has low sensitivity in younger individuals. In addition, Piano et al. (2015) found no changes in MCV values among young adult binge drinkers, and values were within normal range (mean value was 87 fL, normal range: 82–96 fL).
Direct alcohol biomarkers
Direct alcohol biomarkers are formed as a direct consequence of alcohol use. Unlike indirect biomarkers, these biomarkers are not produced as a consequence of the toxic effects of alcohol on cells. In addition, other than blood, several of these biomarkers can be detected in other biologic matrices, such as hair, and some markers such as phosphatidylethanol can be measured using the dried blood spot method.
To our knowledge there are no reports that have examined different drinking patterns and the relationship to the formation of EtG or EtS levels in young adults. Urinary levels of EtG and EtS can be detected up to 3 to 5 days after alcohol consumption and in the blood up to 36 hours after alcohol consumption (Wurst et al., 2010). Given the short time frame for detection, EtG and EtS have been used to determine relapse or recent alcohol consumption in outpatients treated for alcohol-related problems. There are reports, however, of good correlations with the quantity of self-reported alcohol consumption in the 3 days before sample collection. In alcohol-dependent outpatients (26 women, 30 men, Mage = 50 years), Dahl and colleagues (2011) found significant correlations between the self-reported quantity of drinking over the past 3 days (measured using the TLFB) and urinary EtG and EtS levels (EtG r = .662, p < .001 and EtS r = .716, p < .001).
PEth is a direct biomarker of moderate and heavy drinking (Viel et al., 2012) and has been investigated in a number of different U.S. and international populations, including young adult binge drinkers. Others have reported that PEth levels were significantly correlated with validated self-report measures of alcohol consumption (Asiimwe et al., 2015; Francis et al., 2015; Hahn et al., 2012; Jain et al., 2014; Piano et al., 2015; Stewart et al., 2010). Moreover, PEth had a higher sensitivity (88%–100%) and specificity (88.5%–94%) for alcohol use detection compared with other alcohol biomarkers such as GGT, MCV, and CDT (Francis et al., 2015; Hahn et al., 2012; Helander et al., 2012; Jain et al., 2014; Stewart et al., 2010; Wurst et al., 2010). In young adults (Mage = 22 years), whole blood and dried blood spot PEth levels were significantly different among binge drinkers, moderate drinkers, and abstainers—showing that PEth may be a useful alcohol biomarker for binge drinking among young adults—whereas no differences were found in MCV or GGT among young adult groups (Piano et al., 2015). In addition, significant correlations between PEth levels and total AUDIT scores were found in young adult binge drinkers (Piano et al., 2015). Last, in a clinical laboratory setting, Javors et al. (2016) found positive PEth levels (combined levels of homologues 16:0/18:1 and 16:0/18:2) in every subject (Mage = 27 years) who received single doses of ethanol (0.25 g/kg and 0.50 g/kg), demonstrating good sensitivity of PEth even after low doses of ethanol consumption.
Discussion
Limitations
This review provides a broad overview of alcohol consumption questionnaires, tools, and biomarkers used to evaluate binge drinking among young adults. A number of electronic databases were searched using a combination of search terms to ensure that relevant literature was included. However, it is possible that some literature was not identified and therefore not included in this review. We also did not discuss approaches for evaluating or estimating “unrecorded alcohol” consumption, which refers to alcohol that is not taxed in a country where it is consumed and includes homemade or informally produced alcohol. This may be a problem in world regions such as the WHO South-East Asia Region, where unrecorded alcohol consumption is estimated to represent about 50% of total alcohol consumption (WHO, 2014). This review, however, does highlight how, through the use of different questionnaires and biomarkers, in particular PEth, young adult binge drinking can be evaluated and dose-response relationships can be determined.
Conclusions
Considering the increase in intensity of binge drinking among young adults, it is important to use questionnaires with open-ended questions that allow for assessment of intensity (number of drinks) and frequency of binge drinking. Answers to open-ended questions can be used to determine dose-response relationships with respect to specific outcome measures (Mundt et al., 2009). The risk of binge drinking–related harm (health and social consequences) increases with frequency and duration (Esser et al., 2012). Therefore, it is also important to distinguish between a long-term, repeated binge drinking practice and infrequent or rare binge drinking episodes. To this end, several of the tools/questionnaires reviewed above allow for these determinations. Direct ethanol biomarkers may be useful in conjunction with questionnaire data for identifying and categorizing young adult repeated binge drinkers.
Compared with previous generations, the pervasiveness, regularity, and intensity (i.e., 6–7 drinks) of binge drinking may place today’s youth at greater risk for more profound rates of alcohol-related harm and long-term health consequences. More research is needed to elucidate the adverse health effects associated with binge drinking, with the goal of raising population awareness about the dangers of repeated binge drinking and formulating health care messages that discourage binge drinking.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant R21 AA024535.
References
- Allen J. P., Sillanaukee P., Strid N., Litten R. Z. Biomarkers of heavy drinking. In: Allen J. P., Wilson V. B., editors. Assessing alcohol problems: A guide for clinicians and researchers [NIH Publication No. 03-3745] (2nd ed., pp. 37–54) Bethesda, MD: National Institutes of Health; 2004. Retrieved from https://pubs.niaaa.nih.gov/publications/assessingalcohol/ [Google Scholar]
- American Psychiatric Association. 3rd ed. Washington, DC: Author; 1980. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: Author; 1994. Diagnostic and statistical manual ofmental disorders. [Google Scholar]
- Anderson P. Binge drinking and Europe. Hamm, Germany: Deutsche Hauptsteele fur Suchtfragen e.V (DHS) [German Centre for Addiction Issues] 2008 Retrieved from http://www.dhs.de/fileadmin/user_upload/pdf/Pathways_for_Health-Project/binge_drinking_and_europe_report.pdf.
- Asiimwe S. B., Fatch R., Emenyonu N. I., Muyindike W. R., Kekibiina A., Santos G.-M., Hahn J. A. Comparison of traditional and novel self-report measures to an alcohol biomarker for quantifying alcohol consumption among HIV-infected adults in sub-Saharan Africa. Alcoholism: Clinical and Experimental Research. 2015;39:1518–1527. doi: 10.1111/acer.12781. doi:10.1111/acer.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett N. P., Clerkin E. M., Wood M., Monti P. M., O’Leary Tevyaw T., Corriveau D., Kahler C. W. Description and predictors of positive and negative alcohol-related consequences in the first year of college. Journal of Studies on Alcohol and Drugs. 2014;75:103–114. doi: 10.15288/jsad.2014.75.103. doi:10.15288/jsad.2014.75.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conigrave K. M., Davies P., Haber P., Whitfield J. B. Traditional markers of excessive alcohol use. Addiction. 2003;98(Supplement 2):31–43. doi: 10.1046/j.1359-6357.2003.00581.x. doi:10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- Courtney K. E., Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135:142–156. doi: 10.1037/a0014414. doi:10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford J. A., McCabe S. E., Boyd C. J. A new measure of binge drinking: Prevalence and correlates in a probability sample of undergraduates. Alcoholism: Clinical and Experimental Research. 2006;30:1896–1905. doi: 10.1111/j.1530-0277.2006.00234.x. doi:10.1111/j.1530-0277.2006.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H., Hammarberg A., Franck J., Helander A. Urinary ethyl glucuronide and ethyl sulfate testing for recent drinking in alcohol-dependent outpatients treated with acamprosate or placebo. Alcohol and Alcoholism. 2011;46:553–557. doi: 10.1093/alcalc/agr055. doi:10.1093/alcalc/agr055. [DOI] [PubMed] [Google Scholar]
- Davoren M. P., Demant J., Shiely F., Perry I. J. Alcohol consumption among university students in Ireland and the United Kingdom from 2002 to 2014: A systematic review. BMC Public Health. 2016;16:173. doi: 10.1186/s12889-016-2843-1. doi:10.1186/s12889-016-2843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini K. S., Carey K. B. Optimizing the use of the AUDIT for alcohol screening in college students. Psychological Assessment. 2012;24:954–963. doi: 10.1037/a0028519. doi:10.1037/a0028519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser M. B., Kanny D., Brewer R. D., Naimi T. S. Binge drinking intensity: A comparison of two measures. American Journal of Preventive Medicine. 2012;42:625–629. doi: 10.1016/j.amepre.2012.03.001. doi:10.1016/j.amepre.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore M. T., Jude R. Defining binge drinking as five drinks per occasion or drinking to a .08% BAC: Which is more sensitive to risk? American Journal on Addictions. 2011;20:468–475. doi: 10.1111/j.1521-0391.2011.00156.x. doi:10.1111/j.1521-0391.2011.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J. M., Grosskurth H., Changalucha J., Kapiga S. H., Weiss H. A. Systematic review and meta-analysis: Prevalence of alcohol use among young people in eastern Africa. Tropical Medicine & International Health. 2014;19:476–488. doi: 10.1111/tmi.12267. doi:10.1111/tmi.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis J. M., Weiss H. A., Helander A., Kapiga S. H., Changalucha J., Grosskurth H. Comparison of self-reported alcohol use with the alcohol biomarker phosphatidylethanol among young people in northern Tanzania. Drug and Alcohol Dependence. 2015;156:289–296. doi: 10.1016/j.drugalcdep.2015.09.027. doi:10.1016/j.drugalcdep.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Gonzalo P., Radenne S., Gonzalo S. Biomarkers of chronic alcohol use. Current Biomarker Findings. 2014;4:9–22. doi:10.2147/CBF. S37239. [Google Scholar]
- Goslawski M., Piano M. R., Bian J. T., Church E. C., Szczurek M., Phillips S. A. Binge drinking impairs vascular function in young adults. Journal of the American College of Cardiology. 2013;62:201–207. doi: 10.1016/j.jacc.2013.03.049. doi:10.1016/j.jacc.2013.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Dobkin L. M., Mayanja B., Emenyonu N. I., Kigozi I. M., Shiboski S., Wurst F. W. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in subSaharan Africa. Alcoholism: Clinical and Experimental Research. 2012;36:854–862. doi: 10.1111/j.1530-0277.2011.01669.x. doi:10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander A., Péter O., Zheng Y. Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol and Alcoholism. 2012;47:552–557. doi: 10.1093/alcalc/ags065. doi:10.1093/alcalc/ags065. [DOI] [PubMed] [Google Scholar]
- Hingson R., Zha W., Simons-Morton B., White A. Alcohol-induced blackouts as predictors of other drinking related harms among emerging young adults. Alcoholism: Clinical and Experimental Research. 2016;40:776–784. doi: 10.1111/acer.13010. doi:10.1111/acer.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. H., DeJong W., Schneider S. K., Towvim L. G. Measuring college student drinking: Illustrating the feasibility of a composite drinking scale. Substance Abuse. 2006;27:33–45. doi: 10.1300/J465v27n01_05. doi:10.1300/J465v27n01_05. [DOI] [PubMed] [Google Scholar]
- Jain J., Evans J. L., Briceño A., Page K., Hahn J. A. Comparison of phosphatidylethanol results to self-reported alcohol consumption among young injection drug users. Alcohol and Alcoholism. 2014;49:520–524. doi: 10.1093/alcalc/agu037. doi:10.1093/alcalc/agu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javors M. A., Hill-Kapturczak N., Roache J. D., Karns-Wright T. E., Dougherty D. M. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcoholism: Clinical and Experimental Research. 2016;40:1228–1234. doi: 10.1111/acer.13062. doi:10.1111/acer.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanny D., Liu Y., Brewer R. D., Lu H. Binge drinking - United States, 2011. MMWR Supplements. 2013;62(3):77–80. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/su6203a13.htm. [PubMed] [Google Scholar]
- Kechagias S., Dernroth D. N., Blomgren A., Hansson T., Isaksson A., Walther L., Nystrom F. H. Phosphatidylethanol compared with other blood tests as a biomarker of moderate alcohol consumption in healthy volunteers: A prospective randomized study. Alcohol and Alcoholism. 2015;50:399–406. doi: 10.1093/alcalc/agv038. doi:10.1093/alcalc/agv038. [DOI] [PubMed] [Google Scholar]
- Kokotailo P. K., Egan J., Gangnon R., Brown D., Mundt M., Fleming M. Validity of the Alcohol Use Disorders Identification Test in college students. Alcoholism: Clinical and Experimental Research. 2004;28:914–920. doi: 10.1097/01.alc.0000128239.87611.f5. doi:10.1097/01.ALC.0000128239.87611.F5. [DOI] [PubMed] [Google Scholar]
- Luczak S. E., Shea S. H., Carr L. G., Li T. K., Wall T. L. Binge drinking in Jewish and non-Jewish white college students. Alcoholism: Clinical and Experimental Research. 2002;26:1773–1778. doi: 10.1097/01.ALC.0000042150.71818.A0. doi:10.1111/j.1530-0277.2002.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Mason J. E., Starke R. D., Van Kirk J. E. Gamma-glutamyl transferase: A novel cardiovascular risk biomarker. Preventive Cardiology. 2010;13:36–41. doi: 10.1111/j.1751-7141.2009.00054.x. doi:10.1111/j.1751-7141.2009.00054.x. [DOI] [PubMed] [Google Scholar]
- Meerkerk G. J., Njoo K. H., Bongers I. M., Trienekens P., van Oers J. A. Comparing the diagnostic accuracy of carbohydrate-deficient transferrin, gamma-glutamyltransferase, and mean cell volume in a general practice population. Alcoholism: Clinical and Experimental Research. 1999;23:1052–1059. doi:10.1111/j.1530-0277.1999.tb04224.x. [PubMed] [Google Scholar]
- Mehrabian A., Russell J. A. A questionnaire measure of habitual alcohol use. Psychological Reports. 1978;43:803–806. doi: 10.2466/pr0.1978.43.3.803. doi:10.2466/pr0.1978.43.3.803. [DOI] [PubMed] [Google Scholar]
- Mundt M. P., Zakletskaia L. I., Brown D. D., Fleming M. F. Alcohol-induced memory blackouts as an indicator of injury risk among college drinkers. Injury Prevention. 2012;18:44–49. doi: 10.1136/ip.2011.031724. doi:10.1136/ip.2011.031724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt M. P., Zakletskaia L. I., Fleming M. F. Extreme college drinking and alcohol-related injury risk. Alcoholism: Clinical and Experimental Research. 2009;33:1532–1538. doi: 10.1111/j.1530-0277.2009.00981.x. doi:10.1111/j.1530-0277.2009.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanau R. M., Neuman M. G. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339–1385. doi: 10.3390/biom5031339. doi:10.3390/biom5031339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano M. R., Tiwari S., Nevoral L., Phillips S. A. Phosphati-dylethanol levels are elevated and correlate strongly with AUDIT scores in young adult binge drinkers. Alcohol and Alcoholism. 2015;50:519–525. doi: 10.1093/alcalc/agv049. doi:10.1093/alcalc/agv049. [DOI] [PubMed] [Google Scholar]
- Pirro V., Valente V., Oliveri P., De Bernardis A., Salomone A., Vincenti M. Chemometric evaluation of nine alcohol biomarkers in a large population of clinically-classified subjects: Pre-eminence of ethyl glucuronide concentration in hair for confirmatory classification. Analytical and Bioanalytical Chemistry. 2011;401:2153–2164. doi: 10.1007/s00216-011-5314-7. doi:10.1007/ s00216-011-5314-7. [DOI] [PubMed] [Google Scholar]
- Read J. P., Haas A. L., Radomski S., Wickham R. E., Borish S. E. Identification of hazardous drinking with the Young Adult Alcohol Consequences Questionnaire: Relative operating characteristics as a function of gender. Psychological Assessment. 2016;28:1276–1289. doi: 10.1037/pas0000251. doi:10.1037/pas0000251. [DOI] [PubMed] [Google Scholar]
- Read J. P., Kahler C. W., Strong D. R., Colder C. R. Development and preliminary validation of the Young Adult Alcohol Consequences Questionnaire. Journal of Studies on Alcohol. 2006;67:169–177. doi: 10.15288/jsa.2006.67.169. doi:10.15288/jsa.2006.67.169. [DOI] [PubMed] [Google Scholar]
- Rehm J., Baliunas D., Borges G. L. G., Graham K., Irving H., Kehoe T., Taylor B. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. doi:10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. B., Aasland O. G., Babor T. F., de la Fuente J. R., Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. doi:10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sillanaukee P., Massot N., Jousilahti P., Vartiainen E., Sundvall J., Olsson U., Alho H. Dose response of laboratory markers to alcohol consumption in a general population. American Journal of Epidemiology. 2000;152:747–751. doi: 10.1093/aje/152.8.747. doi:10.1093/aje/152.8.747. [DOI] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten R. Z., Allen J., editors. Measuring alcohol consumption: Psychosocial and biological methods (pp. 41–72) Clifton, NJ: Humana Press; 1992. [Google Scholar]
- Sobell M. B., Sobell L. C., Klajner F., Pavan D., Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: Utility for alcohol research. Addictive Behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. doi:10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Stewart S. H., Law T. L., Randall P. K., Newman R. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcoholism: Clinical and Experimental Research. 2010;34:488–492. doi: 10.1111/j.1530-0277.2009.01113.x. doi:10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force on Recommended Alcohol Questions. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. Recommended alcohol questions. Retrieved from https://www.niaaa.nih.gov/research/guidelines-and-resources/recommended-alcohol-questions. [Google Scholar]
- Tavolacci M. P., Boerg E., Richard L., Meyrignac G., Dechelotte P., Ladner J. Prevalence of binge drinking and associated behaviours among 3286 college students in France. BMC Public Health. 2016;16:178. doi: 10.1186/s12889-016-2863-x. doi:10.1186/s12889-016-2863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townshend J. M., Duka T. Patterns of alcohol drinking in a population of young social drinkers: A comparison of questionnaire and diary measures. Alcohol and Alcoholism. 2002;37:187–192. doi: 10.1093/alcalc/37.2.187. doi:10.1093/ alcalc/37.2.187. [DOI] [PubMed] [Google Scholar]
- Townshend J. M., Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcoholism: Clinical and Experimental Research. 2005;29:317–325. doi: 10.1097/01.alc.0000156453.05028.f5. doi:10.1097/01. ALC.0000156453.05028.F5. [DOI] [PubMed] [Google Scholar]
- Viel G., Boscolo-Berto R., Cecchetto G., Fais P., Nalesso A., Ferrara S. D. Phosphatidylethanol in blood as a marker of chronic alcohol use: A systematic review and meta-analysis. International Journal of Molecular Sciences. 2012;13:14788–14812. doi: 10.3390/ijms131114788. doi:10.3390/ijms131114788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H., Davenport A., Dowdall G., Moeykens B., Castillo S. Health and behavioral consequences of binge drinking in college: A national survey of students at 140 campuses. JAMA. 1994;272:1672–1677. doi:10.1001/jama.1994.03520210056032. [PubMed] [Google Scholar]
- Wetherill R. R., Fromme K. Alcohol-induced blackouts: A review of recent clinical research with practical implications and recommendations for future studies. Alcoholism: Clinical and Experimental Research. 2016;40:922–935. doi: 10.1111/acer.13051. doi:10.1111/acer.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. M., Kraus C. L., Swartzwelder H. Many college freshmen drink at levels far beyond the binge threshold. Alcoholism: Clinical and Experimental Research. 2006;30:1006–1010. doi: 10.1111/j.1530-0277.2006.00122.x. doi:10.1111/j.1530-0277.2006.00122.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Geneva, Switzerland: Author; 2014. Global status report on alcohol and health 2014. Retrieved from http://www.who.int/substance_abuse/publications/alcohol_2014/en/ [Google Scholar]
- Wurst F. M., Thon N., Aradottir S., Hartmann S., Wiesbeck G. A., Lesch O., Alling C. Phosphatidylethanol: Normalization during detoxification, gender aspects and correlation with other biomarkers and self-reports. Addiction Biology. 2010;15:88–95. doi: 10.1111/j.1369-1600.2009.00185.x. doi:10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]