Abstract
Objective:
The objective of this study was to examine associations between symptoms of alcohol hangover and depression, both cross-sectionally and prospectively.
Method:
Data were from a survey of young adults (N = 986, 60% female) initially recruited as part of an observational study of youth smoking. Participants reported past-year hangover symptoms, past-year frequency of heavy episodic drinking (HED), and past-week depression symptoms on two occasions separated by 1 year. Path analysis was used to evaluate prospective, directional associations linking symptoms of depression and hangover after taking into account their stabilities and cross-sectional associations. Individual differences in HED frequency were accounted for to permit interpretation of residual hangover score variance in terms of susceptibility to hangover effects.
Results:
Past-week depression and past-year hangover symptoms were associated at Time 1. Path analysis indicated that Time 1 depression symptoms were associated with elevated hangover symptoms a year later at Time 2. In contrast, Time 1 hangover symptoms did not predict future depression.
Conclusions:
Depression symptoms are associated with current and future hangover susceptibility. Hangover and depression overlap symptomatically and are empirically associated with one another, suggesting the possibility that common underlying causal mechanisms may contribute to both phenomena.
Epidemiological studies indicate that heavy alcohol use and depression are robustly associated with one another (Boden & Fergusson, 2011; Hasin et al., 2005), but uncertainty remains regarding the responsible causal mechanism(s). One strategy for gaining improved insight is to investigate how different indicators of alcohol involve- ment associate with depression. Using this approach in a prospective, population-based cohort study of Finnish adults, Paljärvi et al. (2009) found that the frequency of alcohol hangover at baseline emerged as the best predictor of depression symptoms and hospitalization for depression 5 years later. A reversed analysis indicated that elevated depressive symptoms at baseline also forecast frequent hangover at the 5-year follow-up.
Why might frequent hangover be associated with depression? Paljärvi et al. (2009) suggested that hangovers served as markers of heavy episodic drinking (HED). In support of this, they found that other indicators of heavy episodic use, such as frequency of intoxication and frequency of passing out from drinking, also were associated with later depression. In contrast, total monthly volume of ethanol consumed—a measure that is less informative with regard to the heaviness of alcohol use in specific episodes—was more weakly related to depression.
This pattern corresponds with results from a large-scale population-based study of Canadian adults, which indicated that measures of HED were strongly related to depression, total volume of alcohol consumption was a weaker predictor, and frequency of drinking was unrelated to depression (Graham et al., 2007). The presence of a hangover after drinking may be associated with depression because heavy episodic use is the crucial variable related to depression risk, and hangover simply functions as a good proxy for such episodes.
Another possibility is that there is some more direct connection between the hangover syndrome and depressive symptomatology. Notably, the symptoms of hangover (e.g., depressed mood, fatigue, difficulty concentrating) partially overlap with those of depression. Thus, hangover symptoms might summate with ongoing depressive experiences, making them more intense or salient to the sufferer. Symptomatic overlap between the two syndromes may be a clue that some shared underlying mechanism contributes to both conditions. If so, hangover sensitivity might be a trait marker of depression risk (or vice versa).
Alternatively, hangover events could amplify the burden of drinking by interfering with role obligations, thereby generating life stressors that trigger or prolong depression (McBride et al., 2016). To date, studies of alcohol-depression associations have included very limited assessments of hangover (if any), so there is little evidence available for evaluating these sorts of hypotheses.
The goal of the current study was to extend to the literature characterizing links between hangover and depression. Using data from a cohort study of young adults, we examined how symptoms of hangover and depression were related to one another cross-sectionally and prospectively over a 1-year interval. Conceptually, measures of hangover symptoms in a given period can reflect a mixture of individual differences in two distinguishable processes: (a) the frequency of HED and (b) susceptibility to hangover (Piasecki et al., 2010; Slutske et al., 2014; Verster et al., 2010). Frequent HED provides more opportunities for hangover to be experienced, whereas enhanced susceptibility increases the likelihood of hangover effects following the use of any alcohol.
Drinkers differ substantially in their liability to hangover at a constant level of alcohol exposure (Howland et al., 2008; Piasecki et al., 2012; Rohsenow et al., 2012). Laboratory- based alcohol challenge designs represent the gold standard for assessing variability in hangover susceptibility, but this construct can be approximated in survey research by using residual hangover frequency scores after covarying HED frequency (Piasecki et al., 2010; Slutske et al., 2014). In the current analysis, HED was included to explore whether depressive symptoms are primarily linked to heavy alcohol exposures, individual differences in hangover susceptibility, or both. Cross-lagged analyses addressed whether depression symptoms predict subsequent hangover symptoms or vice versa because the information about temporal priority may provide clues about possible causal effects.
Method
Participants
Data were drawn from the Social and Emotional Contexts of Adolescent Smoking Patterns project, a longitudinal observational study of smoking. All 9th and 10th graders attending 16 Chicago-area high schools (N = 12,970) were screened for smoking behavior. Approximately 6–8 weeks later, all current smokers and random samples of never- smokers and individuals who had previously experimented with smoking were invited to participate in the longitudinal cohort study (n = 3,654). A total of 1,344 agreed to participate and 1,263 (94%) completed the baseline survey. This cohort included 213 never-smokers, 304 former experimenters (smoked in the past year but not in the past 90 days, fewer than 100 lifetime cigarettes), 594 current experimenters (smoked in the past 90 days, fewer than 100 lifetime cigarettes), and 152 regular smokers (smoked in the past 30 days, more than 100 lifetime cigarettes). Participants were resurveyed on eight occasions spanning 7 years.
The current study used data from the Year 6 (subsequently referred to as T1) and Year 7 (T2) follow-ups, the two waves that included assessments of hangover symptoms. Specifically, analyses are limited to 986 participants (78% of baseline sample, 95% of the 1,043 completing assessments atT1) who provided complete data on depression symptoms, HED frequency, and hangover symptoms at each of the two final waves. The analyzed sample included 590 women (60%) and averaged 22.4 years of age (range: 20.2–25.5, SD = 0.8) at T1. The racial composition of the analyzed sample was as follows: White (n = 683, 69.3%), Black (n = 180, 18.3%), Asian (n = 43, 4.4%), Native Hawaiian/Pacific Islander (n = 16, 1.6%), Native American (n = 6, 0.6%), and more than one category (n = 58, 5.9%). Hispanic ethnicity was reported by 151 participants (15.3%). The protocol was approved by the Institutional Review Board at the University of Illinois at Chicago.
Measures
Depression symptoms.
The Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977), a widely used instrument developed for use with community samples, was administered at T1 and T2. The CES-D consists of 20 items assessing affective, somatic, and interpersonal symptoms of depression. Participants reported the frequency of each symptom over the past week using a 4-point scale: 0 (rarely or none of the time) to 3 (most or all of the time). Accordingly, scores could range from 0 to 60, with higher scores indicating a higher degree of past-week symptomatology. A cut score of 16 on the CES-D indicates the presence of clinically significant depressive symptomatology (Le- winsohn et al., 1997; Radloff, 1977). Internal consistency of the CES-D was high (T1 α = .90; T2 α = .89).
Hangover symptoms.
A five-item short form of the Hangover Symptoms Scale (HSS; Robertson et al., 2012; Slutske et al., 2003) was administered at T1 and T2. Participants were asked how frequently they experienced five symptoms (more tired than usual, headache, nauseous, very weak, and difficulty concentrating) on mornings after drinking in the past year using a scale ranging from 0 (never) to 5 (every time). Scores on each of the five items were dichotomized to indicate the presence or absence of each symptom in the past year and then summed to indicate total number of symptoms experienced over the past year (Slutske et al., 2003). Internal consistency was high (α = .86 at T1 and T2).
A separate item asked, “During the past 12 months, when you drank alcohol how often did you have a hangover the next morning?” using the same response options. Responses were used for descriptive purposes but were not included in main path analyses. This item was added because the original HSS did not include a simple, face-valid measure of the frequency of self-defined hangovers.
Heavy episodic drinking frequency.
Past-year frequency of HED was assessed at each wave using a single item: “During the last 12 months, how often did you have 5 or more drinks (males) or 4 or more drinks (females) containing any kind of alcohol within a two-hour period?” This item uses the definition of HED recommended by the National Institute on Alcohol Abuse and Alcoholism National Advisory Council, corresponding to a pattern of intake that is likely to yield blood alcohol concentrations (BACs) of .08 g/ dl or higher. Participants answered using a 10-level ordinal response scale (0 = never, 1 = 1 or 2 days, 2 = 3–11 days, 3 = 1 day a month, 4 = 2–3 days a month, 5 = 1 day a week, 6 = 2 days a week, 7 = 3–4 days a week 8 = 5–6 days a week, and 9 = every day).
Other drinking pattern descriptors.
At each time point, participants were asked to report the frequency of drinking any alcohol, the typical number of drinks per drinking day, the maximum number of drinks consumed in a 24-hour period, and the frequency of consuming this maximum number of drinks over the past year. Summary statistics from these items were used to provide a more comprehensive description of drinking behaviors in this sample.
Smoking frequency.
At each wave, participants were asked, “During the last 30 days, on how many days did you smoke cigarettes?” Participants could enter any number from 0 to 30.
Statistical analyses
Descriptive analyses characterized mean levels of depression symptoms, hangover symptoms, smoking frequency, and HED frequency and tested for sex differences in these domains given the well-established sex difference in depression (Hasin et al., 2005) and some findings indicating heightened hangover vulnerability in women compared with men (Piasecki et al., 2010). We also computed bivariate correlations among these measures and examined rates of HED, hangover, smoking, and clinically significant depressive symptoms at each wave.
An autoregressive cross-lagged path analysis was estimated using the SEM command in Stata/SE (Version 13.1, StataCorp LP, College Station, TX). The model included paths representing the stabilities and cross-lagged effects involving scores on CES-D, HSS, and HED frequency at T1 and T2. Sex and smoking frequency at T1 and T2 were included in the model as exogenous covariates. Sex (coded male = 1, female = 0) and T1 smoking frequency were included as predictors of all six endogenous variables (CES-D, HED, and hangover symptoms, all at T1 and T2). T2 smoking was specified as an exogenous predictor of depression symptoms, HED, and hangover symptoms at T2 only. Modeling the covariance between HED frequency and HSS scores at each wave allowed the residual variance in HSS scores to be interpreted more cleanly in terms of individual differences in hangover susceptibility. After fitting the initial model, we trimmed nonsignificant paths and estimated a more parsimonious final model.
Results
Descriptive analyses
At T1, 778 participants (78.9%) reported HED in the past year, 702 (71.2%) reported experiencing a past-year hangover, 520 (52.7%) smoked at least one cigarette in the past 30 days, and 299 (30.3%) scored 16 or higher on the CES-D. AtT2, 773 participants (78.4%) reported HED, 713 (72.3%) reported hangover, 485 (49.2%) reported smoking, and 287 (29.1%) scored 16 or higher on the CES-D. At both T1 and T2, mean and modal responses to other questions about drinking patterns indicated an approximately weekly frequency of drinking, a typical consumption of three to four drinks per drinking day, a maximum consumption in a 24- hour period averaging five to seven drinks, and consumption of the maximum amount 3−11 times per year.
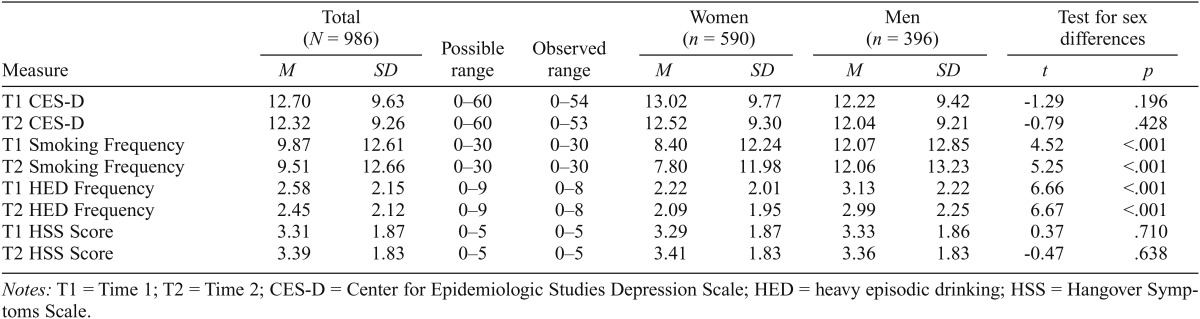
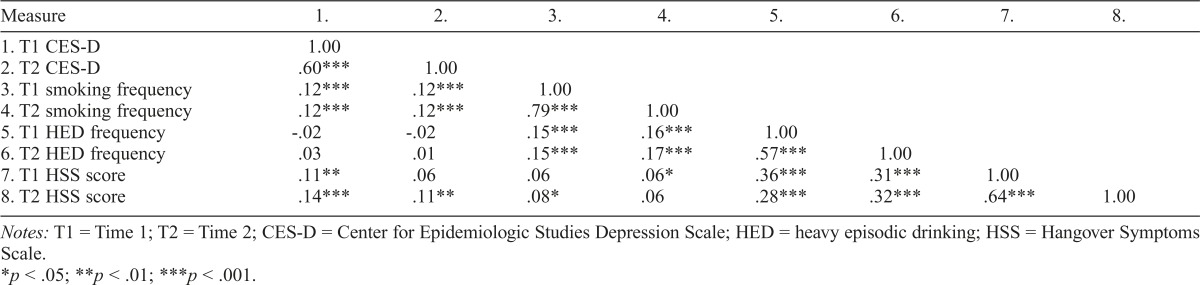
Table 1 presents descriptive statistics for the CES-D, smoking frequency, HED frequency, and HSS at both time points and corresponding tests for sex differences. Relative to men, women reported less frequent smoking and HED at each wave. There were no sex differences in CES-D or HSS scores at either time. Correlational analyses (Table 2) indicated that each measure showed moderate to substantial stability from T1 to T2. Most measures were significantly intercorrelated, with the exceptions that HED frequency was not related to depression symptoms at either time, T1 HSS was not correlated with T2 CES-D, and HSS scores were inconsistently related to smoking.
Table 1.
Descriptive statistics and tests for sex differences
| Measure | Total (N = 986) |
Possible range | Observed range | Women (n = 590) |
Men = 396) |
Test for sex differences |
||||
| M | SD | M | SD | M | SD | t | p | |||
| T1 CES-D | 12.70 | 9.63 | 0–60 | 0–54 | 13.02 | 9.77 | 12.22 | 9.42 | -1.29 | .196 |
| T2 CES-D | 12.32 | 9.26 | 0–60 | 0–53 | 12.52 | 9.30 | 12.04 | 9.21 | -0.79 | .428 |
| T1 Smoking Frequency | 9.87 | 12.61 | 0–30 | 0–30 | 8.40 | 12.24 | 12.07 | 12.85 | 4.52 | <.001 |
| T2 Smoking Frequency | 9.51 | 12.66 | 0–30 | 0–30 | 7.80 | 11.98 | 12.06 | 13.23 | 5.25 | <.001 |
| T1 HED Frequency | 2.58 | 2.15 | 0–9 | 0–8 | 2.22 | 2.01 | 3.13 | 2.22 | 6.66 | <.001 |
| T2 HED Frequency | 2.45 | 2.12 | 0–9 | 0–8 | 2.09 | 1.95 | 2.99 | 2.25 | 6.67 | <.001 |
| T1 HSS Score | 3.31 | 1.87 | 0–5 | 0–5 | 3.29 | 1.87 | 3.33 | 1.86 | 0.37 | .710 |
| T2 HSS Score | 3.39 | 1.83 | 0–5 | 0–5 | 3.41 | 1.83 | 3.36 | 1.83 | -0.47 | .638 |
Notes: T1 = Time 1; T2 = Time 2; CES-D = Center for Epidemiologic Studies Depression Scale; HED = heavy episodic drinking; HSS = Hangover Symptoms Scale.
Table 2.
Correlations among measures of depression, smoking frequency, heavy episodic drinking frequency, and hangover
| Measure | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. |
| 1.T1 CES-D | 1.00 | |||||||
| 2. T2 CES-D | .60*** | 1.00 | ||||||
| 3.T1 smoking frequency | 12*** | .12*** | 1.00 | |||||
| 4. T2 smoking frequency | .12*** | .12*** | 79*** | 1.00 | ||||
| 5. T1 HED frequency | -.02 | -.02 | .15*** | .16*** | 1.00 | |||
| 6. T2 HED frequency | .03 | .01 | .15*** | .17*** | .57*** | 1.00 | ||
| 7. T1 HSS score | .11** | .06 | .06 | .06* | .36*** | .31*** | 1.00 | |
| 8. T2 HSS score | 14*** | 11** | .08* | .06 | .28*** | .32*** | .64*** | 1.00 |
Notes: T1 = Time 1; T2 = Time 2; CES-D = Center for Epidemiologic Studies Depression Scale; HED = heavy episodic drinking; HSS = Hangover Symptoms Scale.
p < .05;
p < .01;
p < .001.
Exploratory cross-sectional correlation analyses were conducted to examine whether other indicators of drinking pattern were associated with depressive symptoms. CES-D scores were associated with a lower frequency of drinking (T1: r = -.09, p = .003; T2: r = -.07, p = .02), lower number of maximum drinks (T1: r = -.11, p = .001; T2: r = -.08, p = .01), and consuming the maximum drink total more frequently (T2: r = .07, p = .04).
Path analysis
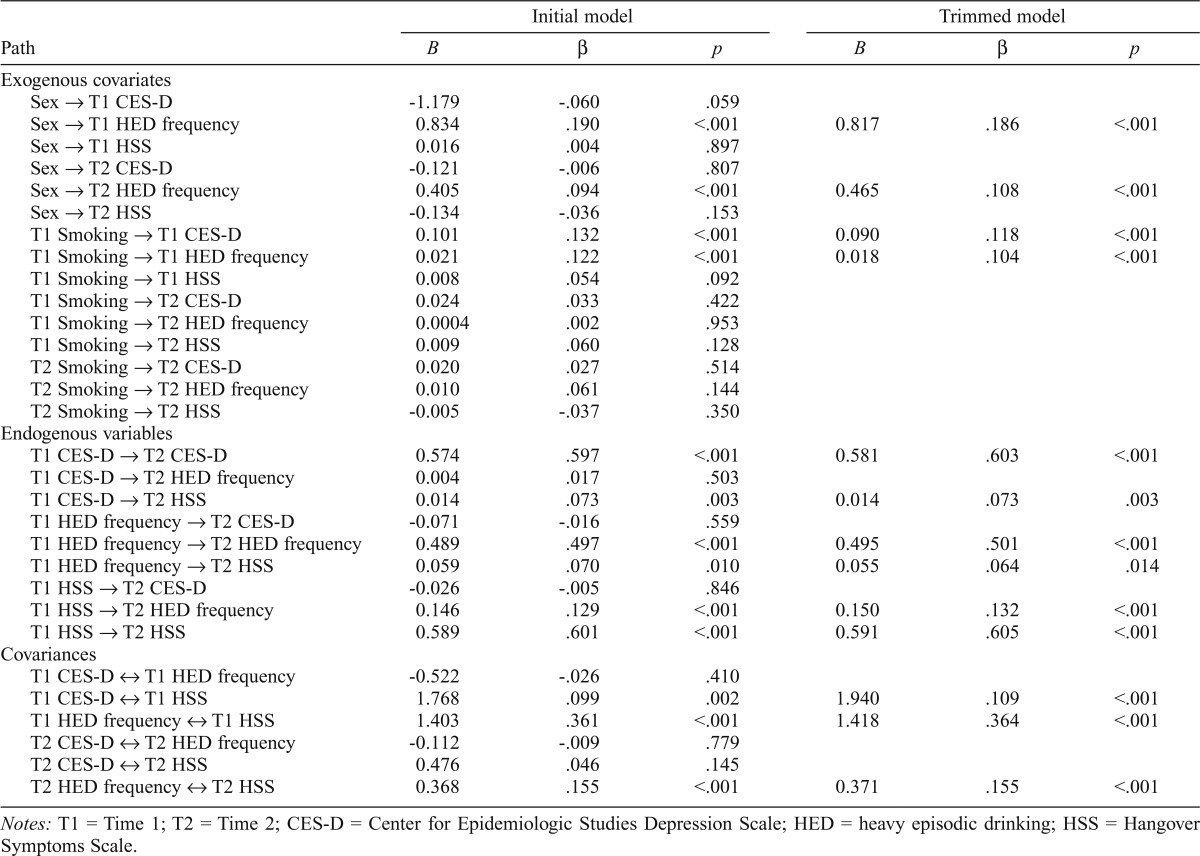
The initial path model fit the data well, χ2(3) = 5.03, p = .170; root mean square error of approximation (RMSEA) = .026; comparative fit index (CFI) = .999. The final model estimated after trimming all nonsignificant paths also fit the data well, χ2(14) = 21.39, p = .092; RMSEA = .023; CFI = .995. Standardized and unstandardized path coefficients from the initial model and final trimmed models are presented in Table 3.
Table 3.
Unstandardized (B) and standardized (p) path coefficients from the initial and final cross-lagged panel models
| Initial model |
Trimmed model |
|||||
| Path | B | β | p | B | β | p |
| Exogenous covariates | ||||||
| Sex → T1 CES-D | -1.179 | -.060 | .059 | |||
| Sex → T1 HED frequency | 0.834 | .190 | <.001 | 0.817 | .186 | <.001 |
| Sex → T1 HSS | 0.016 | .004 | .897 | |||
| Sex → T2 CES-D | -0.121 | -.006 | .807 | |||
| Sex → T2 HED frequency | 0.405 | .094 | <.001 | 0.465 | .108 | <.001 |
| Sex → T2 HSS | -0.134 | -.036 | .153 | |||
| T1 Smoking → T1 CES-D | 0.101 | .132 | <.001 | 0.090 | .118 | <.001 |
| T1 Smoking → T1 HED frequency | 0.021 | .122 | <.001 | 0.018 | .104 | <.001 |
| T1 Smoking → T1 HSS | 0.008 | .054 | .092 | |||
| T1 Smoking → T2 CES-D | 0.024 | .033 | .422 | |||
| T1 Smoking → T2 HED frequency | 0.0004 | .002 | .953 | |||
| T1 Smoking → T2 HSS | 0.009 | .060 | .128 | |||
| T2 Smoking → T2 CES-D | 0.020 | .027 | .514 | |||
| T2 Smoking → T2 HED frequency | 0.010 | .061 | .144 | |||
| T2 Smoking → T2 HSS | -0.005 | -.037 | .350 | |||
| Endogenous variables | ||||||
| T1 CES-D → T2 CES-D | 0.574 | .597 | <.001 | 0.581 | .603 | <.001 |
| T1 CES-D → T2 HED frequency | 0.004 | .017 | .503 | |||
| T1 CES-D → T2 HSS | 0.014 | .073 | .003 | 0.014 | .073 | .003 |
| T1 HED frequency → T2 CES-D | -0.071 | -.016 | .559 | |||
| T1 HED frequency → T2 HED frequency | 0.489 | .497 | <.001 | 0.495 | .501 | <.001 |
| T1 HED frequency → T2 HSS | 0.059 | .070 | .010 | 0.055 | .064 | .014 |
| T1 HSS → T2 CES-D | -0.026 | -.005 | .846 | |||
| T1 HSS → T2 HED frequency | 0.146 | .129 | <.001 | 0.150 | .132 | <.001 |
| T1 HSS → T2HSS | 0.589 | .601 | <.001 | 0.591 | .605 | <.001 |
| Covariances | ||||||
| T1 CES-D o↔ T1 HED frequency | -0.522 | -.026 | .410 | |||
| T1 CES-D ↔ T1 HSS | 1.768 | .099 | .002 | 1.940 | .109 | <.001 |
| T1 HED frequency ↔ T1 HSS | 1.403 | .361 | <.001 | 1.418 | .364 | <.001 |
| T2 CES-D ↔ T2 HED frequency | -0.112 | -.009 | .779 | |||
| T2 CES-D ↔ T2 HSS | 0.476 | .046 | .145 | |||
| T2 HED frequency ↔ T2 HSS | 0.368 | .155 | <.001 | 0.371 | .155 | <.001 |
Notes: T1 = Time 1; T2 = Time 2; CES-D = Center for Epidemiologic Studies Depression Scale; HED = heavy episodic drinking; HSS = Hangover Symptoms Scale.
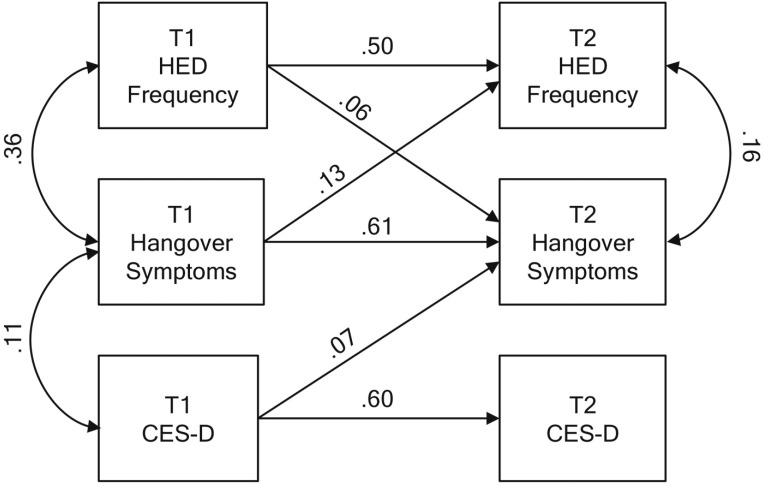
Figure 1 depicts the main results from the cross-lagged portion of the final model. Each of the measures showed moderate stability over time. Depression symptoms at T1 were significantly related to hangover symptoms at T2 (β = .073,p = .003). The path from T1 hangover to T2 depression symptoms was not significant in the initial model (β = -.005, p = .846) and was therefore dropped from the final model. HED frequency at T1 was associated with increased hangover symptoms at T2 and vice versa. No cross-lagged effects involving HED and depression symptoms were significant. At T1, depression and hangover symptoms had significant residual covariance, but this was not observed at T2. At each wave, HED frequency covaried with hangover symptoms but not depression symptoms.
Figure 1.
Main results from the final trimmed cross-lagged panel model. Numerical estimates represent standardized path coefficients. All depicted path coefficients were statistically significant. To simplify presentation, paths from covariates (sex and smoking) are omitted from the diagram. T1 = Time 1; T2 = Time 2; HED = heavy episodic drinking; CES-D = Center for Epidemiologic Studies Depression Scale.
To explore whether the association between T1 depression symptoms and T2 hangover symptoms was moderated by sex, we expanded the final model to include a path from sex to T2 HSS (i.e., sex main effect) and an additional interaction term variable (i.e., the product of sex and T1 CES-D) to T2 HSS. The interaction term path was not significant (β = -.11, p = .151) and the path fromT1 CESD to T2 HSS remained significant (β = .16, p = .015). Thus, the prospective association between depression symptoms and hangover was consistent across men and women.
Discussion
This study examined cross-sectional and prospective associations between hangover and depression symptoms using data from a prospective study of young adults collected across 1 year. HED frequency was included in the model to investigate whether depression symptoms are related to heavy alcohol exposures, individual differences in hangover susceptibility, or both. Past-week depression symptoms and past-year hangover were related at T1, even after accounting for individual differences in HED frequency. In the prospective portion of the model, depression symptoms at T1 forecast hangover susceptibility at T2. In contrast, hangover susceptibility at T1 did not predict depression symptoms a year later.
As expected, HED frequency was robustly associated with hangover symptoms. However, we found no evidence for an association between HED frequency and depression symptoms, even in simple correlational analyses unadjusted for hangover or other covariates (Table 1). The absence of this association is at odds with some prior findings and hypotheses (e.g., Graham et al., 2007; Paljarvi et al., 2009). Some other indicators of drinking pattern were cross-sectionally associated with CES-D scores, but most of these correlations suggested that depression was associated with modestly lower quantity and frequency of consumption. Further research is needed to clarify how diverse measures of alcohol intake relate to depression and to identify possible methodological sources of heterogeneity in these effects.
We conceptualize the residual variance in HSS scores after covarying HED frequency as a measure of hangover susceptibility. Following this logic, the findings from the path model can be interpreted as indicating that depression symptoms are associated with elevated current and future vulnerability to hangover effects after drinking. Why might this be? At present, various hypotheses can be formulated, but future, targeted research will be necessary to address them directly.
One possibility is that this effect is an artifact driven by predictor-criterion overlap. The symptoms of hangover and depression share many features. Thus, a depressed person may tend to achieve elevated scores on the HSS simply because he or she experiences these symptoms frequently. The HSS asks about the presence of these symptoms on mornings after drinking on the assumption that these are attributable to alcohol. This assessment strategy does not take into account the possibility that some individuals chronically experience a similar constellation of symptoms and would therefore endorse them on both mornings after drinking and mornings following abstention. It may be necessary to use refined assessment strategies, such as daily diary studies or reformulated hangover questionnaires more explicitly assessing symptomatic elevations after drinking versus abstention occasions, to better characterize the degree of “genuine” versus artifactual overlap between depression symptoms and hangover.
Another possibility is that the symptomatic overlap is not a nuisance or artifact, but instead a clue that the two syndromes may share some underlying pathological process. There has been relatively little research into the pathophysiology of alcohol hangover, but the limited available evidence implicates inflammatory processes (Penning et al., 2010). Circulating levels of pro-inflammatory cytokines and C- reactive protein are correlated with the intensity of acute hangover symptoms after high-dose alcohol exposure (Kim et al., 2003; Wiese et al., 2004). In preclinical models, systemic administration of the immunogen lipopolysaccharide triggers sickness behaviors closely resembling those produced by acute ethanol withdrawal (a term used synonymously with “hangover” in animal studies; Richey et al., 2012).
Lipopolysaccharide administration has also been used as an animal model of depression (e.g., Kubera et al., 2013; Yirmiya, 1996). More generally, mounting research points to an important role for activation of the inflammatory system in major depressive disorder (Miller & Raison, 2016; Slavich & Irwin, 2014). A dysregulated inflammatory process may contribute to depression, and, perhaps as a consequence, depressed individuals may be more sensitive to hangover—a syndrome that presumably involves a transient inflammatory response to a high dose of alcohol. The current study did not include measures of inflammatory biomarkers, so we could not test this hypothesis directly. Although this inflammation hypothesis is speculative, it appears plausible based on the available evidence and therefore deserves attention in future clinical and experimental research.
Other pathways implicated in depression (e.g., monoamine neurotransmitters; Krishnan & Nestler, 2008) merit attention but have less converging support from available hangover studies.
A final possibility is that hangovers are driven by many of the same psychological and environmental influences that contribute to depression and other forms of affective distress. Harburg et al. (1993) examined predictors of hangover in a large community sample. They found that psychosocial variables such as neuroticism, negative life events, drinking to escape negative emotions, guilt over drinking, and experiencing anger and depression when drunk incrementally predicted hangover after accounting for the amount of alcohol consumed. The authors suggested that hangovers may be better construed as psychosomatic reactions facilitated by drinking rather than inevitable physiologic responses to heavy alcohol exposure. The reproducibility of these findings requires testing because the hangover measure used in this investigation included some symptoms not conventionally considered part of the hangover syndrome (e.g., blackout, suicidal thoughts). Nonetheless, the findings highlight the potential value of investigating individual differences in psychological traits, drinking motives, circumstances of drinking, and life stress as possible explanations for the association between hangover and depression symptoms.
To our knowledge, this study represents the first longitudinal evaluation of the stability of HSS scores. The findings indicate that reports of past-year hangover symptoms were moderately stable across a 1-year period, as indicated by both zero-order correlations (Table 2) and when HED frequency and other covariates were accounted for (Table 3). This indicates that there is both consistency and change in the frequency of hangover events and individual differences in hangover susceptibility in young adulthood.
The findings should be interpreted in the context of study limitations. We examined data from a sample enriched for youthful smoking and risk of tobacco dependence. Smoking behavior was accounted for in the analyses, but it is not certain whether findings from this high-risk cohort will generalize to samples with other characteristics.
Young adulthood is a stage of life associated with especially high levels of depression (Sutin et al., 2013), HED (Centers for Disease Control and Prevention, 2012), and hangover (Piasecki et al., 2005; Tolstrup et al., 2014). Thus, the current analysis focused on a period when these phenomena are prevalent and have ample opportunity to influence one another. On the other hand, the peak prevalences may be achieved by mixing developmentally limited phenocopies with cases of more enduring alcohol problems or depressive conditions. Such a mixture could have the effect of attenuating associations among heavy drinking, hangover, and depression. Therefore, it would be valuable to extend this kind of work to a broader age range to test how well our findings generalize.
We interpreted the residual variation in HSS scores after accounting for HED frequency in terms of individual differences in hangover susceptibility. This is an approximation and is undoubtedly less precise than using a laboratory-based alcohol challenge procedure.
Hangovers are most reliably observed when BACs exceed .11 g/dl (Chapman, 1970; Verster et al., 2010), and hangover events are optimally identified using a higher threshold than is conventionally used to define a heavy drinking episode (about 13 drinks for men, 10 drinks for women; Jackson, 2008). The HED measure used in the current analysis was intended to identify drinking episodes in which an individual was likely to exceed a BAC of .08 g/dl. Some of the residual variance in HSS scores may function as a proxy for very heavy exposures exceeding the .08 g/dl threshold. This might explain why T1 HSS scores predicted T2 heavy drinking, a finding that appears at odds with results of a daily diary study indicating that hangovers do not strongly influence subsequent drinking behaviors (Epler et al., 2014).
Hangover symptoms and HED were assessed with reference to the past year, whereas participants rated depression symptoms over the past week. The asymmetry in the cross- lagged effects could be an artifact of these discrepant time frames. For example, if hangover events occur relatively infrequently and have only time-limited effects on depression, then an annual assessment of past-week depression may infrequently coincide with recent hangovers and thus may not fully capture hangover effects. A single item was used to assess HED frequency. Better resolution of drinking behavior might have been possible using a calendar-based method (e.g., Sobell & Sobell, 1995). The CES-D is a screening instrument intended for use in nonclinical samples. Future work should examine whether the findings using this dimensional symptom measure extend to clinically diagnos- able major depression.
The current study extends the sparse existing literature by demonstrating empirical associations between symptoms of hangover and depression in a sample of young adults. These associations were modest in magnitude, as might be expected given that both depression and hangover are multi- factorially determined. Nonetheless, using prospective data and accounting for individual differences in HED frequency provides potentially important clues about the nature of the overlap between the two syndromes. Specifically, the findings indicate that elevated depression symptoms are associated with current and future hangover susceptibility. Going forward, it will be important to determine whether these effects replicate in independent samples.
Additional research is needed to explore various mechanisms that could account for the overlap between depression and hangover. This might be pursued using survey measures, as done here. A complementary approach would be to test whether depression status moderates hangover responses to alcohol after laboratory-based alcohol challenge or naturalistic drinking. Because several intersecting lines of evidence implicate inflammatory processes in both depression and hangover, it seems promising to assess biomarkers of inflammation in future studies. Further research into the pathophysiology of hangover may yield new clues about potential mechanistic linkages with symptoms of depression. The contributions of psychosocial factors and life events also deserve careful consideration in future studies.
Footnotes
This work was supported by the National Cancer Institute of the National Institutes of Health under award number 5P01CA098262. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Boden J. M., Fergusson D. M. Alcohol and depression. Addiction. 2011;106:906–914. doi: 10.1111/j.1360-0443.2010.03351.x. doi:10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Vital signs: Binge drinking prevalence, frequency, and intensity among adults - United States, 2010. Morbidity and Mortality Weekly Report. 2012;61:14–19. [PubMed] [Google Scholar]
- Chapman L. F. Experimental induction of hangover. Quarterly Journal of Studies on Alcohol, Supplement. 1970;5:67–86. [PubMed] [Google Scholar]
- Epler A. J., Tomko R. L., Piasecki T. M., Wood P. K., Sher K. J., Shiffman S., Heath A. C. Does hangover influence the time to next drink? An investigation using ecological momentary assessment. Alcoholism: Clinical and Experimental Research. 2014;38:1461–1469. doi: 10.1111/acer.12386. doi:10.1111/acer.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K., Massak A., Demers A., Rehm J. Does the association between alcohol consumption and depression depend on how they are measured? Alcoholism: Clinical and Experimental Research. 2007;31:78–88. doi: 10.1111/j.1530-0277.2006.00274.x. doi:10.1111/j.1530-0277.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- Harburg E., Gunn R., Gleiberman L., DiFranceisco W., Schork A. Psychosocial factors, alcohol use, and hangover signs among social drinkers: A reappraisal. Journal of Clinical Epidemiology. 1993;46:413–422. doi: 10.1016/0895-4356(93)90017-u. doi:10.1016/0895-4356(93)90017-U. [DOI] [PubMed] [Google Scholar]
- Hasin D. S., Goodwin R. D., Stinson F. S., Grant B. F. Epidemiology of major depressive disorder: Results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Archives of General Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. doi:10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Howland J., Rohsenow D. J., Edwards E. M. Are some drinkers resistant to hangover? A literature review. Current Drug Abuse Reviews. 2008;1:42–6. doi: 10.2174/1874473710801010042. doi:10.2174/1874473710801010042. [DOI] [PubMed] [Google Scholar]
- Jackson K. M. Heavy episodic drinking: Determining the predictive utility of five or more drinks. Psychology of Addictive Behaviors. 2008;22:68–77. doi: 10.1037/0893-164X.22.1.68. doi:10.1037/0893-164X.22.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-J., Kim W., Yoon S.-J., Choi B.-M., Kim J.-S., Go H. J., Jeong J. Effects of alcohol hangover on cytokine production in healthy subjects. Alcohol. 2003;31:167–170. doi: 10.1016/j.alcohol.2003.09.003. doi:10.1016/j.alcohol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler E. J. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. doi:10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M., Curzytek K., Duda W., Leskiewicz M., Basta-Kaim A., Budziszewska B., Maes M. A new animal model of (chronic) depression induced by repeated and intermittent lipopolysac- charide administration for 4 months. Brain, Behavior, and Immunity. 2013;31:96–104. doi: 10.1016/j.bbi.2013.01.001. doi:10.1016/j.bbi.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Lewinsohn P. M., Seeley J. R., Roberts R. E., Allen N. B. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. doi:10.1037/0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- McBride O., Cheng H. G., Slade T., Lynskey M. T. The role of specific alcohol-related problems in predicting depressive experiences in a cross-sectional national household survey. Alcohol andAlcoholism. 2016;51:655–663. doi: 10.1093/alcalc/agw010. doi:10.1093/alcalc/agw010. [DOI] [PubMed] [Google Scholar]
- Miller A. H., Raison C. L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology. 2016;16:22–34. doi: 10.1038/nri.2015.5. doi:10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paljärvi T., Koskenvuo M., Poikolainen K., Kauhanen J., Sillanmäki L., Mäkelä P. Binge drinking and depressive symptoms: A 5-year population-based cohort study. Addiction. 2009;104:1168–1178. doi: 10.1111/j.1360-0443.2009.02577.x. doi:10.1111/j.1360-0443.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- Penning R., van Nuland M., Fliervoet L. A. L., Olivier B., Verster J. C. The pathology of alcohol hangover. Current Drug Abus e Reviews. 2010;3:68–75. doi: 10.2174/1874473711003020068. doi:10.2174/1874473711003020068. [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Alley K. J., Slutske W. S., Wood P. K., Sher K. J., Shiffman S., Heath A. C. Low sensitivity to alcohol: Relations with hangover occurrence and susceptibility in an ecological momentary assessment investigation. Journal of Studies on Alcohol and Drugs. 2012;73:925–932. doi: 10.15288/jsad.2012.73.925. doi:10.15288/jsad.2012.73.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki T. M., Robertson B. M., Epler A. J. Hangover and risk for alcohol use disorders: Existing evidence and potential mechanisms. Current Drug Abuse Reviews. 2010;3:92–102. doi: 10.2174/1874473711003020092. doi:10.2174/1874473711003020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki T. M., Sher K. J., Slutske W. S., Jackson K. M. Hangover frequency and risk for alcohol use disorders: Evidence from a longitudinal high-risk study. Journal of Abnormal Psychology. 2005;114:223–234. doi: 10.1037/0021-843X.114.2.223. doi:10.1037/0021-843X.114.2.223. [DOI] [PubMed] [Google Scholar]
- Radloff L. S. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Richey L., Doremus-Fitzwater T. L., Buck H. M., Deak T. Acute illness-induced behavioral alterations are similar to those observed during withdrawal from acute alcohol exposure. Pharmacology, Biochemistry, and Behavior. 2012;103:284–294. doi: 10.1016/j.pbb.2012.08.004. doi:10.1016/j. pbb.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. M., Piasecki T. M., Slutske W. S., Wood P. K., Sher K. J., Shiftman S., Heath A. C. Validity of the Hangover Symptoms Scale: Evidence from an electronic diary study. Alcoholism: Clinical and Experimental Research. 2012;36:171–177. doi: 10.1111/j.1530-0277.2011.01592.x. doi:10.1111/j.1530-0277.2011.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow D. J., Howland J., Winter M., Bliss C. A., Littlefield C. A., Heeren T. C., Calise T. V. Hangover sensitivity after controlled alcohol administration as predictor of post-college drinking. Journal of Abnormal Psychology. 2012;121:270–275. doi: 10.1037/a0024706. doi:10.1037/a0024706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G. M., Irwin M. R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140:774–815. doi: 10.1037/a0035302. doi:10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske W. S., Piasecki T. M., Hunt-Carter E. E. Development and initial validation of the Hangover Symptoms Scale: Prevalence and correlates of hangover symptoms in college students. Alcoholism: Clinical and Experimental Research. 2003;27:1442–1450. doi: 10.1097/01.ALC.0000085585.81711.AE. doi:10.1097/01. ALC.0000085585.81711.AE. [DOI] [PubMed] [Google Scholar]
- Slutske W. S., Piasecki T. M., Nathanson L., Statham D. J., Martin N. G. Genetic influences on alcohol-related hangover. Addiction. 2014;109:2027–2034. doi: 10.1111/add.12699. doi:10.1111/add.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. Toronto, Canada: Addiction Research Foundation; 1995. Alcohol TimelineFollowback Users’ Manual. [Google Scholar]
- Sutin A. R., Terracciano A., Milaneschi Y., An Y., Ferrucci L., Zon- derman A. B. The trajectory of depressive symptoms across the adult life span. JAMA Psychiatry. 2013;70:803–811. doi: 10.1001/jamapsychiatry.2013.193. doi:10.1001/jamapsychiatry. 2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstrup J. S., Stephens R., Granbaek M. Does the severity of hangovers decline with age? Survey of the incidence of hangover in different age groups. Alcoholism: Clinical and Experimental Research. 2014;38:466–470. doi: 10.1111/acer.12238. doi:10.1111/acer.12238. [DOI] [PubMed] [Google Scholar]
- Verster J. C., Stephens R., Penning R., Rohsenow D., McGeary J., Levy D., Young M. The Alcohol Hangover Research Group consensus statement on best practice in alcohol hangover research. Current Drug Abuse Reviews. 2010;3:116–126. doi: 10.2174/1874473711003020116. doi:10.2174/1874473711003020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese J., McPherson S., Odden M. C., Shlipak M. G. Effect of Opuntia ficus indica on symptoms of the alcohol hangover. Archives of Internal Medicine. 2004;164:1334–1340. doi: 10.1001/archinte.164.12.1334. doi:10.1001/archinte.164.12.1334. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Research. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. doi:10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]