Abstract
Objective:
Naltrexone has been identified as a promising psychopharmacological treatment for alcohol dependence. Previous studies have suggested that its efficacy may vary based on ethnic background. The current study examined the efficacy of naltrexone in the treatment of alcohol dependence in Latino adults, a previously unexplored population.
Method:
This was a secondary analysis of the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study. The overall COMBINE sample consisted of 1,383 adult participants who met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for alcohol dependence, including 155 Latinos, who are the focus of this report. Consistent with the main trial, primary drinking outcomes, including percentage of days abstinent (PDA) and time to first heavy drinking day (TTHD), were examined. In addition, we examined the effects of naltrexone on a clinically relevant secondary outcome measure, global clinical outcome of alcohol consumption and alcohol-related problems.
Results:
As seen with the subsample of African Americans from the COMBINE Study, results of the present analysis indicated that there were no significant effects of naltrexone on PDA and TTHD despite these significant effects in the original study. However, contrary to findings in the African American subsample, for Latino participants naltrexone was a significant predictor of a good global clinical outcome (i.e., abstinence or moderate drinking without problems).
Conclusions:
Naltrexone was not significantly associated with improvements in the primary drinking outcomes of PDA or TTHD at the end of treatment or at follow-up. However, Latinos appeared to benefit from naltrexone as demonstrated by improved ratings of global clinical outcome. These results indicate mixed findings for the efficacy of naltrexone among Latinos in the COMBINE Study.
Health disparities associated with alcohol use and abuse have been identified among Latino populations, with consequences of alcohol use being more severe for Latino populations compared with their White1 counterparts (Schmidt et al., 2007). Latino drinkers have more alcohol-related health problems, including higher rates of liver cirrhosis, death rates because of cirrhosis, and overall alcohol-related deaths (Caetano, 2003; Yoon et al., 2001). In addition, compared with White adult drinkers, Latino adult drinkers are more likely to report alcohol dependence (according to criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, [DSM-IV; American Psychiatric Association, 1994]), as well as more negative social consequences as a result of drinking (e.g., fights, workplace accidents, legal problems; Mulia et al., 2009).
Given these disparities and the severity of the consequences of alcohol use for Latinos, it is important to identify effective treatments for this population. There is a dearth of research examining psychopharmacological interventions for alcohol dependence in Latino populations (Trujillo et al., 2006). However, previous studies have identified racial/ethnic2 differences in psychopharmacological treatment for other psychiatric disorders including depression (Marin & Escobar, 2001) and schizophrenia (Frackiewicz et al., 2002; Marin & Escobar, 2001). Similarly, it is possible that ethnic differences exist in the psychopharmacological treatment of alcohol dependence.
Naltrexone, an opioid receptor antagonist, has been identified as a promising psychopharmacological intervention for alcohol dependence (Anton et al., 2006). Initial trials with naltrexone have resulted in significantly fewer drinking days and lower rates of relapse when it was provided in combination with behavioral treatments for alcohol disorders. The Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) Study, a large randomized controlled study, examined the effectiveness of naltrexone as well as acamprosate in combination with two different psychosocial interventions on alcohol dependence (Anton et al., 2006). That study found that naltrexone (but not acamprosate) was an efficacious treatment for alcohol dependence in the context of medical management (a low-intensity medical counseling). Specifically, those receiving naltrexone and medication management had a lower percentage of drinking days and a lower risk of returning to a heavy drinking day. Similarly, naltrexone has been found to reduce relapse to heavy drinking days (Guardia et al., 2002; Srisurapanont & Jarusuraisin, 2005), alcohol consumption (Chick et al., 2000), time to relapse (Kiefer et al., 2003), time to first heavy drink (Kiefer et al., 2003), and alcohol cravings (Chick et al., 2000).
Although studies have found positive effects of naltrexone, there is also evidence that treatment response to naltrexone is not uniform and may vary across different populations (e.g., women, ethnic minority groups). For example, in a subsample of African American participants from the COMBINE Study, Ray and Oslin (2009) found that naltrexone did not appear to be efficacious for the reduction of alcohol-related outcomes in this population. To date, there has been only one study examining the effect of naltrexone on alcohol dependence in a Latino sample. Galarza and colleagues (1997) evaluated the efficacy of naltrexone in reducing alcohol cravings among Puerto Rican male veterans who met criteria for alcohol dependence. A sample of 11 participants was randomly assigned to either a naltrexone or placebo group. No significant differences were found between the two groups; however, the statistical analyses may have been underpowered to detect differences with the small sample size.
Given these findings, further research has been needed on the effects of naltrexone on alcohol dependence among ethnic minority populations, including Latinos. The current study aimed to examine the effects of naltrexone on alcohol dependence among Latino participants in the COMBINE Study, using an analytical approach modeled after previously published studies on this data set (Ray & Oslin, 2009).
Method
Participants
This report is a secondary analysis of the COMBINE Study (Anton et al., 2006). Subjects were recruited from 11 clinical research sites. The overall COMBINE sample consisted of 1,383 adult participants, including 155 Latinos who are the focus of this report. The participants all met DSM-IV criteria for alcohol dependence. Inclusion criteria included drinking an average weekly minimum of 14 drinks (for women) or 21 drinks (for men) with a minimum of 2 heavy drinking days (4 or more drinks for women and 5 or more drinks for men per drinking day) within a 30-day period in the 90 days before the baseline screening; and abstinence for at least 96 consecutive hours. Exclusion criteria are described in detail elsewhere (Anton et al., 2006). Participants underwent full physical examinations, including blood and urine tests to screen for potential safety concerns.
Measures
Drinking outcomes.
The Form 90 (Miller & Del Boca, 1994; Tonigan et al., 1997) was the primary measure of drinking outcomes. Variables included in the current analyses included (a) percentage of days abstinent (PDA) and (b) time to first heavy drinking day (TTHD), operationalized as five or more drinks in a day for men and four or more drinks for women. In addition, we examined the effects of naltrexone on global clinical outcome, a composite measure of alcohol treatment outcome that takes into account alcohol consumption and alcohol-related problems (Cisler & Zweben, 1999). Global clinical outcome classifies participants into one of four categories: 1 = abstinent, 2 = moderate drinking without problems, 3 = heavy drinking or problems, and 4 = heavy drinking and problems, which are further combined into either good clinical outcome (1 and 2) or not (3 and 4).
Procedure
An overview of the methods including assessments, randomization, medical, and behavioral treatments in the COMBINE Study are described in detail elsewhere (Anton et al., 2006). Participants were randomly assigned to one of nine treatment conditions and received 16 weeks of active treatment. Eight of these groups received medical management plus either active/placebo naltrexone or active/placebo acamprosate. These four medication groups were then further divided by two levels of behavioral counseling (i.e., combined behavioral intervention [CBI] vs. no CBI). A ninth group (n =17) received CBI alone, without medical management or pills, and was not included in the analyses presented herein given the focus on naltrexone response. In this study, Latino individuals who received naltrexone during the 16- week treatment trial (n = 69) were compared with those who received placebo (n = 69) regardless of psychotherapy intervention or use of acamprosate.
Results
Demographics
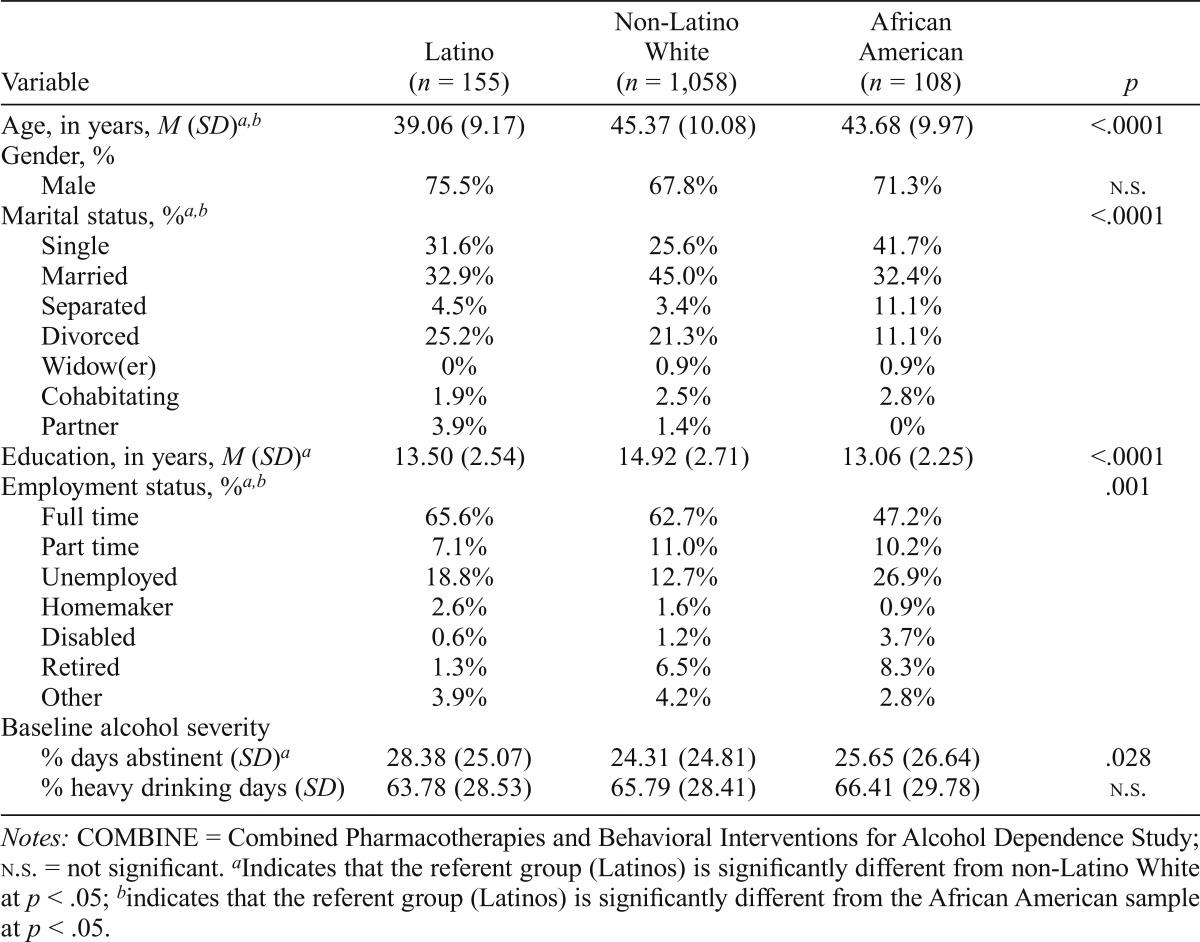
Participants in this subsample of the COMBINE Study included 155 Latinos who were primarily male (75.5%) with an average age of 39 years. Demographic variables and baseline alcohol severity indicators of the Latino subgroup compared with the African American and non-Latino White subgroups (i.e., 96% of the full sample) are presented in Table 1. Differences between Latinos and other ethnic/racial subgroups were found for several demographic variables, including age, marital status, years of education, and employment status. Specifically, Latinos were younger, tended to be nonmarried, and were less educated but were more likely to be employed full time. Of note, significant differences were also found in the baseline alcohol severity indicator PDA but not in percentage of heavy drinking days. Thus, PDA is included as a covariate in all analyses.
Table 1.
Demographics of COMBINE participants by race and ethnicity
| Variable | Latino (n = 155) | Non-Latino White (n = 1,058) | African American (n = 108) | p |
| Age, in years, M (SD)ab | 39.06 (9.17) | 45.37 (10.08) | 43.68 (9.97) | <.0001 |
| Gender, % | ||||
| Male | 75.5% | 67.8% | 71.3% | n.s. |
| Marital status, %ab | ||||
| Single | 31.6% | 25.6% | 41.7% | <.0001 |
| Married | 32.9% | 45.0% | 32.4% | |
| Separated | 4.5% | 3.4% | 11.1% | |
| Divorced | 25.2% | 21.3% | 11.1% | |
| Widow(er) | 0% | 0.9% | 0.9% | |
| Cohabitating | 1.9% | 2.5% | 2.8% | |
| Partner | 3.9% | 1.4% | 0% | |
| Education, in years, M (SD)a | 13.50 (2.54) | 14.92 (2.71) | 13.06 (2.25) | <.0001 |
| Employment status, %a,b | ||||
| Full time | 65.6% | 62.7% | 47.2% | .001 |
| Part time | 7.1% | 11.0% | 10.2% | |
| Unemployed | 18.8% | 12.7% | 26.9% | |
| Homemaker | 2.6% | 1.6% | 0.9% | |
| Disabled | 0.6% | 1.2% | 3.7% | |
| Retired | 1.3% | 6.5% | 8.3% | |
| Other | 3.9% | 4.2% | 2.8% | |
| Baseline alcohol severity | ||||
| % days abstinent (SD)a | 28.38 (25.07) | 24.31 (24.81) | 25.65 (26.64) | .028 |
| % heavy drinking days (SD) | 63.78 (28.53) | 65.79 (28.41) | 66.41 (29.78) | n.s. |
Notes: COMBINE = Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence Study; n.s. = not significant.
Indicates that the referent group (Latinos) is significantly different from non-Latino White at p < .05;
indicates that the referent group (Latinos) is significantly different from the African American sample at p < .05.
Percentage of days abstinent
Mixed-model analyses were used to determine the effects of naltrexone on PDA during treatment. Results revealed no significant effects of naltrexone, t(140) = 0.34, p = .74, B = 1.65, SE = 4.87, after controlling for PDA at baseline, t(140) = 2.85, p = .005, B = 0.28, SE = 0.10, acamprosate, t(140) = 0.52, p = .61, B = 2.52, SE = 4.88, and therapy, t(140) = 0.15, p = .88, B = 0.72, SE = 4.89, as fixed effects and time (month since randomization) as a repeated-measures effect. PDA at baseline was the only significant predictor of PDA at Week 16 (i.e., the end of 4-month treatment). The average PDA at the end of treatment for the naltrexone group was 74.51 (SD = 3.54) and was not determined to be significantly different from the average PDA for those not receiving naltrexone (M = 76.16, SD = 3.34).
Time to first drinking day
A proportional hazards model was evaluated using Cox Regression Survival Analysis to determine whether the use of naltrexone was significantly predictive of time to the first heavy drinking day in this subsample of Latinos. Results of the proportional hazards model revealed no significant main effect of naltrexone for time to the first heavy drinking day (HR, .97, 95% CI [0.65, 1.45], p = .87), after controlling for PDA at baseline (HR, .99, 95% CI [0.99, 1.00], p = .08), acamprosate (HR, .84, 95% CI [0.57, 1.24], p = .38), and therapy (HR, .98, 95% CI [0.66, 1.47], p = .93).
Global clinical outcome
Logistic regression analysis of the composite clinical outcome measure at the end of treatment revealed a significant main effect of naltrexone (odds ratio [OR] = 2.44, 95% CI [1.06, 5.62], p = .036), after controlling for baseline PDA (OR = 1.01, 95% CI [0.990, 1.024], p = .42), acamprosate (OR = 1.29, 95% CI [0.552, 2.99], p = .56), and therapy (OR = 1.13, 95% CI [0.49, 2.62], p = .78). Contrary to other outcome measures of the study, naltrexone was a significant predictor of a good clinical outcome (i.e., abstinence or moderate drinking without problems) for Latino participants.
Discussion
The current study extends to Latinos with alcohol dependence the findings of Ray and Oslin (2009), who demonstrated that naltrexone operated differently in the African American subsample of the larger COMBINE Study. Similar to the findings of Ray and Oslin (2009), the present analyses of the Latino subsample revealed differences in results from the main overall sample. Although Latinos appeared to benefit from the treatment received during their participation in the COMBINE Study as demonstrated by improved ratings of global clinical outcomes, naltrexone was not significantly associated with improvements in PDA or time to first drinking day at the end of treatment or at follow-up, the primary outcome variables of the COMBINE Study.
The potential difference in the efficacy of naltrexone across ethnic groups highlights the importance of the need for further research on mechanisms of action that may be accounting for these variances. Anton and colleagues (2008) found evidence that a Gene x Medication interaction may account for differences in the treatment of alcohol dependence. Specifically, they found a better treatment response to naltrexone for carriers of the Asp40 allele of the gene coding for the µ-opioid receptor (OPRM1). Ray and Oslin (2009) suggested that the low prevalence of this specific polymorphism of the OPRM1 gene among individuals of African descent (<5%) may explain the lack of benefit from naltrexone observed within their sample. However, the Asp40 allele was present in 34% of the Latinos included in the analyses by Anton et al. (2008). This fact suggests that, although this specific genetic variation may explain some of the observed treatment differences, it likely does not account for the majority of the discrepancies of naltrexone treatment effects by race and ethnicity.
However, it is also important to note that the lack of efficacy of the study’s primary outcome variables may be related to insufficient power to detect a statistical difference. Although this was a large sample of Latinos, relatively speaking, it represented only 11% of the overall sample, and this is a recognized limitation of this study. Because of the sample size limitation, this study cannot decisively conclude that naltrexone is not effective alcohol treatment for Latinos. Such a conclusion would be both premature and unwarranted, and even possibly interpreted incorrectly, thereby discouraging Latinos from receiving naltrexone treatment.
Conversely, the analysis of this secondary outcome suggests that naltrexone has a statistically significant effect, similar to that seen in the main trial on improving a measure of global drinking outcome. This validated measure, the global clinical outcome, takes into account both drinking behavior and drinking consequences. This finding, although preliminary, is of potential clinical importance. It suggests that Latinos may benefit from naltrexone treatment, particularly in the area of problems associated with drinking. This result is notable, given that Latinos tend to experience more negative consequences associated with drinking than do other populations (e.g., fights, workplace accidents, legal problems; Mulia et al., 2009). These initial findings warrant further investigation because they also have the potential to contribute to recent discussions critiquing the use of single-outcome alcohol consumption–based cutoffs (e.g., the current 4+/5+ heavy drinking day definition) as a proxy for problematic drinking in clinical samples (Pearson et al., 2016). The use of the 4+/5+ heavy drinking cutoff can have negative effects on how researchers and clinicians determine treatment success and treatment failures, conceivably having implications for health care disparities in Latinos.
Based on traditional alcohol consumption–based cutoffs in this study, naltrexone in Latino participants might not be considered effective despite preliminary evidence of naltrexone’s potential to improve general clinical outcome, because it was not significantly associated with improvements in PDA or time to first heavy drinking day. Although the positive benefit of naltrexone may be true as well for traditional drinking outcome measures, larger sample sizes will be necessary because the effect size is not large enough to detect an ethnic difference. Nonetheless, initial findings suggest a significant effect of naltrexone for Latino participants on an index (global clinical outcome) that accounts for related psychosocial functioning (e.g., drinking consequences). These findings may help corroborate recent messages from larger studies (e.g., Wilson et al., 2016) to review current U.S. Food and Drug Administration guidelines that use only the 4+/5+ criterion and other alcohol consumption–based cutoffs for treatment “failures.” Studies solely using alcohol consumption–based cutoffs may consider similar results as treatment “failures,” partially because of the lack of consideration of these methods of accounting for impactful psychosocial improvements made by people who continue to occasionally drink heavily after treatment without problematic drinking associations (e.g., family/occupational problems, depression, anxiety).
In addition to revising criteria for treatment “success,” further work examining potential differences in the efficacy of treatment interventions for substance abuse among different ethnic groups is needed. As noted in a review by Amaro and colleagues (2006), more empirical research is needed to determine which treatments are efficacious with Latino adults. Given that Latino subgroups differ on alcohol outcomes (Jetelina et al., 2016), treatment effects should be determined across several nationalities (e.g., Puerto Ricans, Cubans, Mexicans) as well as across several generations of immigration status (e.g., foreign born vs. U.S. born; Alegria et al., 2007). Health and health care disparities also vary across nationalities and may intersect with effects of acculturation on gender roles (e.g., Puerto Rican women tend to drink more as they become more acculturated; Marks et al., 1990) and access to treatment (e.g., language barriers, stigma; Caetano et al., 2014; Lara et al., 2005; Zemore et al., 2009).
Subsequent studies should incorporate designs and be powered sufficiently both to determine whether differences in treatment efficacy exist and to identify potential contributors such as genotype (Anton et al., 2008, 2012; Ray & Oslin, 2009) to these differences. Inclusion of these sociodemographic variables in traditional time-to-event analyses and clinically relevant outcomes such as percentage of heavy drinking days is crucial as we work toward identifying and then addressing disparities in health and health care among ethnic minority populations. More descriptives (e.g., nativity, nationality) of the heterogeneous Latino sample can better determine whether the demographic differences observed between this Latino subsample and other racial/ethnic groups are notable, especially in light of the fact that, although the Latinos were on average nearly 5 years younger, they exhibited a similar level of alcohol severity as other subgroups. However, this significant finding may be because Latinos seek treatment at an earlier age than other racial/ethnic groups because of their tendency to experience more negative health and social consequences as a result of drinking.
Footnotes
This research was supported by National Institute on Drug Abuse Grant R01DA025616-04S1. Preparation of the manuscript was also supported by Grant K12HD055885 from the National Institute of Child Health And Human Development and the Office of Research on Women’s Health. Views expressed in this article do not necessarily reflect those of the funding agencies acknowledged.
We use the terms non-Latino White, non-Latino African American, and Latinos to be consistent with labeling used in other databases that are nationally representative (e.g., U.S. Census Bureau). Although definitions of race and ethnicity vary, the U.S. Census refers to White and Black/African American as racial categories and refers to Hispanic/Latino as an ethnicity. According to the U.S. Census Bureau, the term Hispanic/Latino refers to anyone “of Cuban, Mexican, Puerto Rican, South or Central American, or other Spanish culture or origin regardless of race”; the term White “refers to a person having origins in any of the original peoples of Europe, the Middle East, or North Africa”; and the term Black/African American “refers to a person having origins in any of the Black racial groups of Africa” (U.S. Census Bureau, 2011). As such, although we understand that various terms exist to describe different racial, ethnic, and cultural groups, in the current manuscript non-Latino African American refers to someone who is Black and not of Hispanic/Latino origin, non-Latino White similarly refers to someone who is White and not of Hispanic/Latino origin, and Latino refers to someone of Hispanic/Latino origin who may be of any race.
When discussing differences between non-Latino African American, non-Latino White, and Latino groups, we use the term racial/ethnic differences to reflect that the descriptors of each group refer to race and ethnicity per the U.S. Census Bureau (2011).
References
- Alegria M., Sribney W., Woo M., Torres M., Guarnaccia P. Looking beyond nativity: The relation of age at immigration, length of residence, and birth cohorts to the risk of onset of psychiatric disorders for Latinos. Research in Human Development. 2007;4:19–47. doi: 10.1080/15427600701480980. doi:10.1080/15427600701480980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaro H., Arevalo S., Gonzalez G., Szapocznik J., Iguchi M. Y. Needs and scientific opportunities for research on substance abuse treatment among Hispanic adults. Drug and Alcohol Dependence. 2006;84(Supplement 1):S64–S75. doi: 10.1016/j.drugalcdep.2006.05.008. doi:10.1016/j.drugalcdep.2006.05.008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Anton R. F., O’Malley S. S., Ciraulo D. A., Cisler R. A., Couper D., Donovan D. M, Zweben A. the COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. doi:10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton R. F., Oroszi G., O’Malley S., Couper D., Swift R., Pettinati H., Goldman D. An evaluation of µ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: Results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Archives of General Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. doi:10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton R. F., Voronin K. K., Randall P. K., Myrick H., Tiffany A. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: Influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcoholism: Clinical and Experimental Research. 2012;36:2000–2007. doi: 10.1111/j.1530-0277.2012.01807.x. doi:10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano R. Alcohol-related health disparities and treatment-related epidemiological findings among whites, blacks, and Hispanics in the United States. Alcoholism: Clinical and Experimental Research. 2003;27:1337–1339. doi: 10.1097/01.ALC.0000080342.05229.86. doi:10.1097/01.ALC.0000080342.05229.86. [DOI] [PubMed] [Google Scholar]
- Caetano R., Vaeth P, A. C., Chartier K. G., Mills B. A. Chapter 37—Epidemiology of drinking, alcohol use disorders, and related problems in US ethnic minority groups. Handbook of Clinical Neurology. 2014;125:629–648. doi: 10.1016/B978-0-444-62619-6.00037-9. doi:10.1016/B978-0-444-62619-6.00037-9. [DOI] [PubMed] [Google Scholar]
- Chick J., Anton R., Checinski K., Croop R., Drummond D. C., Farmer R., Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol and Alcoholism. 2000;35:587–593. doi: 10.1093/alcalc/35.6.587. doi:10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Cisler R. A., Zweben A. Development of a composite measure for assessing alcohol treatment outcome: Operationalization and validation. Alcoholism: Clinical and Experimental Research. 1999;23:263–271. doi:10.1111/j.1530-0277.1999.tb04109.x. [PubMed] [Google Scholar]
- Frackiewicz E. J., Herrera J. M., Sramek J. J., Collazo Y., Lawson W. B. Risperidone in the treatment of Hispanic inpatients with schizophrenia: A pilot study. Psychiatry: Interpersonal and Biological Processes. 2002;65:371–374. doi: 10.1521/psyc.65.4.371.20237. doi:10.1521/psyc.65.4.371.20237. [DOI] [PubMed] [Google Scholar]
- Galarza N. J., Diaz Ramirez D., Guzman F., Caballero J. A., Martinez A. J. The use of naltrexone to treat ambulatory patients with alcohol dependence. Boletin de la Asociacion Medica de Puerto Rico. 1997;89:157–160. [PubMed] [Google Scholar]
- Guardia J., Caso C., Arias F., Gual A., Sanahuja J., Ramkez M., Casas M. A double-blind, placebo-controlled study of naltrexone in the treatment of alcohol-dependence disorder: Results from a multicenter clinical trial. Alcoholism: Clinical and Experimental Research. 2002;26:1381–1387. doi: 10.1097/01.ALC.0000030561.15921.A9. doi:10.1111/j.1530-0277.2002.tb02682.x. [DOI] [PubMed] [Google Scholar]
- Jetelina K. K., Reingle Gonzalez J. M., Vaeth P. A., Mills B. A., Caetano R. An investigation of the relationship between alcohol use and major depressive disorder across Hispanic national groups. Alcoholism: Clinical and Experimental Research. 2016;40:536–542. doi: 10.1111/acer.12979. doi:10.1111/acer.12979. [DOI] [PubMed] [Google Scholar]
- Kiefer F., Jahn H., Tarnaske T., Helwig H., Briken P., Holzbach R., Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: A double-blind, placebo-controlled study. Archives of General Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. doi:10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- Lara M., Gamboa C., Kahramanian M. I., Morales L. S., Bautista D. E. Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annual Review of Public Health. 2005;26:367–397. doi: 10.1146/annurev.publhealth.26.021304.144615. doi:10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin H., Escobar J. I. Special issues in the psychopharmacological management of Hispanic Americans. Psychopharmacology Bulletin. 2001;35:197–212. [PubMed] [Google Scholar]
- Marks G., Garcia M., Solis J. M. Health risk behaviors of Hispanics in the United States: Findings from HHANES, 1982–84. American Journal of Public Health. 1990;80(Supplement):20–26. doi: 10.2105/ajph.80.suppl.20. doi:10.2105/AJPH.80.Suppl.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. R., Del Boca F. K. Measurement of drinking behavior using the Form 90 family of instruments. Journal of Studies on Alcohol, Supplement. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Mulia N., Ye Y., Greenfield T. K., Zemore S. E. Disparities in alcohol-related problems among white, black, and Hispanic Americans. Alcoholism: Clinical and Experimental Research. 2009;33:654–662. doi: 10.1111/j.1530-0277.2008.00880.x. doi:10.1111/j.1530-0277.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. R., Kirouac M., Witkiewitz K. Questioning the validity of the 4+/5+ binge or heavy drinking criterion in college and clinical populations. Addiction. 2016;111:1720–1726. doi: 10.1111/add.13210. doi:10.1111/add.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. A., Oslin D. W. Naltrexone for the treatment of alcohol dependence among African Americans: Results from the COMBINE Study. Drug and Alcohol Dependence. 2009;105:256–258. doi: 10.1016/j.drugalcdep.2009.07.006. doi:10.1016/j.drugalcdep.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. A., Ye Y., Greenfield T. K., Bond J. Ethnic disparities in clinical severity and services for alcohol problems: Results from the National Alcohol Survey. Alcoholism: Clinical and Experimental Research. 2007;31:48–56. doi: 10.1111/j.1530-0277.2006.00263.x. doi:10.1111/j.1530-0277.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M., Jarusuraisin N. Naltrexone for the treatment of alcoholism: A meta-analysis of randomized controlled trials. International Journal of Neuropsychopharmacology. 2005;8:267–280. doi: 10.1017/S1461145704004997. doi:10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- Tonigan J. S., Miller W. R., Brown J. M. The reliability of Form 90: An instrument for assessing alcohol treatment outcome. Journal of Studies on Alcohol. 1997;58:358–364. doi: 10.15288/jsa.1997.58.358. doi:10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- Trujillo K. A., Castaneda E., Martinez D., Gonzalez G. Biological research on drug abuse and addiction in Latinos: Current status and future directions. Drug and Alcohol Dependence. 2006;84(Supplement):S17–S28. doi: 10.1016/j.drugalcdep.2006.05.004. doi:10.1016/j.drugalcdep.2006.05.004. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. Overview of race and Hispanic origin: 2010. (2010 Census Briefs, C2010BR-02) 2011 Retrieved from https://www.census.gov/prod/cen2010/briefs/c2010br-02.pdf.
- Wilson A. D., Bravo A. J., Pearson M. R., Witkiewitz K. Finding success in failure: Using latent profile analysis to examine heterogeneity in psychosocial functioning among heavy drinkers following treatment. Addiction. 2016;111:2145–2154. doi: 10.1111/add.13518. doi:10.1111/add.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y.-H., Yi H.-y., Grant B. F., Dufour M. C. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2001. Surveillance Report #57: Liver cirrhosis mortality in the United States, 1970–98. Retrieved from https://pubs.niaaa.nih.gov/publications/Cirr98.pdf. [PMC free article] [PubMed] [Google Scholar]
- Zemore S. E., Mulia N., Ye Y., Borges G., Greenfield T. K. Gender, acculturation, and other barriers to alcohol treatment utilization among Latinos in three National Alcohol Surveys. Journal of Substance Abuse Treatment. 2009;36:446–456. doi: 10.1016/j.jsat.2008.09.005. doi:10.1016/j.jsat.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]